Construction and Immunogenicity Comparison of Three Virus-Like Particles Carrying Different Combinations of Structural Proteins of Avian Coronavirus Infectious Bronchitis Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Generation and Analysis of Recombinant Protein Expression
2.3. Preparation and Purification of VLPs
2.4. Identification of Virus-Like Particles
2.5. Immunization and Challenge
2.6. Detection of IBV-Specific Antibodies
2.7. Detection of IBV-Neutralizing Antibody Titers
2.8. Flow Cytometry
2.9. Cytokine Assay
2.10. Detection of Secretory IgA(sIgA)
2.11. Detection of Virus Loads by Real-Time Quantitative PCR
2.12. Ciliostasis Test
2.13. Statistical Analysis
3. Results
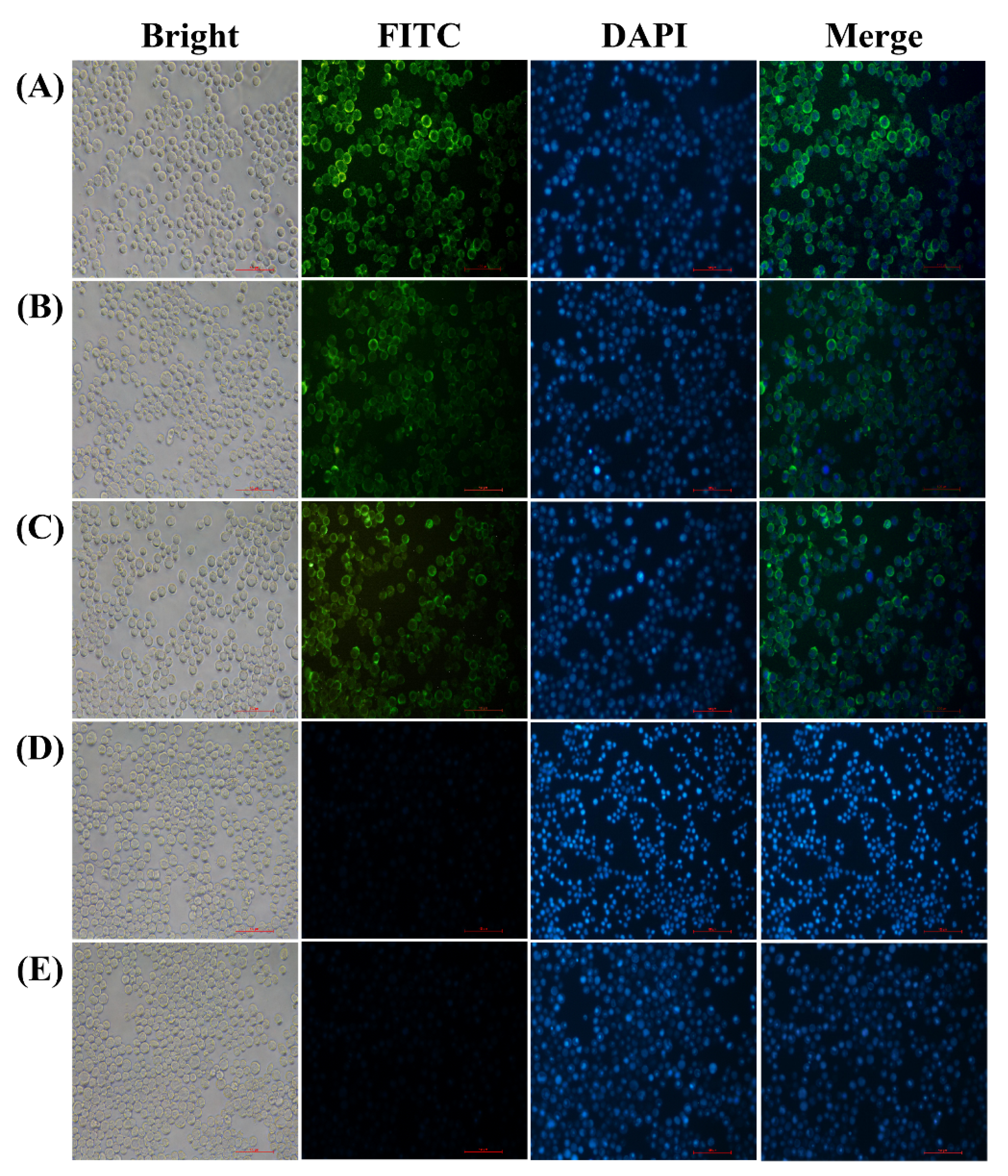
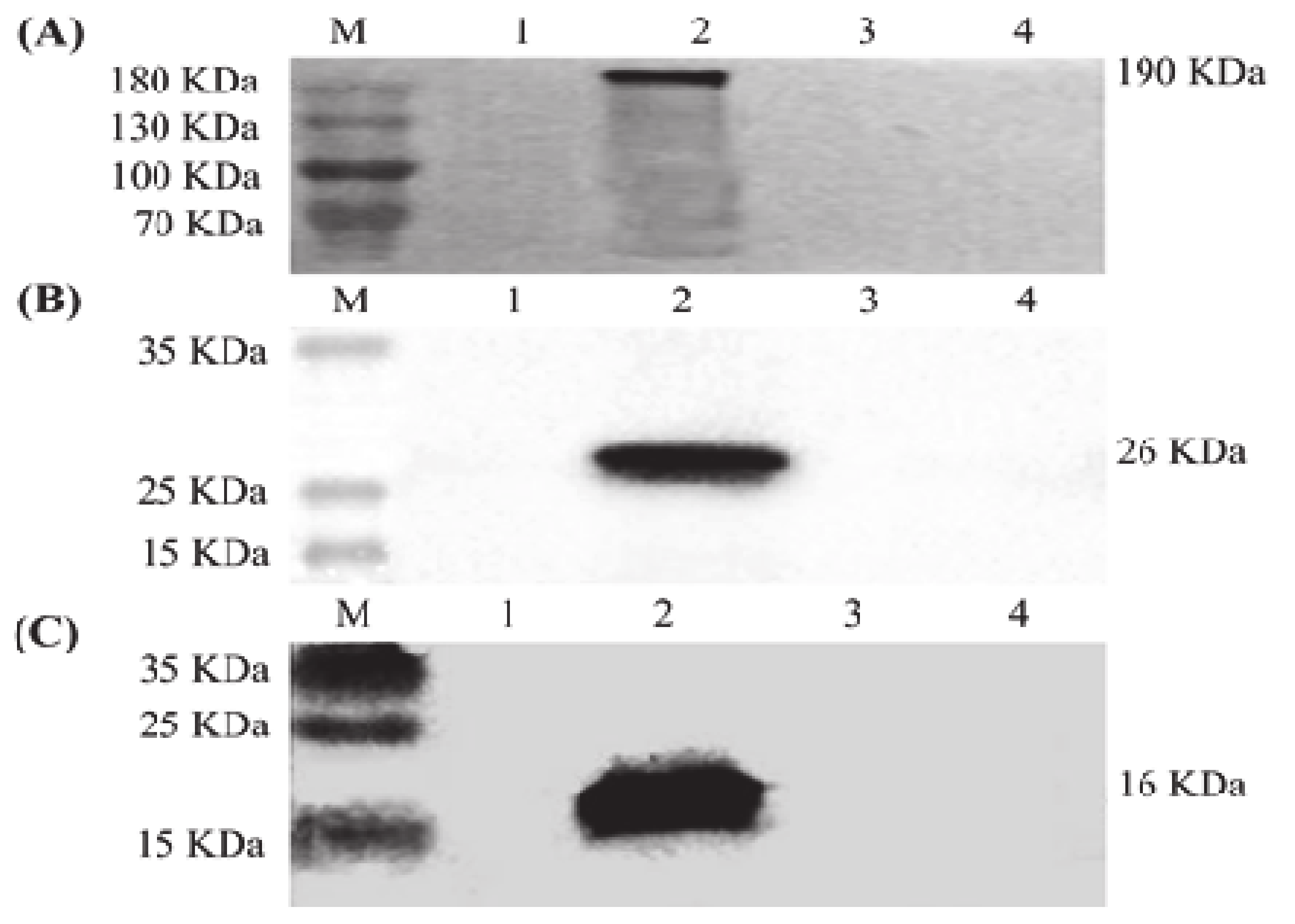
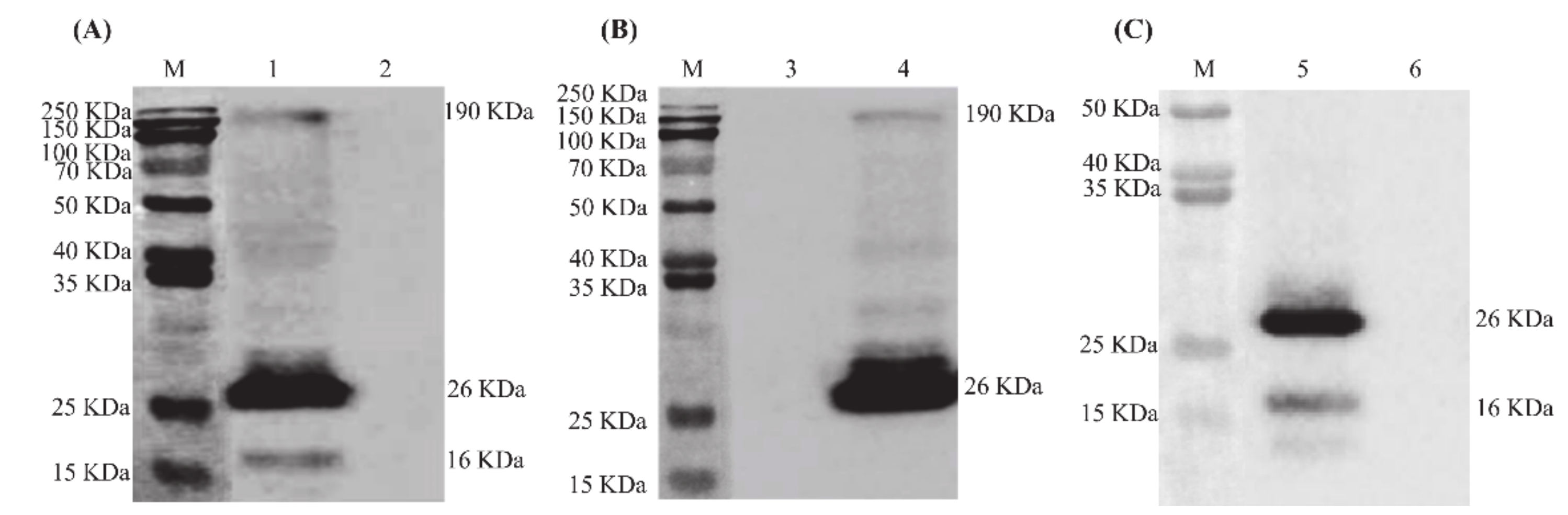
3.1. Expression of S, M and E Proteins in Sf9 Cells
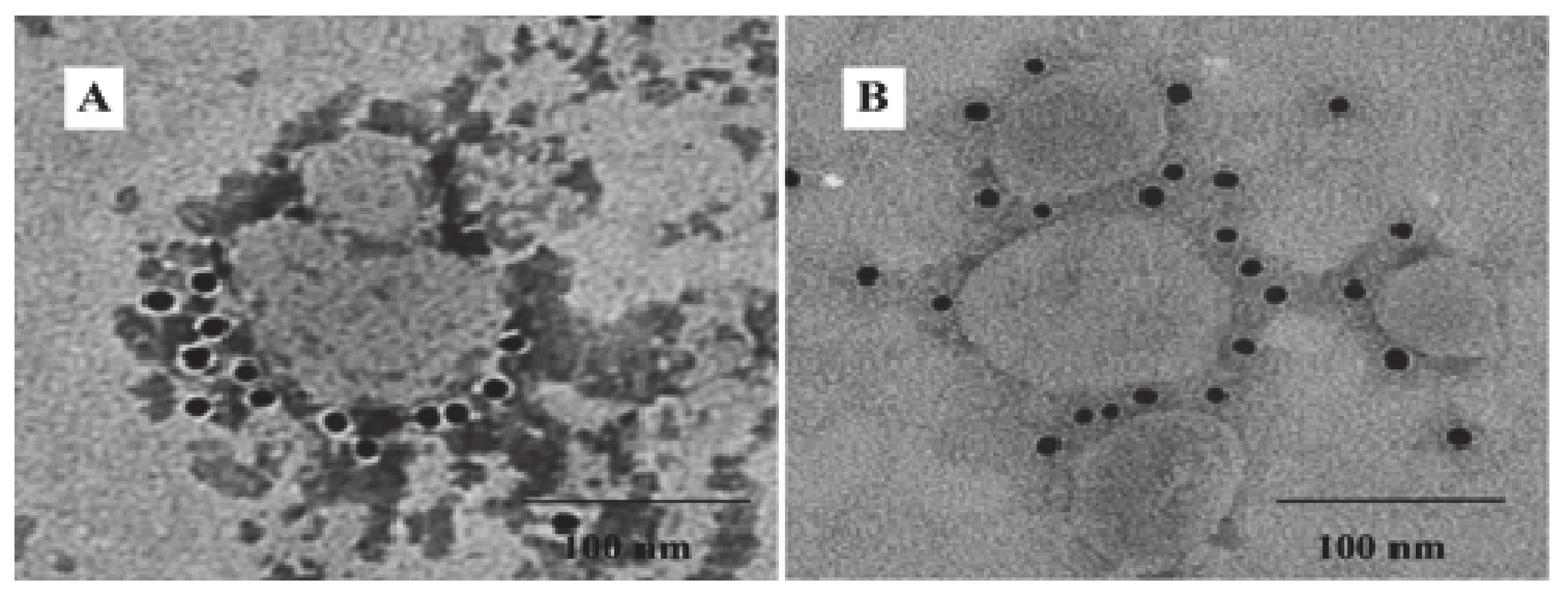
3.2. Generation and Characterization of IBV VLPs
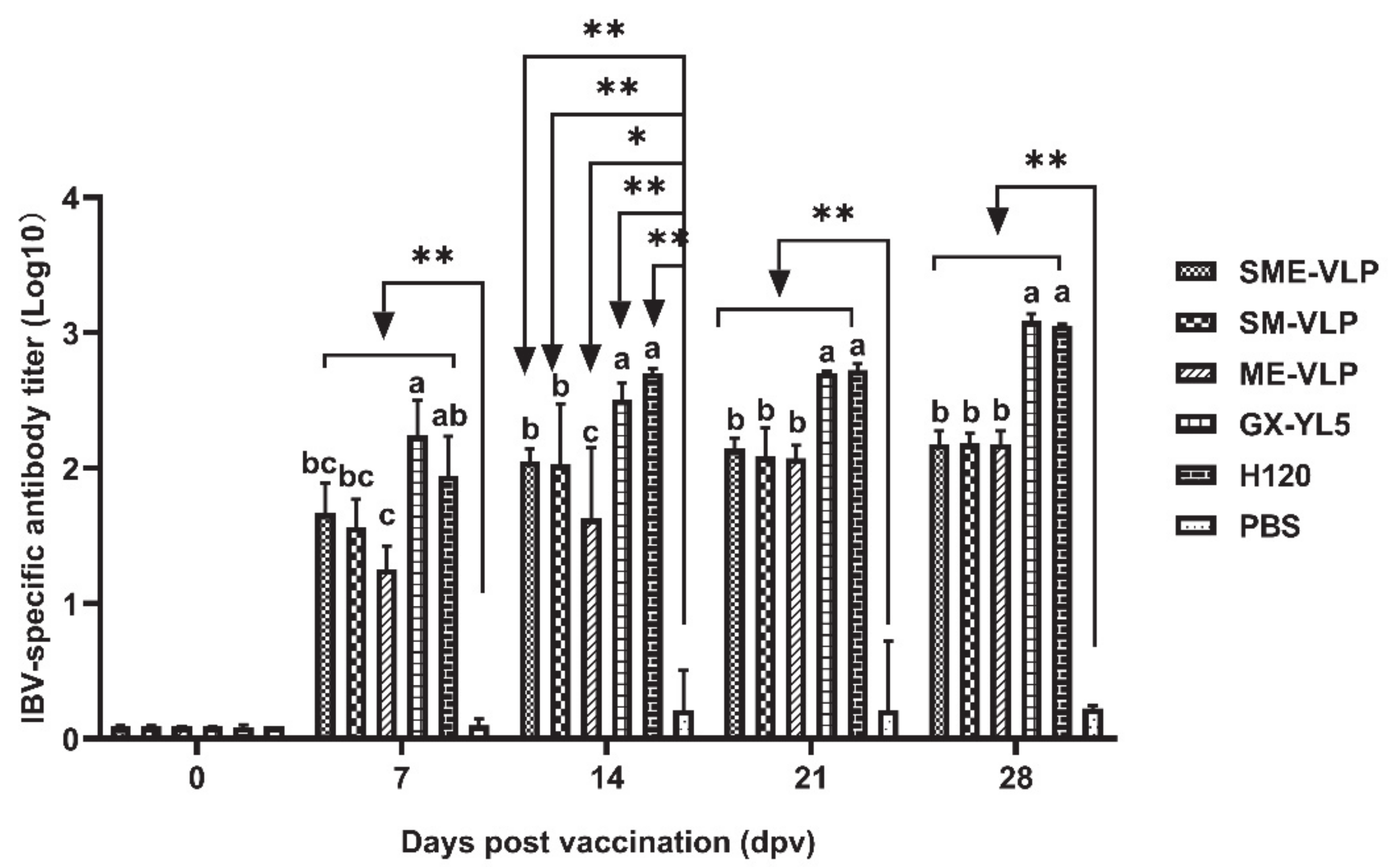
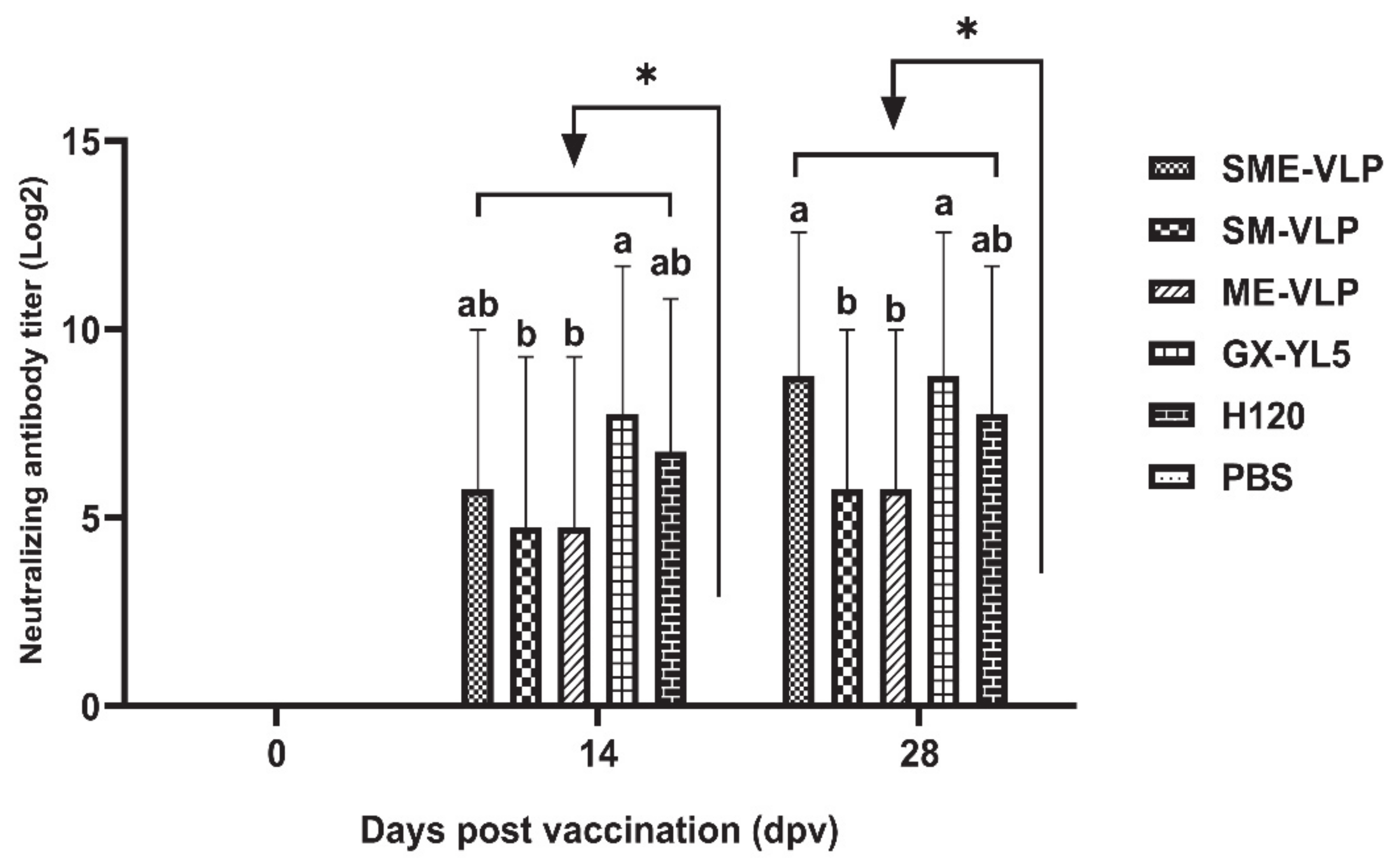
3.3. Humoral Immune Responses in Immunized Chickens
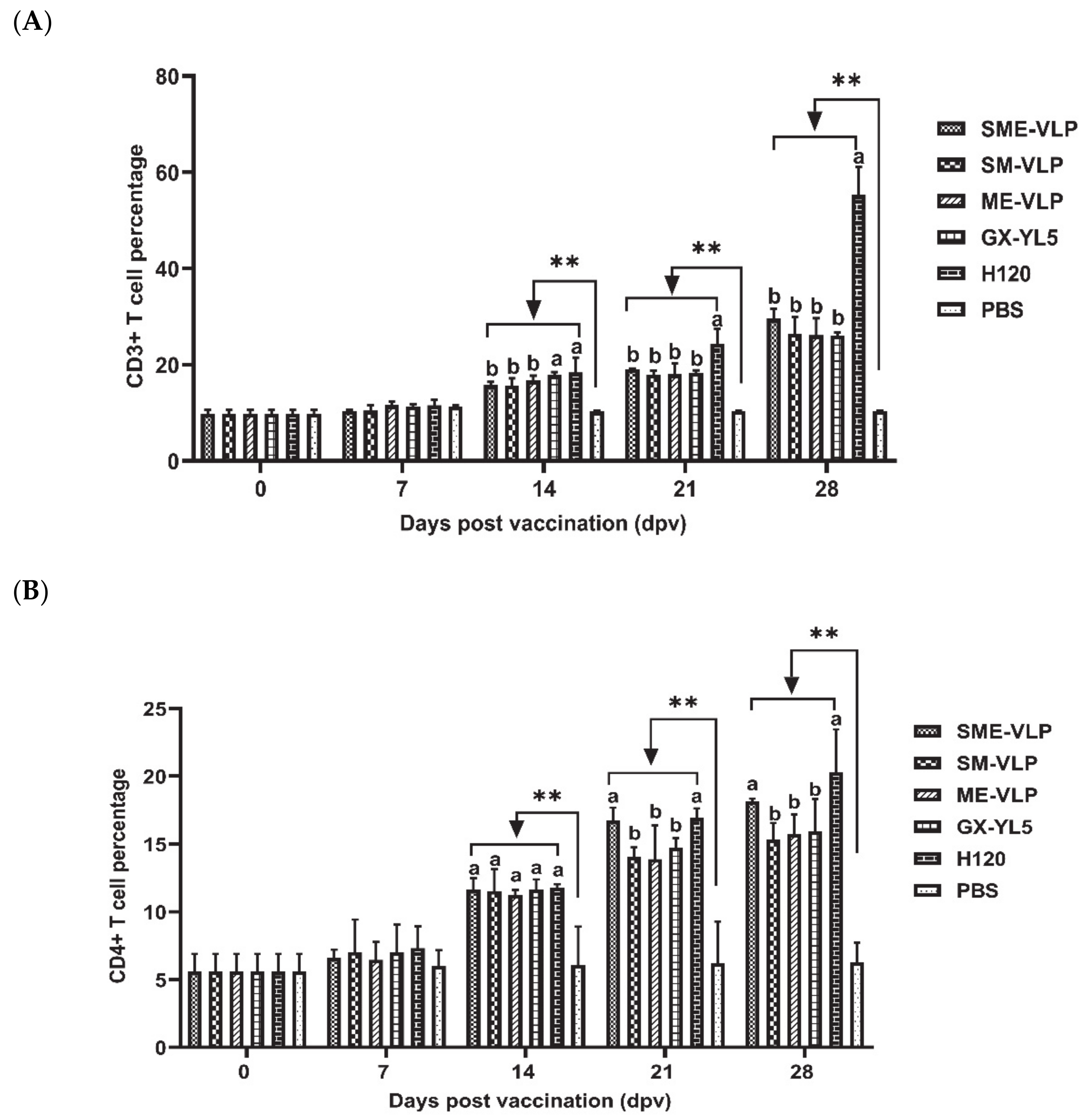
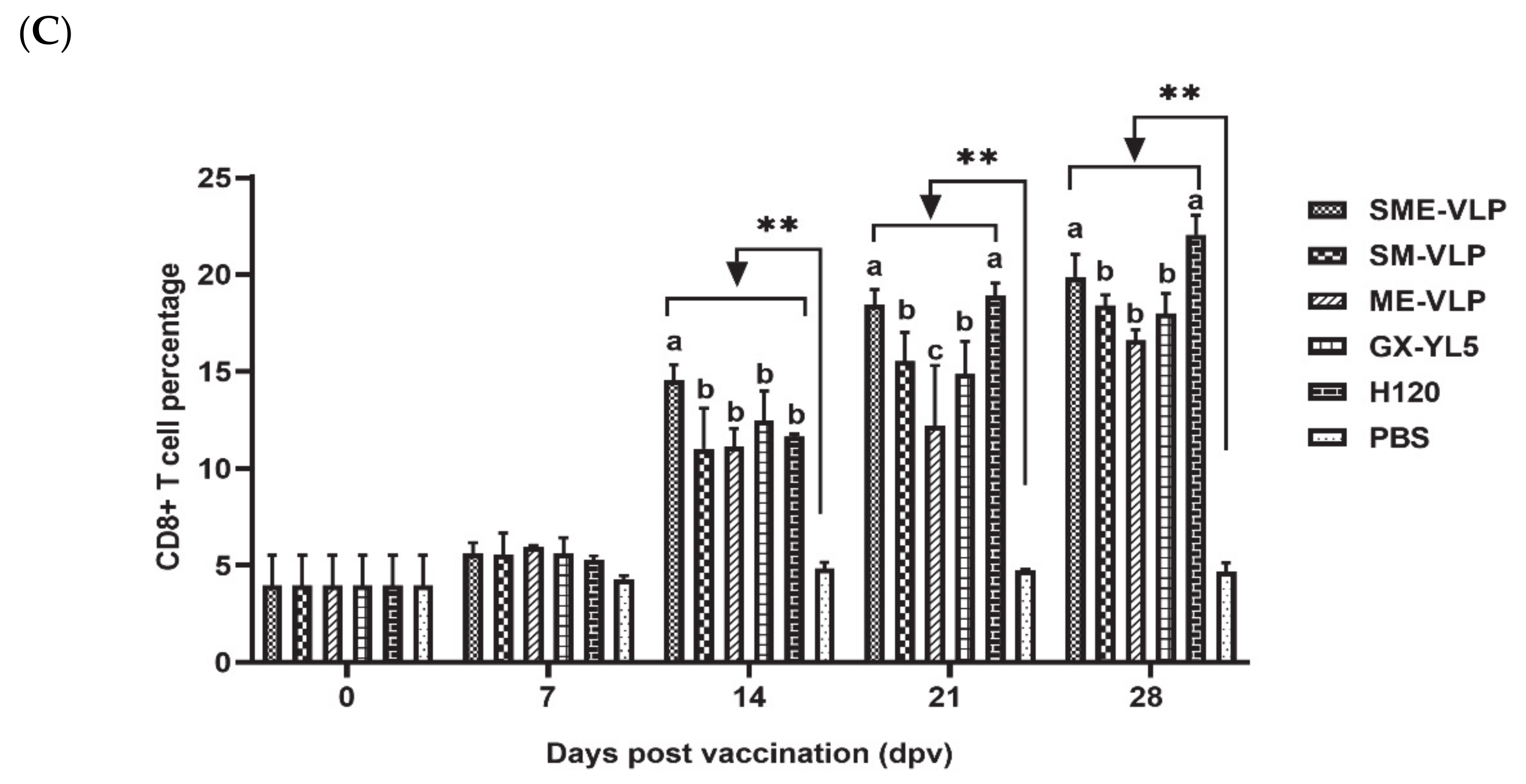
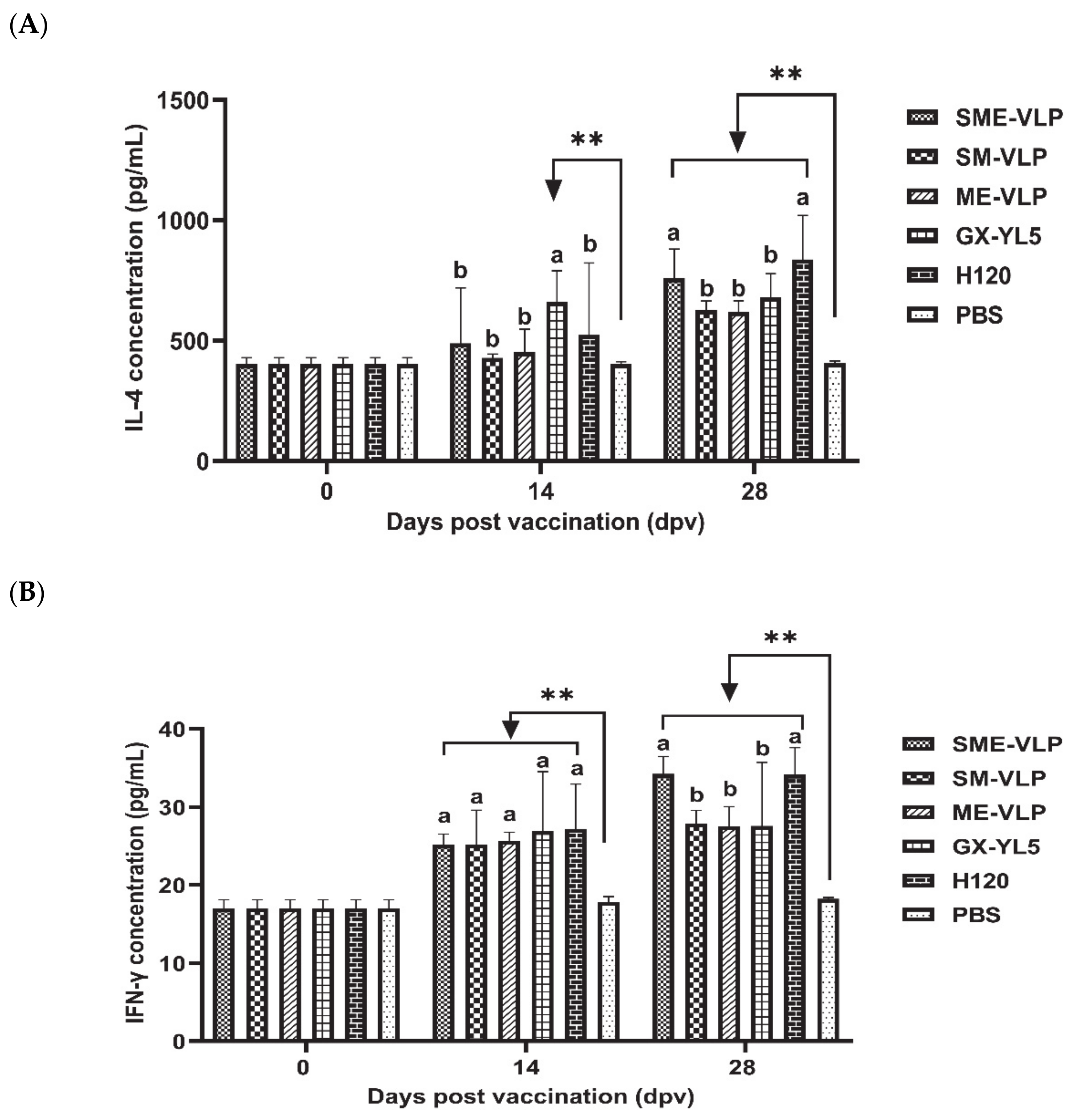
3.4. Cellular Immune Responses in Immunized Chickens
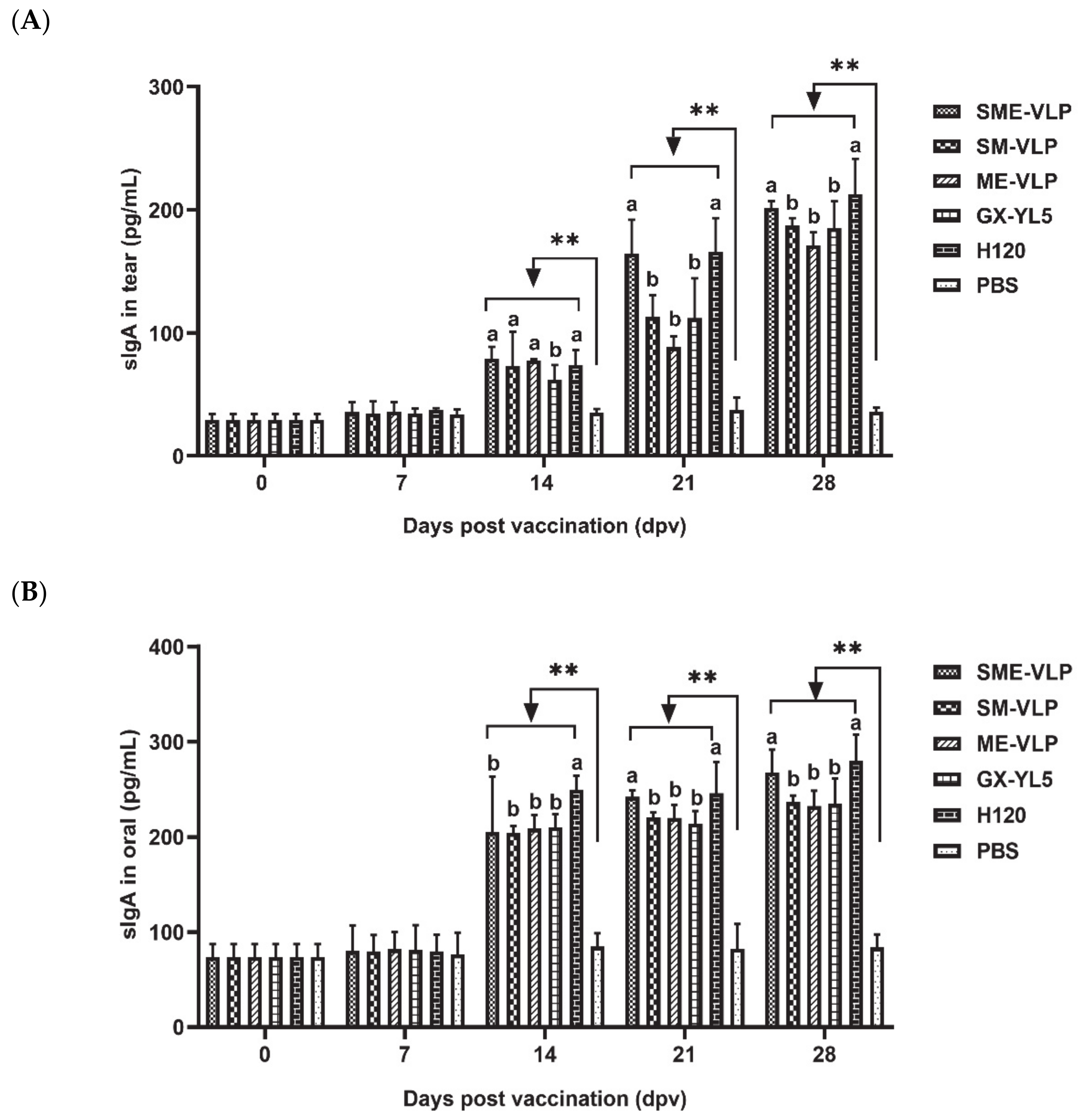
3.5. Mucosal Immune Responses in Immunized Chickens
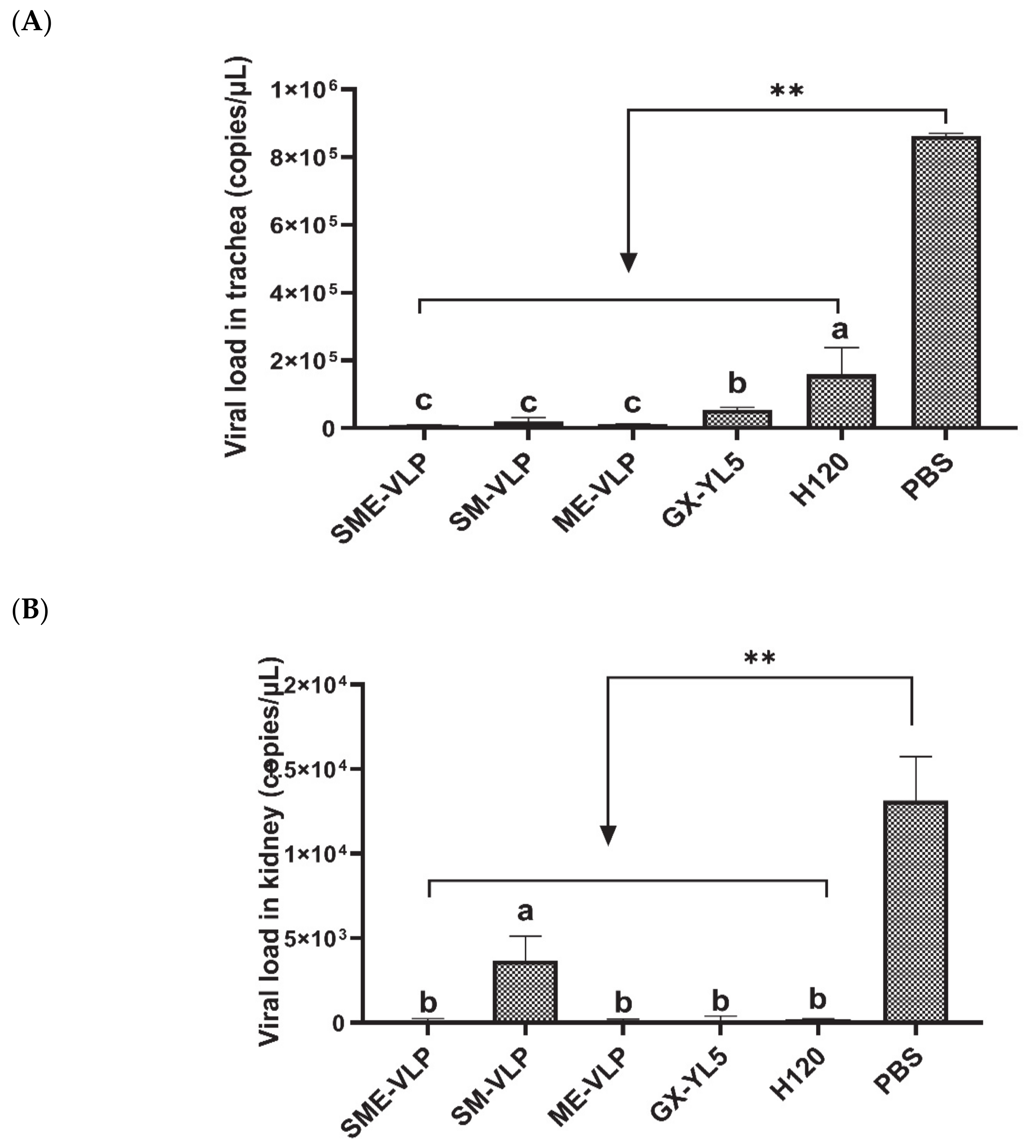
3.6. Protection against the IBV Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef]
- Cook, J.; Jackwood, M.; Jones, R. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef]
- McMullin, P. Diseases of poultry 14th edition. Avian Pathol. 2020, 49, 167–188. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Xu, Q.; Sun, N.; Han, Z.; Sun, C.; Guo, H.; Shao, Y.; Kong, X.; Liu, S. Characterization of a recombinant coronavirus infectious bronchitis virus with distinct S1 subunits of spike and nucleocapsid genes and a 3’ untranslated region. Vet. Microbiol. 2013, 162, 429–436. [Google Scholar] [CrossRef]
- Leyson, C.; Franca, M.; Jackwood, M.; Jordan, B. Polymorphisms in the S1 spike glycoprotein of Arkansas-type infectious bronchitis virus (IBV) show differential binding to host tissues and altered antigenicity. Virology 2016, 498, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D.; Davis, P.; Cook, J.; Li, D.; Kant, A.; Koch, G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992, 21, 33–43. [Google Scholar] [CrossRef] [PubMed]
- De Haan, C.; Vennema, H.; Rottier, P. Assembly of the coronavirus envelope: Homotypic interactions between the M proteins. J. Virol. 2000, 74, 4967–4978. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Hogue, B. Protein interactions during coronavirus assembly. J. Virol. 1997, 71, 9278–9284. [Google Scholar] [CrossRef] [PubMed]
- Ruch, T.; Machamer, C. A single polar residue and distinct membrane topologies impact the function of the infectious bronchitis coronavirus E protein. PLoS Pathog. 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Masters, P.S.; Kuo, L.; Ye, R.; Hurst, K.R.; Koetzner, C.A.; Hsue, B. Genetic and molecular biological analysis of protein-protein interactions in coronavirus assembly. Adv. Exp. Med. Biol. 2006, 581, 163–173. [Google Scholar]
- Casais, R.; Dove, B.; Cavanagh, D.; Britton, P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 2003, 77, 9084–9089. [Google Scholar] [CrossRef] [PubMed]
- Niesters, H.; Bleumink-Pluym, N.; Osterhaus, A.; Horzinek, M.; Van Der Zeijst, B. Epitopes on the peplomer protein of infectious bronchitis virus strain M41 as defined by monoclonal antibodies. Virology 1987, 161, 511–519. [Google Scholar] [CrossRef]
- Shapouri, M.R.S.A.; Mayahi, M.; Assasi, K.; Charkhkar, S. A survey of the prevalence of infectious bronchitis virus type 4/91 in Iran. Acta Vet. Hung. 2004, 52, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, V.L.; Toro, H. Rapid selection in chickens of subpopulations within ArkDPI-derived infectious bronchitis virus vaccines. Avian Pathol. 2009, 37, 293–306. [Google Scholar] [CrossRef]
- McKinley, E.; Hilt, D.; Jackwood, M. Avian coronavirus infectious bronchitis attenuated live vaccines undergo selection of subpopulations and mutations following vaccination. Vaccine 2008, 26, 1274–1284. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, Y.; Gao, H.; Jing, Z.; Wang, M.; Cui, H.; Tong, G.; Wang, Y. Evaluation of recombinant fowlpox virus expressing infectious bronchitis virus S1 gene and chicken interferon-γ gene for immune protection against heterologous strains. Vaccine 2011, 29, 1576–1582. [Google Scholar] [CrossRef]
- Zhang, T.; Han, Z.; Xu, Q.; Wang, Q.; Gao, M.; Wu, W.; Shao, Y.; Li, H.; Kong, X.; Liu, S. Serotype shift of a 793/B genotype infectious bronchitis coronavirus by natural recombination. Infect. Genet. Evol. 2015, 32, 377–387. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Xu, P.; Zhou, L.; Zhang, Z.; Wang, H. Genome sequence and origin analyses of the recombinant novel IBV virulent isolate SAIBK2. Virus Genes 2016, 52, 509–520. [Google Scholar] [CrossRef]
- Ladman, B.; Pope, C.; Ziegler, A.; Swieczkowski, T.; Callahan, J.M.; Davison, S.; Gelb, J. Protection of chickens after live and inactivated virus vaccination against challenge with nephropathogenic infectious bronchitis virus PA/Wolgemuth/98. Avian Dis. 2002, 46, 938–944. [Google Scholar] [CrossRef]
- Meeusen, E.; Walker, J.; Peters, A.; Pastoret, P.; Jungersen, G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007, 20, 489–510. [Google Scholar] [CrossRef]
- Bande, F.; Arshad, S.; Bejo, M.H.; Moeini, H.; Omar, A.R. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J. Immunol. Res. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Li, M.; Huang, B.; Fan, W.; Wei, P.; Wei, T.; Cheng, Q.; Wei, Z.; Lang, Y. Molecular characterization of major structural protein genes of avian coronavirus infectious bronchitis virus isolates in southern China. Viruses 2013, 5, 3007–3020. [Google Scholar] [CrossRef]
- Li, M.; Mo, M.; Huang, B.; Fan, W.; Wei, Z.; Wei, T.; Li, K.; Wei, P. Continuous evolution of avian infectious bronchitis virus resulting in different variants co-circulating in southern China. Arch. Virol. 2013, 158, 1783–1786. [Google Scholar] [CrossRef]
- Feng, K.; Wang, F.; Xue, Y.; Zhou, Q.; Chen, F.; Bi, Y.; Xie, Q. Epidemiology and characterization of avian infectious bronchitis virus strains circulating in southern China during the period from 2013–2015. Sci. Rep. UK 2017, 7, 6576. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Wei, P.; Chen, Q.; Wei, Z.; Mo, M. Serotype and genotype diversity of infectious bronchitis viruses isolated during 1985–2008 in Guangxi, China. Arch. Virol. 2012, 157, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, M.; Sun, R.; Wu, Z.; He, K.; Mo, M.; Wei, T.; Wei, P. Genotypes and serotypes of avian infectious bronchitis viruses isolated during 2009–2011 in Guangxi, China. Bing Du Xue Bao 2014, 30, 162–170. [Google Scholar] [PubMed]
- Fan, W.; Tang, N.; Dong, Z.; Chen, J.; Zhang, W.; Zhao, C.; He, Y.; Li, M.; Wang, C.; Wei, T.; et al. Genetic analysis of avian coronavirus infectious bronchitis virus in yellow chickens in southern China over the past decade: Revealing the changes of genetic diversity, dominant genotypes, and selection pressure. Viruses 2019, 11, 898. [Google Scholar] [CrossRef]
- Fan, W.; Li, H.; He, Y.; Tang, N.; Zhang, L.; Wang, H.; Zhong, L.; Chen, J.; Wei, T.; Huang, T.; et al. Immune protection conferred by three commonly used commercial live attenuated vaccines against the prevalent local strains of avian infectious bronchitis virus in southern China. J. Vet. Med. Sci. 2018, 80, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Chroboczek, J.; Szurgot, I.; Szolajska, E. Virus-like particles as vaccine. Acta Biochim. Pol. 2014, 61, 531–539. [Google Scholar] [CrossRef]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef]
- Beyer, T.; Herrmann, M.; Reiser, C.; Bertling, W.; Hess, J. Bacterial carriers and virus-like-particles as antigen delivery devices: Role of dendritic cells in antigen presentation. Current drug targets. Infect. Disord. 2001, 1, 287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Roden, R. Virus-like particles for the prevention of human papillomavirus-associated malignancies. Expert Rev. Vaccines 2014, 12, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, Y.; Huang, L.; Li, B.; Zheng, Z.; Chen, Z.; Chen, J.; Hu, Q.; Wang, H. Effect of mucosal and systemic immunization with virus-like particles of severe acute respiratory syndrome coronavirus in mice. Immunology 2010, 130, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, X.; Gai, W.; Zhao, Y.; Wang, H.; Wang, H.; Feng, N.; Chi, H.; Qiu, B.; Li, N.; et al. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget 2017, 8, 12686–12694. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Takami, Y.; Kumar, V.; Park, E. Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J. Biotechnol. 2019, 306, 177–184. [Google Scholar] [CrossRef]
- Ho, Y.; Lin, P.; Liu, C.; Lee, S.; Chao, Y. Assembly of human severe acute respiratory syndrome coronavirus-like particles. Biochem. Biophys. Res. Commun. 2004, 318, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Siu, Y.; Teoh, K.; Lo, J.; Chan, C.; Kien, F.; Escriou, N.; Tsao, S.; Nicholls, J.; Altmeyer, R.; Peiris, J.; et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008, 82, 11318–11330. [Google Scholar] [CrossRef]
- Liu, G.; Lv, L.; Yin, L.; Li, X.; Luo, D.; Liu, K.; Xue, C.; Cao, Y. Assembly and immunogenicity of coronavirus-like particles carrying infectious bronchitis virus M and S proteins. Vaccine 2013, 31, 5524–5530. [Google Scholar] [CrossRef]
- Xu, P.; Wu, X.; Wang, H.; Ma, B.; Ding, M.; Yang, X. Assembly and immunogenicity of baculovirus-derived infectious bronchitis virus–like particles carrying membrane, envelope and the recombinant spike proteins. Biotechnol. Lett. 2016, 38, 299–304. [Google Scholar] [CrossRef]
- Lv, L.; Li, X.; Liu, G.; Li, R.; Liu, Q.; Shen, H.; Wang, W.; Xue, C.; Cao, Y. Production and immunogenicity of chimeric virus-like particles containing the spike glycoprotein of infectious bronchitis virus. J. Vet. Sci. 2014, 15, 209. [Google Scholar] [CrossRef]
- Wu, X.; Zhai, X.; Lai, Y.; Zuo, L.; Zhang, Y.; Mei, X.; Xiang, R.; Kang, Z.; Zhou, L.; Wang, H. Construction and immunogenicity of novel chimeric virus-like particles bearing antigens of infectious bronchitis virus and newcastle disease virus. Viruses 2019, 11, 254. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Z.; He, Y.; Fan, W.; Dong, Z.; Zhang, L.; Sun, X.; Song, L.; Wei, T.; Mo, M.; et al. Protection against virulent infectious bronchitis virus challenge conferred by a recombinant baculovirus co-expressing S1 and N proteins. Viruses 2018, 10, 347. [Google Scholar] [CrossRef]
- Zhu, W.; Wen, Y.; Lin, S.; Chen, T.; Chen, H. Anti-influenza protective efficacy of a H6 virus-like particle in chickens. Vaccines 2020, 8, 465. [Google Scholar] [CrossRef]
- He, Y.; Xie, Z.; Dai, J.; Cao, Y.; Hou, J.; Zheng, Y.; Wei, T.; Mo, M.; Wei, P. Responses of the Toll-like receptor and melanoma differentiation-associated protein 5 signaling pathways to avian infectious bronchitis virus infection in chicks. Virol. Sin. 2016, 31, 57–68. [Google Scholar] [CrossRef]
- Cook, J.; Orbell, S.; Woods, M.; Huggins, M. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999, 28, 477–485. [Google Scholar] [CrossRef]
- De Wit, J.; Cook, J. Factors influencing the outcome of infectious bronchitis vaccination and challenge experiments. Avian Pathol. 2014, 43, 485–497. [Google Scholar] [CrossRef]
- Lee, S.; Markham, P.; Coppo, M.; Legione, A.; Markham, J.; Noormohammadi, A.; Browning, G.; Ficorilli, N.; Hartley, C.; Devlin, J. Attenuated vaccines can recombine to form virulent field viruses. Science 2012, 337, 188. [Google Scholar] [CrossRef]
- Tarpey, I.; Orbell, S.; Britton, P.; Casais, R.; Hodgson, T.; Lin, F.; Hogan, E.; Cavanagh, D. Safety and efficacy of an infectious bronchitis virus used for chicken embryo vaccination. Vaccine 2006, 24, 6830–6838. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000, 24, 187–200. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Tong, T.; Ye, Y.; Liao, M.; Fan, H. BacMam virus-based surface display of the infectious bronchitis virus (IBV) S1 glycoprotein confers strong protection against virulent IBV challenge in chickens. Vaccine 2014, 32, 664–670. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yuan, Y.; Zhang, L.-H.; Zhu, D.; Wang, L.; Wei, L.-P.; Fan, W.-S.; Zhao, C.-R.; Su, Y.-J.; Liao, J.-Q.; et al. Construction and Immunogenicity Comparison of Three Virus-Like Particles Carrying Different Combinations of Structural Proteins of Avian Coronavirus Infectious Bronchitis Virus. Vaccines 2021, 9, 146. https://doi.org/10.3390/vaccines9020146
Zhang Y, Yuan Y, Zhang L-H, Zhu D, Wang L, Wei L-P, Fan W-S, Zhao C-R, Su Y-J, Liao J-Q, et al. Construction and Immunogenicity Comparison of Three Virus-Like Particles Carrying Different Combinations of Structural Proteins of Avian Coronavirus Infectious Bronchitis Virus. Vaccines. 2021; 9(2):146. https://doi.org/10.3390/vaccines9020146
Chicago/Turabian StyleZhang, Yu, Yuan Yuan, Li-Hua Zhang, Dan Zhu, Lu Wang, Lan-Ping Wei, Wen-Sheng Fan, Chang-Run Zhao, Yan-Jing Su, Jian-Qi Liao, and et al. 2021. "Construction and Immunogenicity Comparison of Three Virus-Like Particles Carrying Different Combinations of Structural Proteins of Avian Coronavirus Infectious Bronchitis Virus" Vaccines 9, no. 2: 146. https://doi.org/10.3390/vaccines9020146
APA StyleZhang, Y., Yuan, Y., Zhang, L.-H., Zhu, D., Wang, L., Wei, L.-P., Fan, W.-S., Zhao, C.-R., Su, Y.-J., Liao, J.-Q., Yong, L., Wei, T.-C., Wei, P., & Mo, M.-L. (2021). Construction and Immunogenicity Comparison of Three Virus-Like Particles Carrying Different Combinations of Structural Proteins of Avian Coronavirus Infectious Bronchitis Virus. Vaccines, 9(2), 146. https://doi.org/10.3390/vaccines9020146




