OvHV-2 Glycoprotein B Delivered by a Recombinant BoHV-4 Is Immunogenic and Induces Partial Protection against Sheep-Associated Malignant Catarrhal Fever in a Rabbit Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mammalian Cells
2.2. Construction of a BoHV-4 Recombinant Virus
2.3. Reconstitution of Recombinant Viruses
2.4. OvHV-2 gB Expression
2.5. Viral Stocks and Titration
2.6. Viral Growth Curves
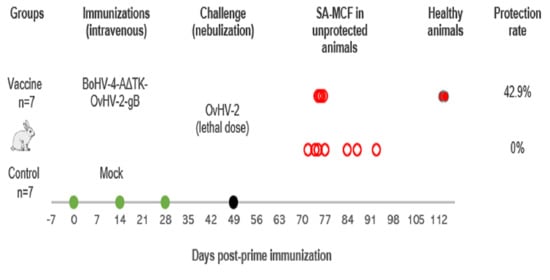
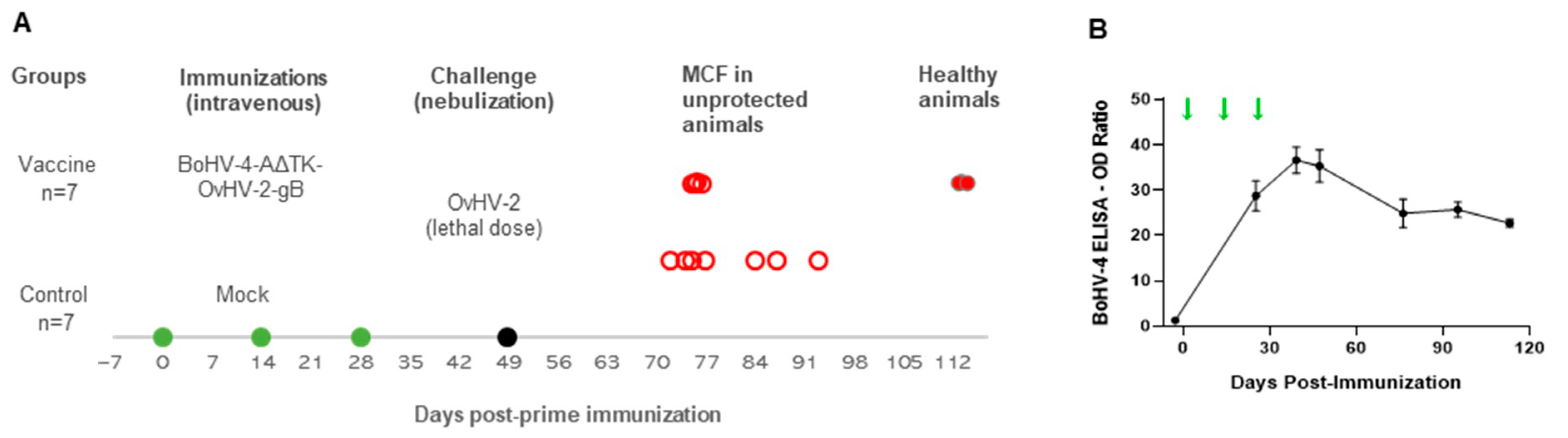
2.7. Animal Trial
2.7.1. Immunization Protocol
2.7.2. OvHV-2 Challenge
2.7.3. Pathology
2.7.4. Viral Detection
2.7.5. BoHV-4 and OvHV-2 gB ELISA
2.7.6. Viral Neutralization Assay
2.8. Data Analyses
3. Results
3.1. Recombinant BoHV-4 Construction and In Vitro Characterization
3.2. Vaccine Trial in a Rabbit Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, G.C.; Stewart, J.P.; Haig, D.M. Malignant catarrhal fever: A review. Vet. J. 2009, 179, 324–335. [Google Scholar] [CrossRef] [PubMed]
- O′Toole, D.; Li, H. The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet. Pathol. 2014, 51, 437–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Taus, N.S.; Jones, C.; Murphy, B.; Evermann, J.F.; Crawford, T.B. A devastating outbreak of malignant catarrhal fever in a bison feedlot. J. Vet. Diagn. Investig. 2006, 18, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.A.; Kohrs, P.; Baszler, T.; Faux, C.; Sathre, P.; Wenz, J.R.; Eldridge, L.; Li, H. Outbreak of malignant catarrhal fever in cattle associated with a state livestock exhibition. J. Am. Vet. Med. Assoc. 2010, 237, 87–92. [Google Scholar] [CrossRef] [PubMed]
- AlHajri, S.M.; Cunha, C.W.; Nicola, A.V.; Aguilar, H.C.; Li, H.; Taus, N.S. Ovine herpesvirus 2 glycoproteins B, H, and L are sufficient for, and viral glycoprotein Ov8 can enhance, cell-cell membrane fusion. J. Virol. 2017, 91, e02454-16. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.W.; Knowles, D.P.; Taus, N.S.; O′Toole, D.; Nicola, A.V.; Aguilar, H.C.; Li, H. Antibodies to ovine herpesvirus 2 glycoproteins decrease virus infectivity and prevent malignant catarrhal fever in rabbits. Vet. Microbiol. 2015, 175, 349–355. [Google Scholar] [CrossRef]
- Jorge, S.; Dellagostin, O.A. The development of veterinary vaccines: A review of traditional methods and modern biotechnology approaches. Biotechnol. Res. Innov. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Franceschi, V.; Capocefalo, A.; Calvo-Pinilla, E.; Redaelli, M.; Mucignat-Caretta, C.; Mertens, P.; Ortego, J.; Donofrio, G. Immunization of knock-out +¦/+¦ interferon receptor mice against lethal bluetongue infection with a BoHV-4-based vector expressing BTV-8 VP2 antigen. Vaccine 2011, 29, 3074–3082. [Google Scholar] [CrossRef]
- Williams, L.B.A.; Fry, L.M.; Herndon, D.R.; Franceschi, V.; Schneider, D.A.; Donofrio, G.; Knowles, D.P. A recombinant bovine herpesvirus-4 vectored vaccine delivered via intranasal nebulization elicits viral neutralizing antibody titers in cattle. PLoS ONE 2019, 14, e0215605. [Google Scholar] [CrossRef] [Green Version]
- Pedrera, M.; Macchi, F.; McLean, R.K.; Franceschi, V.; Thakur, N.; Russo, L.; Medfai, L.; Todd, S.; Tchilian, E.Z.; Audonnet, J.-C.; et al. Bovine Herpesvirus-4-Vectored Delivery of Nipah Virus Glycoproteins Enhances T Cell Immunogenicity in Pigs. Vaccines (Basel) 2020, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Donofrio, G.; Sartori, C.; Franceschi, V.; Capocefalo, A.; Cavirani, S.; Taddei, S.; Flammini, C.F. Double immunization strategy with a BoHV-4-vectorialized secreted chimeric peptide BVDV-E2/BoHV-1-gD. Vaccine 2008, 26, 6031–6042. [Google Scholar] [CrossRef] [PubMed]
- Warming, S.; Costantino, N.; Court, D.; Jenkins, N.A.; Copeland, N.G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005, 33, e36. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.W.; O′Toole, D.; Taus, N.S.; Shringi, S.; Knowles, D.P.; Hong, L. A Rabbit Model for Sheep-Associated Malignant Catarrhal Fever Research: From Virus Infection to Pathogenesis Studies and Vaccine Development. Curr. Clin. Microbiol. Rep. 2019, 6, 148–155. [Google Scholar] [CrossRef]
- Egyed, L.; Ballagi-Pordány, A.; Bartha, A.; Belák, S. Studies of in vivo distribution of bovine herpesvirus type 4 in the natural host. J. Clin. Microbiol. 1996, 34, 1091–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egyed, L.; Sassi, G.; Tibold, J.; Mádl, I.; Szenci, O. Symptomless intrauterine transmission of bovine herpesvirus 4 to bovine fetuses. Microb. Pathog. 2011, 50, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Hussy, D.; Stauber, N.; Leutenegger, C.M.; Rieder, S.; Ackermann, M. Quantitative fluorogenic PCR assay for measuring ovine herpesvirus 2 replication in sheep. Clin. Diagn. Lab. Immunol. 2001, 8, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traul, D.L.; Taus, N.S.; Oaks, J.L.; O′Toole, D.; Rurangirwa, F.R.; Baszler, T.V.; Li, H. Validation of non-nested and real-time PCR for diagnosis of sheep-associated malignant catarrahal fever in clinical samples. J. Vet. Diagn. Investig. 2007, 19, 405–408. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.W.; Taus, N.S.; Dewals, B.G.; Vanderplasschen, A.; Knowles, D.P.; Li, H. Replacement of glycoprotein B in alcelaphine herpesvirus 1 by Its ovine herpesvirus 2 homolog: Implications in vaccine development for sheep-associated malignant catarrhal fever. mSphere 2016, 1, e00108–e00116. [Google Scholar] [CrossRef] [Green Version]
- Donofrio, G.; Franceschi, V.; Capocefalo, A.; Taddei, S.; Sartori, C.; Bonomini, S.; Cavirani, S.; Cabassi, C.S.; Flammini, C.F. Cellular Targeting of Engineered Heterologous Antigens Is a Determinant Factor for Bovine Herpesvirus 4-Based Vaccine Vector Development. Clin. Vaccine Immunol 2009, 16, 1675–1686. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, C.A.; Traesel, C.K.; Music, N.; Laroche, J.; Tison, N.; Auger, J.P.; Music, S.; Provost, C.; Bellehumeur, C.; Abrahamyan, L.; et al. Whole Genome Sequencing of a Canadian Bovine Gammaherpesvirus 4 Strain and the Possible Link between the Viral Infection and Respiratory and Reproductive Clinical Manifestations in Dairy Cattle. Front. Vet. Sci. 2017, 4, 92. [Google Scholar] [CrossRef] [Green Version]
- Areda, D.; Chigerwe, M.; Crossley, B. Bovine herpes virus type-4 infection among postpartum dairy cows in California: Risk factors and phylogenetic analysis. Epidemiol. Infect. 2018, 146, 904–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, F.A.; Reed, D.E. Experimental inoculation of cattle with bovine herpesvirus-4: Evidence for a lymphoid-associated persistent infection. Am. J. Vet. Res. 1983, 44, 975–980. [Google Scholar] [PubMed]
- Chastant-Maillard, S. Impact of Bovine Herpesvirus 4 (BoHV-4) on Reproduction. Transbound. Emerg. Dis. 2015, 62, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Pascottini, O.B.; Xie, J.; Desmarets, L.; Cui, T.; Opsomer, G.; Nauwynck, H.J. Presence of gammaherpesvirus BoHV-4 in endometrial cytology samples is not associated with subclinical endometritis diagnosed at artificial insemination in dairy cows. Vet. Microbiol. 2019, 229, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.A.; Reed, D.E.; Rock, D.L. Experimental infection of rabbits with bovine herpesvirus-4: Acute and persistent infection. Vet. Microbiol. 1982, 7, 503–513. [Google Scholar] [CrossRef]
- Naeem, K.; Murtaugh, M.P.; Goyal, S.M. Tissue distribution of bovid herpesvirus-4 in inoculated rabbits and its detection by DNA hybridization and polymerase chain reaction. Arch. Virol. 1991, 119, 239–255. [Google Scholar] [CrossRef]
- Lankester, F.; Lugelo, A.; Werling, D.; Mnyambwa, N.; Keyyu, J.; Kazwala, R.; Grant, D.; Smith, S.; Parameswaran, N.; Cleaveland, S.; et al. The efficacy of alcelaphine herpesvirus-1 (AlHV-1) immunization with the adjuvants Emulsigen- and the monomeric TLR5 ligand FliC in zebu cattle against AlHV-1 malignant catarrhal fever induced by experimental virus challenge. Vet. Microbiol. 2016, 195, 144–153. [Google Scholar] [CrossRef]
- Parameswaran, N.; Russell, G.C.; Bartley, K.; Grant, D.M.; Deane, D.; Todd, H.; Dagleish, M.P.; Haig, D.M. The effect of the TLR9 ligand CpG-oligodeoxynucleotide on the protective immune response to alcelaphine herpesvirus-1-mediated malignant catarrhal fever in cattle. Vet. Res. 2014, 45, 59. [Google Scholar] [CrossRef] [Green Version]
- Russell, G.; Benavides, J.; Grant, D.; Todd, H.; Deane, D.; Percival, A.; Thomson, J.; Connelly, M.; Haig, D. Duration of protective immunity and antibody responses in cattle immunised against alcelaphine herpesvirus-1-induced malignant catarrhal fever. Vet. Res. 2012, 43, 51. [Google Scholar] [CrossRef] [Green Version]
- Cook, E.; Russell, G.; Grant, D.; Mutisya, C.; Omoto, L.; Dobson, E.; Lankester, F.; Nene, V. A randomised vaccine field trial in Kenya demonstrates protection against wildebeest-associated malignant catarrhal fever in cattle. Vaccine 2019, 37, 5946–5953. [Google Scholar] [CrossRef]
- Haig, D.M.; Grant, D.; Deane, D.; Campbell, I.; Thomson, J.; Jepson, C.; Buxton, D.; Russell, G.C. An immunisation strategy for the protection of cattle against alcelaphine herpesvirus-1-induced malignant catarrhal fever. Vaccine 2008, 26, 4461–4468. [Google Scholar] [CrossRef] [PubMed]


| Vaccine | Total | ||
|---|---|---|---|
| BoHV-4-A∆TK-OvHV-2-gB | Mock | ||
| Protected | 3 (42.9%) | 0 (0%) | 3 |
| Unprotected (MCF) | 4 (57.1%) | 7 (100%) | 11 |
| Total | 7 | 7 | 14 |
| Vaccine 1 | Challenge 2 | Animal ID | Neutralizing Antidodies 3 | DPC | Health Status | Lesion Scores 4 | |||
|---|---|---|---|---|---|---|---|---|---|
| Lung | Liver | Spleen | Mes LN | ||||||
| BoHV-4-A∆TK- OvHV-2gB | OvHV-2 | 3020 | pos (16) | 64 | Healthy | NVL | NVL | NVL | NVL |
| 3021 | pos (32) | 27 | MCF | NA | ++ | NVL | ++ | ||
| 3022 | pos (16) | 26 | MCF | + | ++ | NVL | ++ | ||
| 3023 | pos (128) | 64 | Healthy | NVL | NVL | NVL | NVL | ||
| 3024 | pos (32) | 26 | MCF | + | ++ | NVL | NVL | ||
| 3025 | pos (128) | 27 | MCF | + | +++ | NVL | NVL | ||
| 3026 | pos (64) | 64 | Healthy | NVL | NVL | NVL | NVL | ||
| Mock | OvHV-2 | 3030 | Neg | 26 | MCF | + | +++ | NVL | NVL |
| 3031 | Neg | 38 | MCF | + | + | NVL | NVL | ||
| 3032 | Neg | 35 | MCF | ++ | ++ | NVL | NVL | ||
| 3033 | Neg | 25 | MCF | + | ++ | NVL | NVL | ||
| 3034 | Neg | 28 | MCF | + | ++ | NVL | NVL | ||
| 3035 | Neg | 44 | MCF | + | + | NVL | NVL | ||
| 3036 | Neg | 23 | MCF | ++ | +++ | NVL | NVL | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shringi, S.; O’Toole, D.; Cole, E.; Baker, K.N.; White, S.N.; Donofrio, G.; Li, H.; Cunha, C.W. OvHV-2 Glycoprotein B Delivered by a Recombinant BoHV-4 Is Immunogenic and Induces Partial Protection against Sheep-Associated Malignant Catarrhal Fever in a Rabbit Model. Vaccines 2021, 9, 90. https://doi.org/10.3390/vaccines9020090
Shringi S, O’Toole D, Cole E, Baker KN, White SN, Donofrio G, Li H, Cunha CW. OvHV-2 Glycoprotein B Delivered by a Recombinant BoHV-4 Is Immunogenic and Induces Partial Protection against Sheep-Associated Malignant Catarrhal Fever in a Rabbit Model. Vaccines. 2021; 9(2):90. https://doi.org/10.3390/vaccines9020090
Chicago/Turabian StyleShringi, Smriti, Donal O’Toole, Emily Cole, Katherine N. Baker, Stephen N. White, Gaetano Donofrio, Hong Li, and Cristina W. Cunha. 2021. "OvHV-2 Glycoprotein B Delivered by a Recombinant BoHV-4 Is Immunogenic and Induces Partial Protection against Sheep-Associated Malignant Catarrhal Fever in a Rabbit Model" Vaccines 9, no. 2: 90. https://doi.org/10.3390/vaccines9020090
APA StyleShringi, S., O’Toole, D., Cole, E., Baker, K. N., White, S. N., Donofrio, G., Li, H., & Cunha, C. W. (2021). OvHV-2 Glycoprotein B Delivered by a Recombinant BoHV-4 Is Immunogenic and Induces Partial Protection against Sheep-Associated Malignant Catarrhal Fever in a Rabbit Model. Vaccines, 9(2), 90. https://doi.org/10.3390/vaccines9020090