GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of GMMA and Antigens
2.2. Synthesis and Characterization of the GMMA Conjugates
2.2.1. Linkage of Heterologous Saccharides to GMMA
Conjugation via SH-Maleimido Chemistry
Conjugation via Reductive Amination Chemistry
Conjugation through BS3 Chemistry
Synthesis of the Bivalent Conjugate
2.2.2. Linkage of Heterologous Saccharides to GMMA
Conjugation via SIDEA (Adipic Acid Bis(N-hydroxysuccinimmide)) Chemistry
2.3. Conjugate Characterization
2.4. Amino Acid Analysis
2.5. SDS-PAGE/Western Blot
2.6. Immunogenicity Studies in Mice and Rats
2.7. Statistical Analysis
3. Results
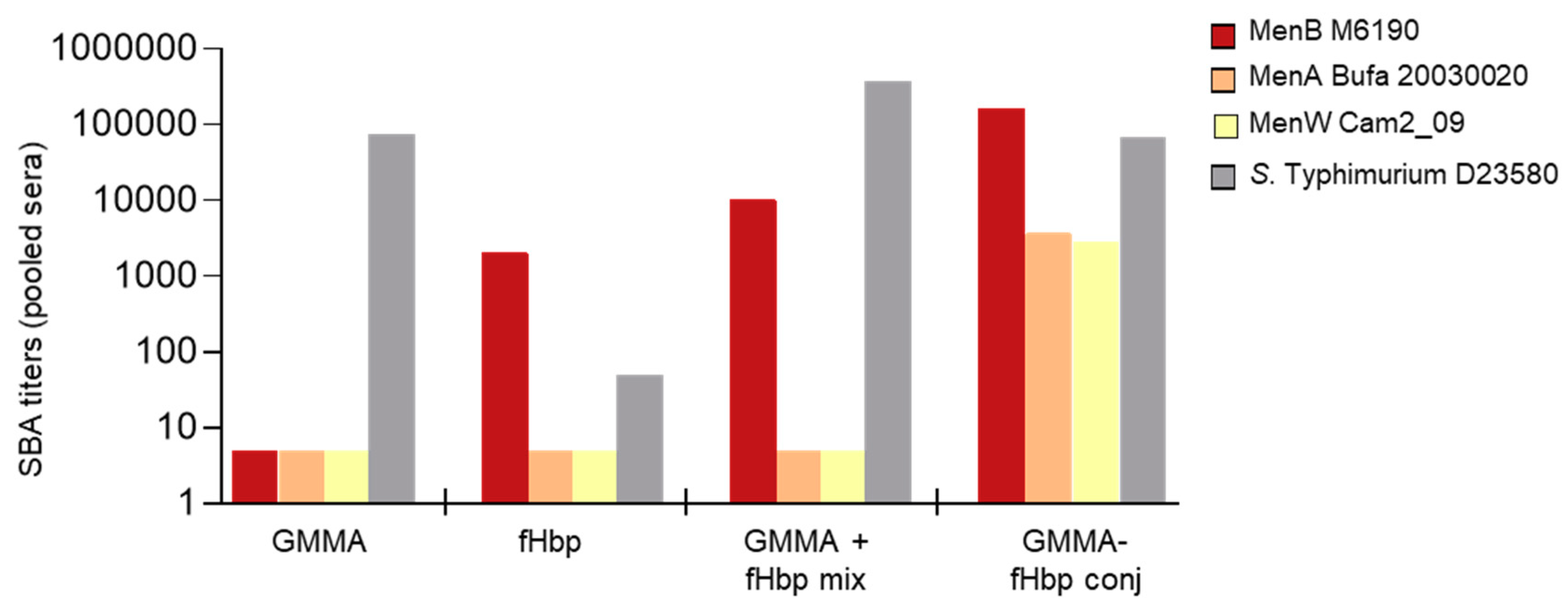
3.1. GMMA as a Carrier for Protein Antigens
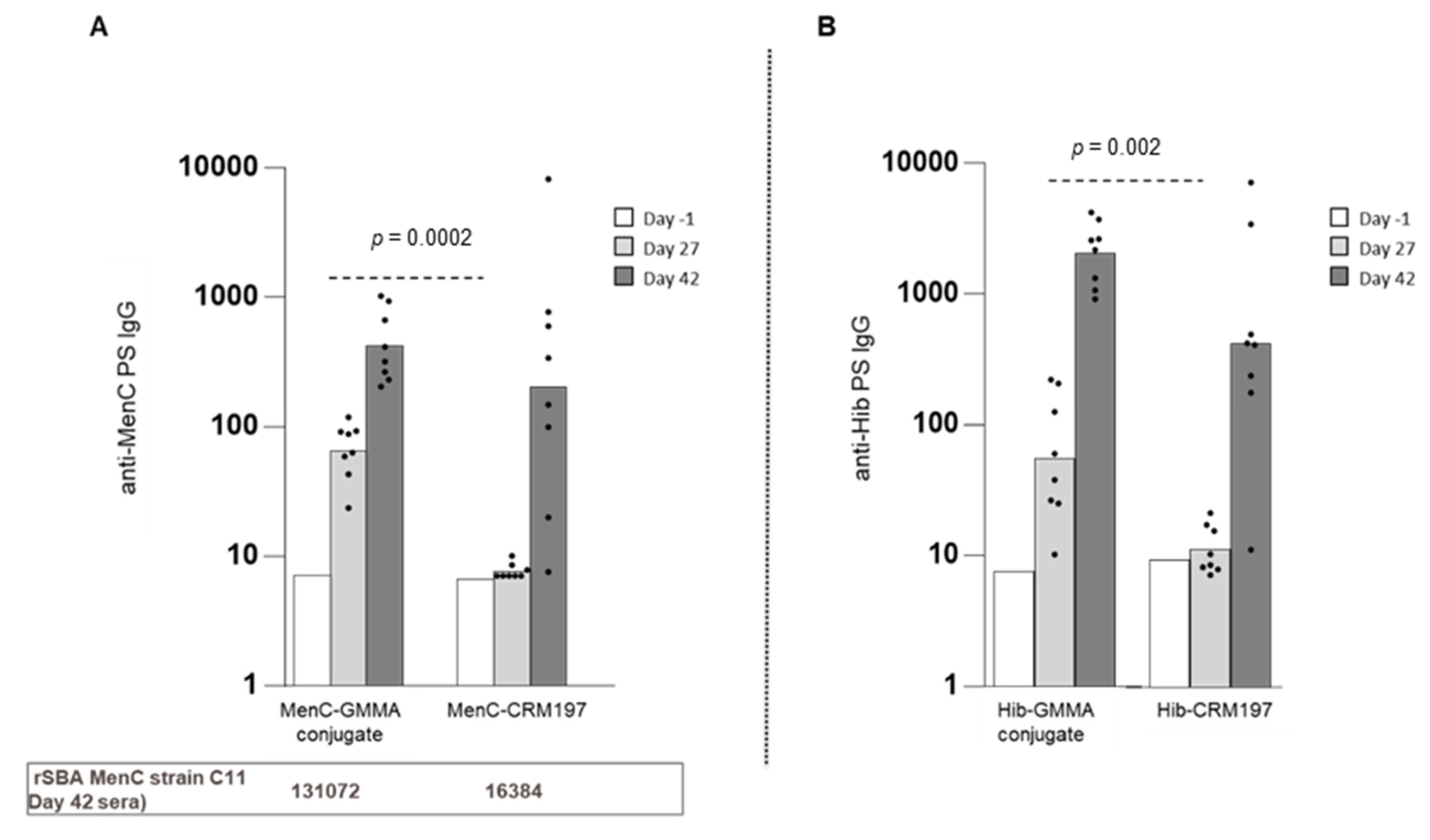
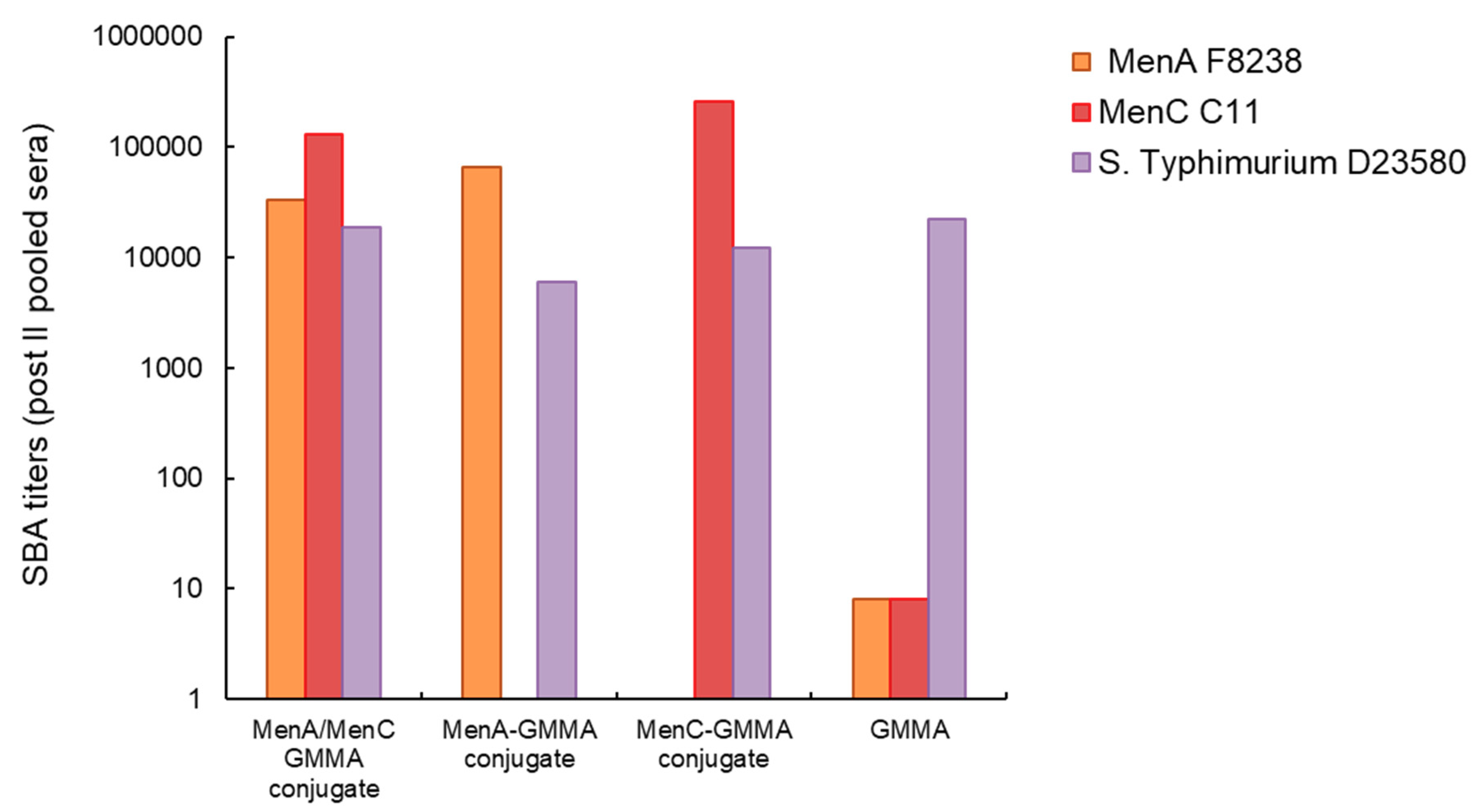
3.2. GMMA as an Effective Carrier for Polysaccharides
3.3. Chemical Linkage of Two Different Proteins or Saccharides on the Same GMMA Did Not Generate Immunointerference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging technologies for low-cost, rapid vaccine manufacture. Biotechnol. J. 2019, 14, e1800376. [Google Scholar] [CrossRef]
- Collins, B.S. Gram-negative outer membrane vesicles in vaccine development. Discov. Med. 2011, 12, 7–15. [Google Scholar] [PubMed]
- Gerritzen, M.J.H.; Martens, D.E.; Wijffels, R.H.; Van Der Pol, L.; Stork, M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017, 35, 565–574. [Google Scholar] [CrossRef]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.; Alfini, R.; Cescutti, P.; Caboni, M.; Lanzilao, L.; Necchi, F.; Saul, A.; MacLennan, C.; Rondini, S.; Micoli, F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 2017, 35, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; Pesce, I.; Giannelli, C.; Aprea, S.; Caboni, M.; Citiulo, F.; Valentini, S.; Ferlenghi, I.; MacLennan, C.A.; D’Oro, U.; et al. Modulation of endotoxicity of shigella generalized modules for membrane antigens (GMMA) by genetic lipid a modifications: Relative activation of tlr4 and tlr2 pathways in different mutants. J. Biol. Chem. 2014, 289, 24922–24935. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; Caboni, M.; Negrea, A.; Necchi, F.; Alfini, R.; Micoli, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Gerke, C. Toll-like receptor activation by generalized modules for membrane antigens from lipid a mutants of salmonella enterica serovars typhimurium and enteritidis. Clin. Vaccine Immunol. 2016, 23, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Lewis, D.J.; Anemona, A.; Loulergue, P.; Leahy, J.; Sciré, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety profile and immunologic responses of a novel vaccine against shigella sonnei administered intramuscularly, intradermally and intranasally: Results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Ndiaye, A.G.W.; Conti, V.; Loulergue, P.; Sciré, A.S.; Landre, A.M.; Ferruzzi, P.; Nedjaai, N.; Schütte, L.D.; Auerbach, J.; et al. Booster vaccination with GVGH Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: Results from a phase 1 clinical trial. Front. Immunol. 2019, 10, 335. [Google Scholar] [CrossRef]
- Obiero, C.W.; Ndiaye, A.G.W.; Sciré, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schütte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A.; et al. A Phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Wu, Y.; Przysiecki, C.; Flanagan, E.; Bello-Irizarry, S.N.; Ionescu, R.; Muratova, O.; Dobrescu, G.; Lambert, L.; Keister, D.; Rippeon, Y.; et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. USA 2006, 103, 18243–18248. [Google Scholar] [CrossRef]
- Schneerson, R.; Barrera, O.; Sutton, A.; Robbins, J.B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980, 152, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Perrett, K.P.; Beverley, P. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R. Glycoconjugate vaccines: Principles and mechanisms. Sci. Transl. Med. 2018, 10, eaat4615. [Google Scholar] [CrossRef] [PubMed]
- Kaslow, D.C.; Black, S.; Bloom, D.E.; Datla, M.; Salisbury, D.; Rappuoli, R. Vaccine candidates for poor nations are going to waste. Nature 2018, 564, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Bloom, D.E.; Black, S.; Salisbury, D.; Rappuoli, R. Antimicrobial resistance and the role of vaccines. Proc. Natl. Acad. Sci. USA 2018, 115, 12868–12871. [Google Scholar] [CrossRef]
- WHO’s Mission and Vision in Immunization and Vaccines (2015–2030). Available online: https://www.who.int/immunization/documents/general/WHO_mission_vision_immunization_vaccines_2015_2030/en/ (accessed on 15 September 2020).
- De Benedetto, G.; Cescutti, P.; Giannelli, C.; Rizzo, R.; Micoli, F. Multiple techniques for size determination of generalized modules for membrane antigens from salmonella typhimurium and salmonella enteritidis. ACS Omega 2017, 2, 8282–8289. [Google Scholar] [CrossRef]
- Satake, K.; Okuyama, T.; Ohashi, M.; Shinoda, T. The spectrophotometric determination of amine, amino acid and peptide with 2,4,6-trinitrobenzene 1-sulfonic acid. J. Biochem. 1960, 47, 654–660. [Google Scholar] [CrossRef]
- Costantino, P.; Viti, S.; Podda, A.; Velmonte, M.A.; Nencioni, L.; Rappuoli, R. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine 1992, 10, 691–698. [Google Scholar] [CrossRef]
- Micoli, F.; Rondini, S.; Pisoni, I.; Giannelli, C.; Di Cioccio, V.; Costantino, P.; Saul, A.; Martin, L. Production of a conjugate vaccine for Salmonella enterica serovar Typhi from Citrobacter Vi. Vaccine 2012, 30, 853–861. [Google Scholar] [CrossRef]
- Kabanova, A.; Margarit, I.; Berti, F.; Romano, M.R.; Grandi, G.; Bensi, G.; Chiarot, E.; Proietti, D.; Swennen, E.; Cappelletti, E.; et al. Evaluation of a Group A Streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine 2010, 29, 104–114. [Google Scholar] [CrossRef]
- Ho, M.M.; Mawas, F.; Bolgiano, B.; Lemercinier, X.; Crane, D.T.; Huskisson, R.; Corbel, M.J. Physico-chemical and immunological examination of the thermal stability of tetanus toxoid conjugate vaccines. Vaccine 2002, 20, 3509–3522. [Google Scholar] [CrossRef]
- Ispasanie, E.; Micoli, F.; Lamelas, A.; Keller, D.; Berti, F.; De Riccio, R.; Di Benedettoi, R.; Rondini, S.; Pluschke, G. Spontaneous point mutations in the capsule synthesis locus leading to structural and functional changes of the capsule in serogroup A meningococcal populations. Virulence 2018, 9, 1138–1149. [Google Scholar] [CrossRef]
- Ho, M.M.; Bolgiano, B.; Corbel, M.J. Assessment of the stability and immunogenicity of meningococcal oligosaccharide C-CRM197 conjugate vaccines. Vaccine 2000, 19, 716–725. [Google Scholar] [CrossRef]
- Bardotti, A.; Ravenscroft, N.; Ricci, S.; D’Ascenzi, S.; Guarnieri, V.; Averani, G.; Constantino, P. Quantitative determination of saccharide in Haemophilus influenzae type b glycoconjugate vaccines, alone and in combination with DPT, by use of high-performance anion-exchange chromatography with pulsed amperometric detection. Vaccine 2000, 18, 1982–1993. [Google Scholar] [CrossRef]
- Rondini, S.; Micoli, F.; Lanzilao, L.; Hale, C.; Saul, A.; Martin, L.B. Evaluation of the immunogenicity and biological activity of the citrobacter freundiivi-CRM197 conjugate as a vaccine for salmonella enterica serovar typhi. Clin. Vaccine Immunol. 2011, 18, 460–468. [Google Scholar] [CrossRef]
- Necchi, F.; Saul, A.; Rondini, S. Setup of luminescence-based serum bactericidal assay against Salmonella Paratyphi A. J. Immunol. Methods 2018, 461, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.M.; Santini, L.; Brunelli, B.; Biolchi, A.; Aricò, B.; Di Marcello, F.; Cartocci, E.; Comanducci, M.; Masignani, V.; Lozzi, L.; et al. The region comprising amino acids 100 to 255 of neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infect. Immun. 2005, 73, 1151–1160. [Google Scholar] [CrossRef]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Levine, M.M. Invasive nontyphoidalSalmonelladisease in Africa: Current status. Expert Rev. Anti Infect. Ther. 2013, 11, 443–446. [Google Scholar] [CrossRef]
- Mabey, D.; Brown, A.; Greenwood, B.M. Plasmodium falciparum malaria and salmonella infections in gambian children. J. Infect. Dis. 1987, 155, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Keister, D.B.; Muratova, O.V.; Sattabongkot, J.; Long, C.A.; Saul, A. Transmission-blocking activity induced by malaria vaccine candidates pfs25/pvs25 is a direct and predictable function of antibody titer. Malar. J. 2007, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Nussenzweig, V.; Nussenzweig, R. Experimental basis for the development of a synthetic vaccine against plasmodium falciparum malaria sporozoites. Ciba Found. Symp. 1986, 119, 150–163. [Google Scholar] [CrossRef]
- Shimp, R.L.; Rowe, C.; Reiter, K.; Chen, B.; Nguyen, V.; Aebig, J.; Rausch, K.M.; Kumar, K.; Wu, Y.; Jin, A.; et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 2013, 31, 2954–2962. [Google Scholar] [CrossRef][Green Version]
- Kastenmüller, K.; Espinosa, D.A.; Trager, L.; Stoyanov, C.; Salazar, A.M.; Pokalwar, S.; Singh, S.; Dutta, S.; Ockenhouse, C.F.; Zavala, F.; et al. Full-length plasmodium falciparum circumsporozoite protein administered with long-chain poly(I·C) or the toll-like receptor 4 agonist glucopyranosyl lipid adjuvant-stable emulsion elicits potent antibody and CD4+T cell immunity and protection in mice. Infect. Immun. 2013, 81, 789–800. [Google Scholar] [CrossRef]
- Koeberling, O.; Ispasanie, E.; Hauser, J.; Rossi, O.; Pluschke, G.; Caugant, D.A.; Saul, A.; MacLennan, C.A. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine 2014, 32, 2688–2695. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Martin, L.B.; Micoli, F. Vaccines against invasive salmonella disease: Current status and future directions. Hum. Vaccin. Immunother. 2014, 10, 1478–1493. [Google Scholar] [CrossRef]
- Uche, I.; MacLennan, C.A.; Saul, A. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal salmonella (iNTS) disease in Africa (1966 to 2014). PLOS Negl. Trop. Dis. 2017, 11, e0005118. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Borrow, R.; Miller, E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 2003, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Hirve, S.; Bavdekar, A.; Pandit, A.; Juvekar, S.; Patil, M.; Preziosi, M.-P.; Tang, Y.; Marchetti, E.; Martellet, L.; Findlow, H.; et al. Immunogenicity and safety of a new meningococcal A conjugate vaccine in Indian children aged 2–10 years: A Phase II/III double-blind randomized controlled trial. Vaccine 2012, 30, 6456–6460. [Google Scholar] [CrossRef] [PubMed]
- Valeri, M.; Paccani, S.R.; Kasendra, M.; Nesta, B.; Serino, L.; Pizza, M.; Soriani, M. Pathogenic E. coli exploits SslE Mucinase activity to translocate through the mucosal barrier and get access to host cells. PLoS ONE 2015, 10, e0117486. [Google Scholar] [CrossRef] [PubMed]
- Nesta, B.; Valeri, M.; Spagnuolo, A.; Rosini, R.; Mora, M.; Donato, P.; Alteri, C.J.; Del Vecchio, M.; Buccato, S.; Pezzicoli, A.; et al. SslE elicits functional antibodies that impair in vitro mucinase activity and in vivo colonization by both intestinal and extraintestinal escherichia coli strains. PLoS Pathog. 2014, 10, e1004124. [Google Scholar] [CrossRef] [PubMed]
- Nesta, B.; Spraggon, G.; Alteri, C.J.; Moriel, D.G.; Rosini, R.; Veggi, D.; Smith, S.; Bertoldi, I.; Pastorello, I.; Ferlenghi, I.; et al. FdeC, a Novel broadly conserved escherichia coli adhesin eliciting protection against urinary tract infections. mBio 2012, 3, e00010-12. [Google Scholar] [CrossRef]
- Walker, R. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 2015, 33, 954–965. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Baker, S.J.; Payne, D.J.; Rappuoli, R.; De Gregorio, E. Technologies to address antimicrobial resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 12887–12895. [Google Scholar] [CrossRef]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Micoli, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P.; et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, 10428–10433. [Google Scholar] [CrossRef]
- Schager, A.E.; Dominguez-Medina, C.C.; Necchi, F.; Micoli, F.; Goh, Y.S.; Goodall, M.; Flores-Langarica, A.; Bobat, S.; Cook, C.N.L.; Arcuri, M.; et al. IgG responses to porins and lipopolysaccharide within an outer membrane-based vaccine against nontyphoidal salmonella develop at discordant rates. mBio 2018, 9, e02379-17. [Google Scholar] [CrossRef]
- Valguarnera, E.; Feldman, M.F. Glycoengineered outer membrane vesicles as a platform for vaccine development. Methods Enzym. 2017, 597, 285–310. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Del Bino, L.; Alfini, R.; Carboni, F.; Romano, M.R.; Adamo, R. Glycoconjugate vaccines: Current approaches towards faster vaccine design. Expert Rev. Vaccines 2019, 18, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [PubMed]
- Granoff, D.M.; Holmes, S.J. Comparative immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugate vaccines. Vaccine 1991, 9, S30–S34, discussion S42–S33. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Deck, R.R.; Liu, M.A. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J. Immunol. 1990, 145, 3071–3079. [Google Scholar]
- Scaria, P.V.; Rowe, C.G.; Chen, B.B.; Muratova, O.V.; Fischer, E.R.; Barnafo, E.K.; Anderson, C.F.; Zaidi, I.U.; Lambert, L.E.; Lucas, B.J.; et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. npj Vaccines 2019, 4, 24. [Google Scholar] [CrossRef]
- Micoli, F.; Adamo, R.; Costantino, P. Protein carriers for glycoconjugate vaccines: History, selection criteria, characterization and new trends. Molecules 2018, 23, 1451. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G.; et al. GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines. Vaccines 2020, 8, 540. https://doi.org/10.3390/vaccines8030540
Micoli F, Alfini R, Di Benedetto R, Necchi F, Schiavo F, Mancini F, Carducci M, Palmieri E, Balocchi C, Gasperini G, et al. GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines. Vaccines. 2020; 8(3):540. https://doi.org/10.3390/vaccines8030540
Chicago/Turabian StyleMicoli, Francesca, Renzo Alfini, Roberta Di Benedetto, Francesca Necchi, Fabiola Schiavo, Francesca Mancini, Martina Carducci, Elena Palmieri, Cristiana Balocchi, Gianmarco Gasperini, and et al. 2020. "GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines" Vaccines 8, no. 3: 540. https://doi.org/10.3390/vaccines8030540
APA StyleMicoli, F., Alfini, R., Di Benedetto, R., Necchi, F., Schiavo, F., Mancini, F., Carducci, M., Palmieri, E., Balocchi, C., Gasperini, G., Brunelli, B., Costantino, P., Adamo, R., Piccioli, D., & Saul, A. (2020). GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines. Vaccines, 8(3), 540. https://doi.org/10.3390/vaccines8030540






