Evaluation of a Virus-like Nanoparticle Porcine Circovirus Type-2 (PCV2) Capsid Protein Fused with the Pig Immunoglobulin Fc Fragment as a Novel Vaccine Candidate against PCV2 in Mice
Abstract
1. Introduction
2. Materials and Methods
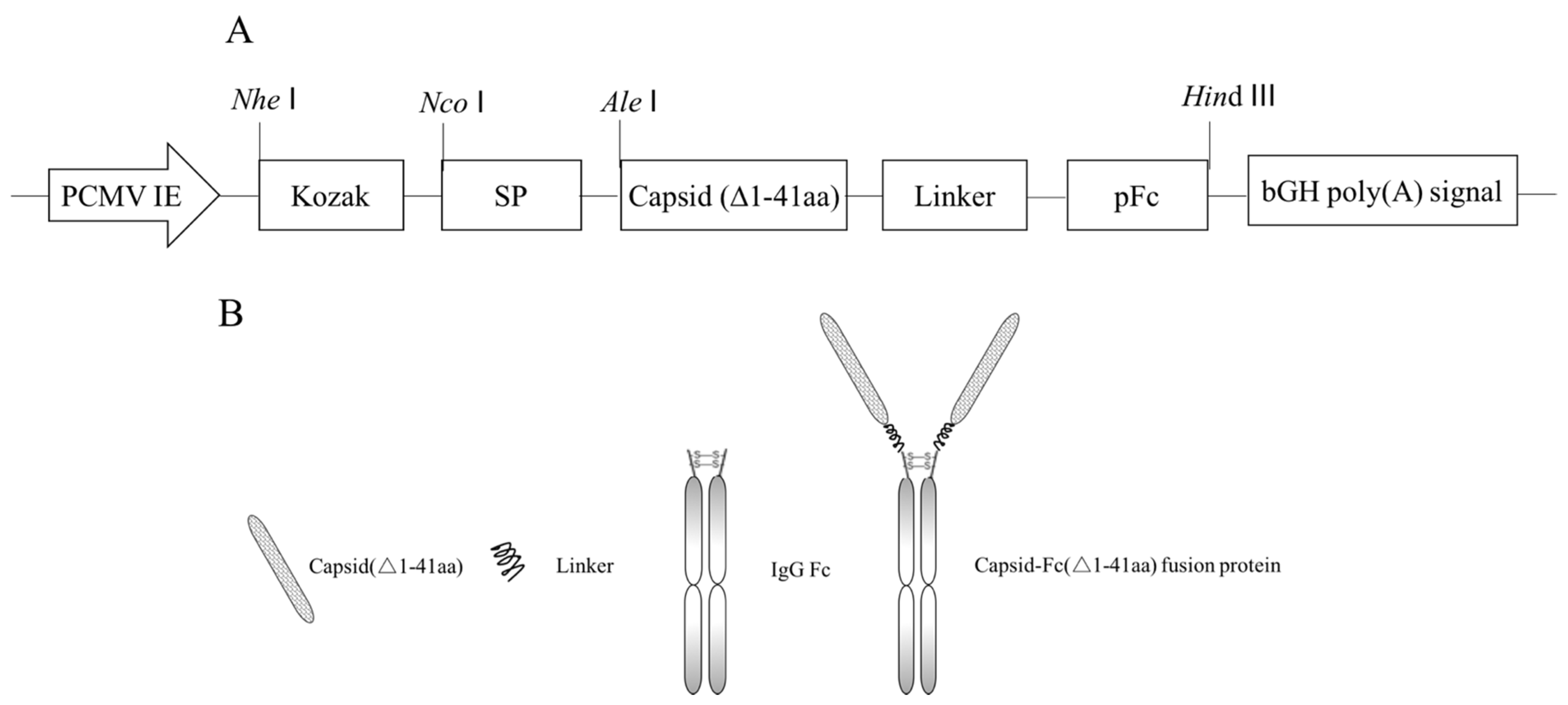
2.1. Vector Construction
2.2. Cell Culture
2.3. Transfection Condition
2.4. Expression and Purification of Recombinant PCFP Fusion Protein in HEK293F Cells
2.5. SDS-PAGE and Western Blot Analysis
2.6. Morphology Analysis by Transmission Electron Microscope
2.7. Immunization of Mice with Recombinant Fusion Protein
2.8. Evaluation of Specific Antibodies
2.9. Proliferation Assay and Cytokine Determination
2.10. Flow Cytometry Analysis
2.11. Statistical Analysis
3. Results
3.1. Construction of Recombinant Plasmid pEM-PCFP
3.2. Production and Purification of PCFP Fusion Protein
3.3. PCFP Fusion Protein Spontaneously Assembled into Virus-Like Nanoparticles
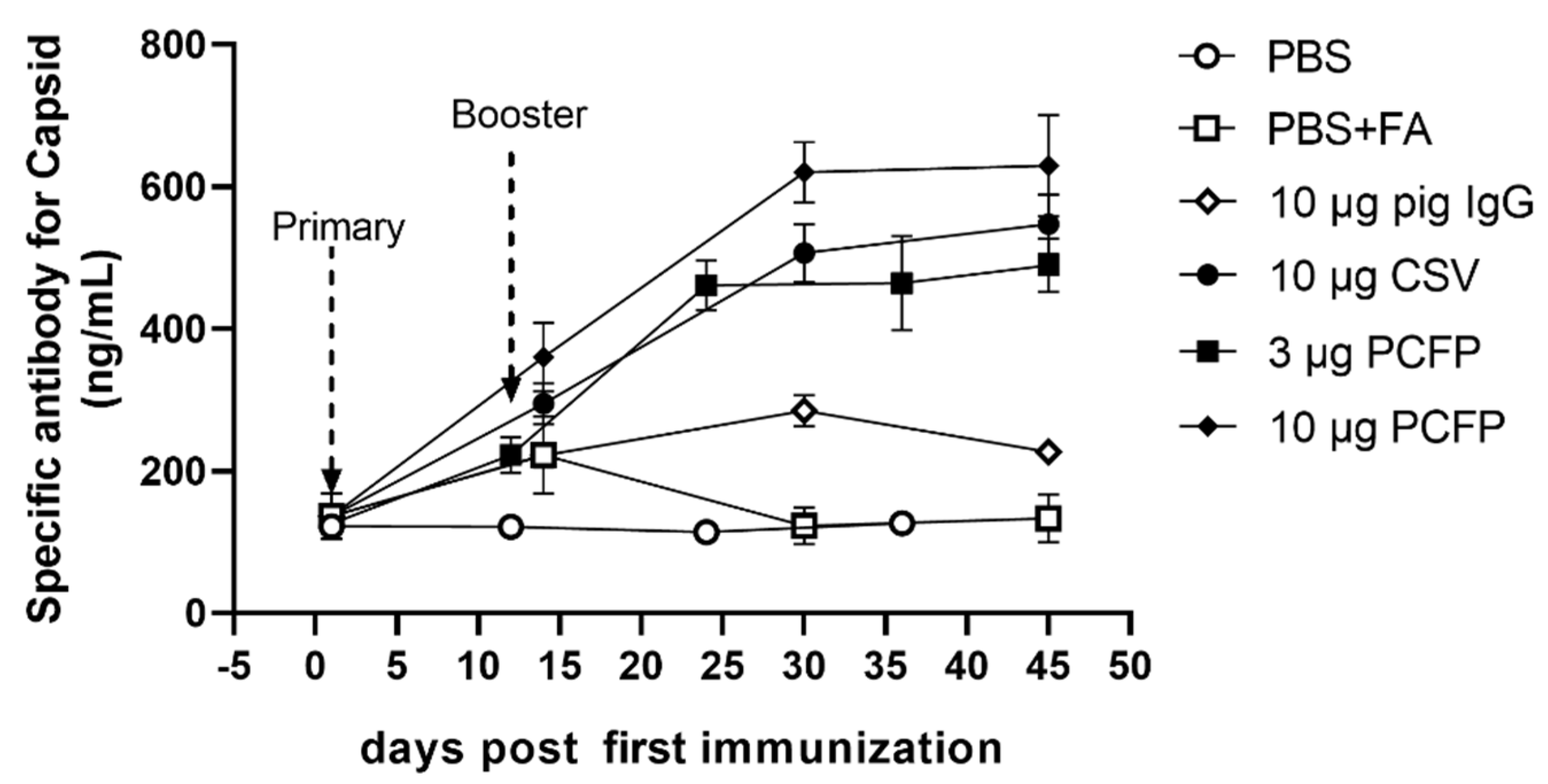
3.4. PCFP Induced Neutralizing Antibodies in Immunized Mice
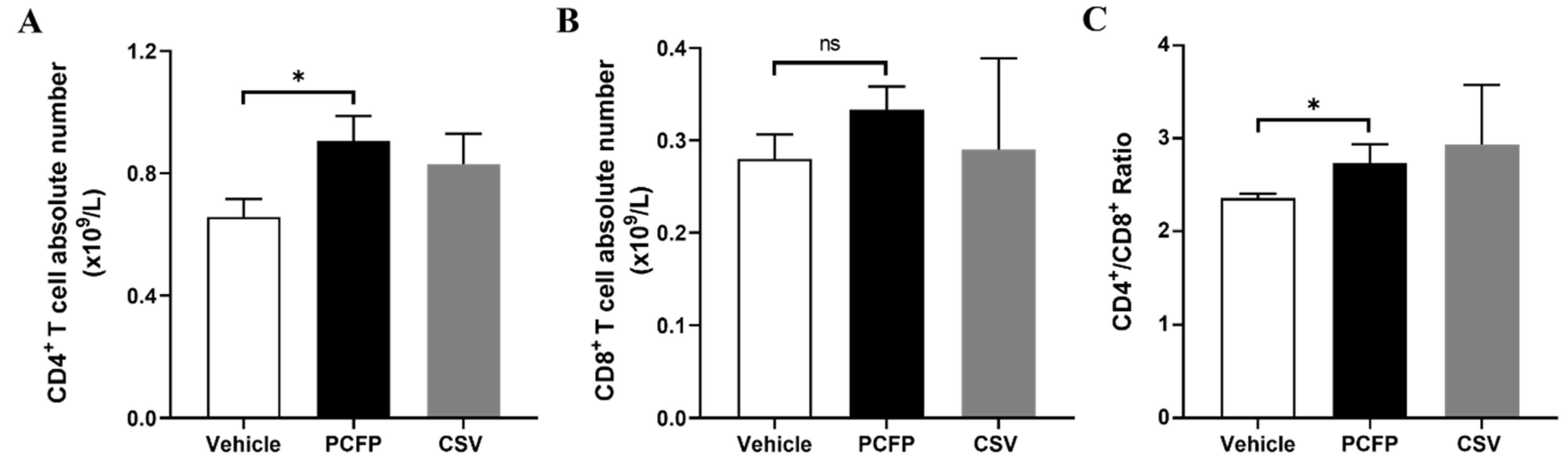
3.5. PCFP Changed Immuno-Profiling of Peripheral T Lymphocytes in Immunized Mice
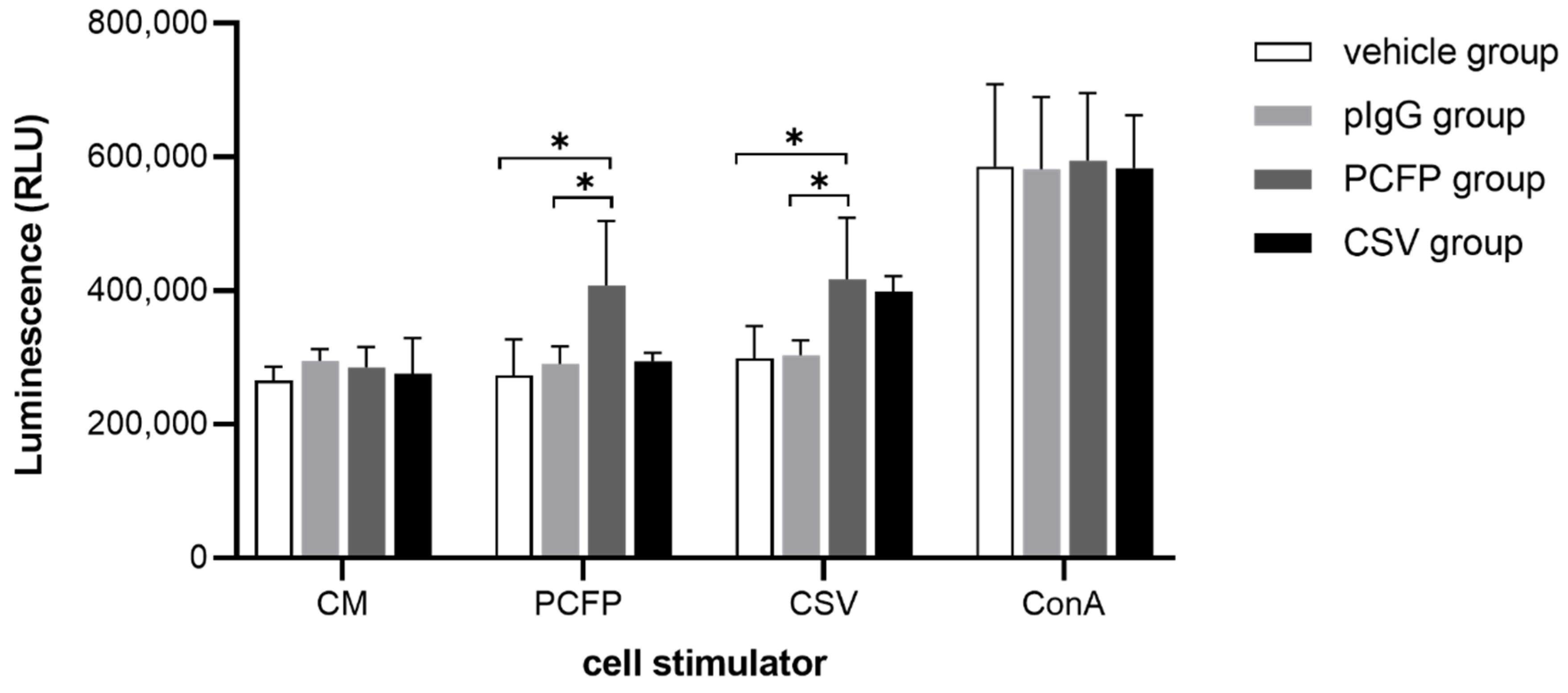
3.6. PCFP Immunization Elicited Specific Lymphocyte Proliferative Responses
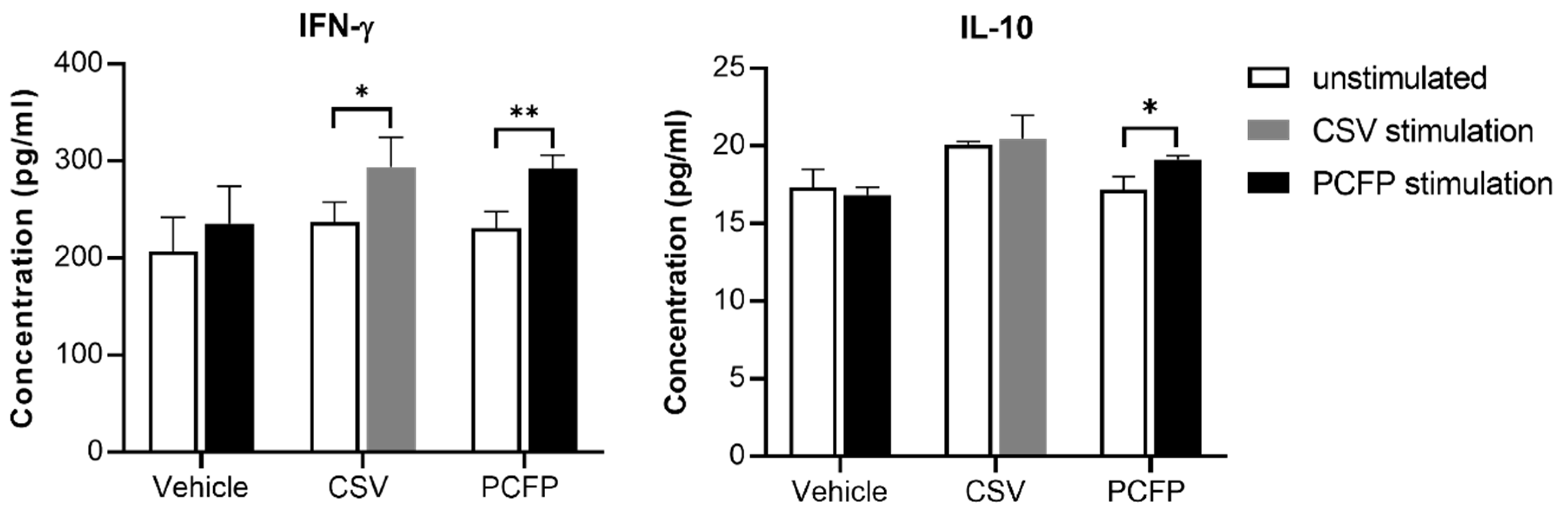
3.7. Cytokine Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Boisséson, C.; Béven, V.; Bigarré, L.; Thiéry, R.; Rose, N.; Eveno, E.; Madec, F.; Jestin, A. Molecular characterization of Porcine circovirus type 2 isolates from post-weaning multisystemic wasting syndrome-affected and non-affected pigs. J. Gen. Virol. 2004, 85, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.; Opriessnig, T.; Meng, X.; Pelzer, K.; Buechner-Maxwell, V. Porcine Circovirus Type 2 and Porcine Circovirus-Associated Disease. J. Vet. Intern. Med. 2009, 23, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Meehan, B.M.; Allan, G.M.; Hassard, L.E.; Clark, E.G.; Todd, D.; McNeilly, F.; Haines, D.M.; Jewhurst, V.A.; Kennedy, S.; Ellis, J.A. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J. Gen. Virol. 1998, 79, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, C.; Dai, A.; Lin, Z.; Fan, K.; Fan, J.; Liu, J.; Luo, M.; Yang, X. Detection of PCV2e strains in Southeast China. PeerJ 2018, 6, e4476. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-L.; Zhao, Y.; Zheng, H.-H.; Tian, R.-B.; Han, H.-Y.; Chen, H.-Y.; Zheng, L.-L. Analysis of genetic variation of porcine circovirus type 2 within pig populations in central China. Arch. Virol. 2019, 164, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Meng, X.-J. Porcine circoviruses: A minuscule yet mammoth paradox. Anim. Health Res. Rev. 2008, 10, 1–20. [Google Scholar] [CrossRef]
- Xiao, C.-T.; Harmon, K.M.; Halbur, P.G.; Opriessnig, T. PCV2d-2 is the predominant type of PCV2 DNA in pig samples collected in the U.S. during 2014–2016. Vet. Microbiol. 2016, 197, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.M.; McNeilly, F.; Kennedy, S.; Daft, B.; Ellis, J.A.; Haines, D.M.; Meehan, B.M.; Adair, B.M. Isolation of Porcine Circovirus-like Viruses from Pigs with a Wasting Disease in the USA and Europe. J. Vet. Diagn. Investig. 1998, 10, 3–10. [Google Scholar] [CrossRef]
- Firth, C.; Charleston, M.A.; Duffy, S.; Shapiro, B.; Holmes, E. Insights into the Evolutionary History of an Emerging Livestock Pathogen: Porcine Circovirus 2. J. Virol. 2009, 83, 12813–12821. [Google Scholar] [CrossRef]
- Constans, M.; Ssemadaali, M.; Kolyvushko, O.; Ramamoorthy, S. Antigenic Determinants of Possible Vaccine Escape by Porcine Circovirus Subtype 2b Viruses. Bioinform. Biol. Insights 2015, 9, 1–12. [Google Scholar] [CrossRef]
- Wang, K.; Huang, L.; Kong, J.; Zhang, X. Expression of the capsid protein of porcine circovirus type 2 in Lactococcus lactis for oral vaccination. J. Virol. Methods 2008, 150, 1–6. [Google Scholar] [CrossRef]
- Yin, S.; Sun, S.; Yang, S.; Shang, Y.; Cai, X.; Liu, X. Self-assembly of virus-like particles of porcine circovirus type 2 capsid protein expressed from Escherichia coli. Virol. J. 2010, 7, 166. [Google Scholar] [CrossRef]
- Kong, W.; Kong, J.; Hu, S.; Lu, W.; Wang, K.; Ji, M. Enhanced expression of PCV2 capsid protein in Escherichia coli and Lactococcus lactis by codon optimization. World J. Microbiol. Biotechnol. 2010, 27, 651–657. [Google Scholar] [CrossRef]
- Silva, J.G.; Coimbra, E.C.; Jesus, A.L.; Mariz, F.C.; Silva, K.M.; Lobato, Z.; Campos, A.C.; Coutinho, L.C.; Castro, R.S.; Freitas, A.C. Secretory expression of Porcine Circovirus Type 2 capsid protein in Pichia pastoris. J. Virol. Methods 2014, 207, 226–231. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, Y.; Wang, G.; Wu, J.; Liu, Y.; Wang, S.; Jiang, C.; Cai, X. High-level expression and immunogenicity of a porcine circovirus type 2 capsid protein through codon optimization in Pichia pastoris. Appl. Microbiol. Biotechnol. 2012, 97, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Javier, L.V.; Silvia, G.S.; Juan, B.; Nuñez, M.d.C.; Diego, M.A.; Benoit, D. Improved Production Efficiency of Virus-Like Particles by the Baculovirus Expression Vector System. PLoS ONE 2015, 10, e0140039. [Google Scholar] [CrossRef]
- Tao, Y.; Li, G.; Zheng, W.; Shu, J.; Chen, J.; Yang, F.; Wu, Y.; He, Y. Development of a Combined Genetic Engineering Vaccine for Porcine Circovirus Type 2 and Mycoplasma Hyopneumoniae by a Baculovirus Expression System. Int. J. Mol. Sci. 2019, 20, 4425. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yi, X.; Zhuang, Y.; Chu, J. Regulation of porcine circovirus type 2-like particles expressed in baculovirus expression system. Bioresour. Bioprocess 2016, 3, 4565. [Google Scholar] [CrossRef]
- Li, D.; Du, Q.; Wu, B.; Li, J.; Chang, L.; Zhao, X.; Huang, Y.; Tong, D. Immunogenicity of adenovirus vaccines expressing the PCV2 capsid protein in pigs. Vaccine 2017, 35, 4722–4729. [Google Scholar] [CrossRef]
- Trible, B.R.; Suddith, A.W.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Hesse, R.A.; Rowland, R.R.R. Recognition of the Different Structural Forms of the Capsid Protein Determines the Outcome following Infection with Porcine Circovirus Type 2. J. Virol. 2012, 86, 13508–13514. [Google Scholar] [CrossRef]
- Mahé, D.; Blanchard, P.; Truong, C.; Arnauld, C.; LE Cann, P.; Cariolet, R.; Madec, F.; Albina, E.; Jestin, A. Differential recognition of ORF2 protein from type 1 and type 2 porcine circoviruses and identification of immunorelevant epitopes. J. Gen. Virol. 2000, 81, 1815–1824. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Shang, S.-B.; Gong, H.; Chen, Q.-X.; Wu, J.-X.; Shen, H.-G.; Chen, T.-F.; Guo, J.-Q. In vitro expression, monoclonal antibody and bioactivity for capsid protein of porcine circovirus type II without nuclear localization signal. J. Biotechnol. 2005, 118, 201–211. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhang, Y.; Zhu, P.; Deng, Y.; Liu, Q. Secreted expression of truncated capsid protein from porcine circovirus type 2 in Pichia pastoris. Biotechnol. Lett. 2016, 38, 959–967. [Google Scholar] [CrossRef] [PubMed]
- George, N.C.; Darin, J.S.; Sharon, J.C.; Alison, M.B.; Elizabeth, A.C.; Daniel, H.D. Enhanced circulating half-life and hematopoietic properties of a human granulocyte colony-stimulating factor/immunoglobulin fusion protein. Exp. Hematol. 2004, 32, 441–449. [Google Scholar]
- Zhang, M.-Y.; Wang, Y.; Mankowski, M.K.; Ptak, R.G.; Dimitrov, D.S. Cross-reactive HIV-1-neutralizing activity of serum IgG from a rabbit immunized with gp41 fused to IgG1 Fc: Possible role of the prolonged half-life of the immunogen. Vaccine 2009, 27, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Z.; Zhu, S.-L.; Zu, X.; Wang, Z.; Zhang, L.-K.; Wang, W.; Xiao, G. A novel RSV F-Fc fusion protein vaccine reduces lung injury induced by respiratory syncytial virus infection. Antivir. Res. 2019, 165, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Senthilkumar, P.; Zeng, R.Y.; Yu, B.; Liu, X.D.; Wang, Y.S. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J. Virol. 2011, 85, 10542–10553. [Google Scholar] [CrossRef]
- Konduru, K.; Bradfute, S.B.; Jacques, J.; Manangeeswaran, M.; Nakamura, S.; Morshed, S.; Wood, S.C.; Bavari, S.; Kaplan, G.G. Ebola virus glycoprotein Fc fusion protein confers protection against lethal challenge in vaccinated mice. Vaccine 2011, 29, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Patterson, A.R.; Madson, D.M.; Pal, N.; Ramamoorthy, S.; Meng, X.; Halbur, P.G. Comparison of the effectiveness of passive (dam) versus active (piglet) immunization against porcine circovirus type 2 (PCV2) and impact of passively derived PCV2 vaccine-induced immunity on vaccination. Vet. Microbiol. 2010, 142, 177–183. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J.; Choi, K.; Park, S.-J.; Kang, I.; Chae, C. Development of porcine circovirus 2 (PCV2) open reading frame 2 DNA vaccine with different adjuvants and comparison with commercial PCV2 subunit vaccine in an experimental challenge. Can. J. Vet. Res. 2017, 81, 171–177. [Google Scholar]
- Zhang, H.; Qian, P.; Peng, B.; Shi, L.; Chen, H.; Li, X. A novel subunit vaccine co-expressing GM-CSF and PCV2b Cap protein enhances protective immunity against porcine circovirus type 2 in piglets. Vaccine 2015, 33, 2449–2456. [Google Scholar] [CrossRef]
- Fu, F.; Li, X.; Lang, Y.; Yang, Y.; Tong, G.; Li, G.; Zhou, Y.; Li, X. Co-expression of Ubiquitin gene and capsid protein gene enhances the potency of DNA immunization of PCV2 in mice. Virol. J. 2011, 8, 264. [Google Scholar] [CrossRef]
- Seo, H.W.; Han, K.; Kim, D.; Oh, Y.; Kang, I.; Park, C.; Jang, H.; Chae, C. Effects of an Inactivated Porcine Circovirus Type 2 (PCV2) Vaccine on PCV2 Virus Shedding in Semen from Experimentally Infected Boars. Clin. Vaccine Immunol. 2011, 18, 1091–1096. [Google Scholar] [CrossRef]
- Khayat, R.; Brunn, N.; Speir, J.A.; Hardham, J.M.; Ankenbauer, R.G.; Schneemann, A.; Johnson, J.E. The 2.3-Angstrom Structure of Porcine Circovirus 2. J. Virol. 2011, 85, 11542. [Google Scholar] [CrossRef]
- Mo, X.; Li, X.; Yin, B.; Deng, J.; Tian, K.; Yuan, A. Structural roles of PCV2 capsid protein N-terminus in PCV2 particle assembly and identification of PCV2 type-specific neutralizing epitope. PLoS Pathog. 2019, 15, e1007562. [Google Scholar] [CrossRef] [PubMed]
- Ohkuri, T.; Nagatomo, S.; Oda, K.; So, T.; Imoto, T.; Ueda, T. A Protein’s Conformational Stability Is an Immunologically Dominant Factor: Evidence That Free-Energy Barriers for Protein Unfolding Limit the Immunogenicity of Foreign Proteins. J. Immunol. 2010, 185, 4199–4205. [Google Scholar] [CrossRef] [PubMed]
- So, T.; Ito, H.-O.; Hirata, M.; Ueda, T.; Imoto, T. Contribution of conformational stability of hen lysozyme to induction of type 2 T-helper immune responses. Immunology 2001, 104, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Thai, R.; Moine, G.; Desmadril, M.; Servent, D.; Tarride, J.-L.; Ménez, A.; Léonetti, M. Antigen Stability Controls Antigen Presentation. J. Biol. Chem. 2004, 279, 50257–50266. [Google Scholar] [CrossRef] [PubMed]
- Soleimanpour, S.; Farsiani, H.; Mosavat, A.; Ghazvini, K.; Eydgahi, M.R.A.; Sankian, M.; Sadeghian, H.; Meshkat, Z.; Rezaee, S.A. APC targeting enhances immunogenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice. Appl. Microbiol. Biotechnol. 2015, 99, 10467–10480. [Google Scholar] [CrossRef]
- Loureiro, S.; Ren, J.; Phapugrangkul, P.; Colaco, C.A.; Bailey, C.R.; Shelton, H.; Molesti, E.; Temperton, N.J.; Barclay, W.S.; Jones, I.M. Adjuvant-Free Immunization with Hemagglutinin-Fc Fusion Proteins as an Approach to Influenza Vaccines. J. Virol. 2011, 85, 3010–3014. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Ahmed, W.; Dai, Y.; Mohsin, A.; Hang, H.; Zhuang, Y.; Guo, M. Evaluation of a Virus-like Nanoparticle Porcine Circovirus Type-2 (PCV2) Capsid Protein Fused with the Pig Immunoglobulin Fc Fragment as a Novel Vaccine Candidate against PCV2 in Mice. Vaccines 2021, 9, 1128. https://doi.org/10.3390/vaccines9101128
Luo Q, Ahmed W, Dai Y, Mohsin A, Hang H, Zhuang Y, Guo M. Evaluation of a Virus-like Nanoparticle Porcine Circovirus Type-2 (PCV2) Capsid Protein Fused with the Pig Immunoglobulin Fc Fragment as a Novel Vaccine Candidate against PCV2 in Mice. Vaccines. 2021; 9(10):1128. https://doi.org/10.3390/vaccines9101128
Chicago/Turabian StyleLuo, Qingping, Waqas Ahmed, Yichen Dai, Ali Mohsin, Haifeng Hang, Yingping Zhuang, and Meijin Guo. 2021. "Evaluation of a Virus-like Nanoparticle Porcine Circovirus Type-2 (PCV2) Capsid Protein Fused with the Pig Immunoglobulin Fc Fragment as a Novel Vaccine Candidate against PCV2 in Mice" Vaccines 9, no. 10: 1128. https://doi.org/10.3390/vaccines9101128
APA StyleLuo, Q., Ahmed, W., Dai, Y., Mohsin, A., Hang, H., Zhuang, Y., & Guo, M. (2021). Evaluation of a Virus-like Nanoparticle Porcine Circovirus Type-2 (PCV2) Capsid Protein Fused with the Pig Immunoglobulin Fc Fragment as a Novel Vaccine Candidate against PCV2 in Mice. Vaccines, 9(10), 1128. https://doi.org/10.3390/vaccines9101128







