Field Study on the Immunological Response and Protective Effect of a Licensed Autogenous Vaccine to Control Streptococcus suis Infections in Post-Weaned Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Farm and Clinical History
2.2. Vaccine Preparation
2.3. Immunization Protocol
2.4. Blood Sampling
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) for Pig Immunoglobulin (Ig) Titers
2.6. ELISA for Pig Antibodies against Serotype 7 S. suis Capsular Polysaccharide (CPS)
2.7. Opsonophagocytosis Assay (OPA)
2.8. Clinical Evaluation of Animals
2.9. Statistical Analyses
3. Results
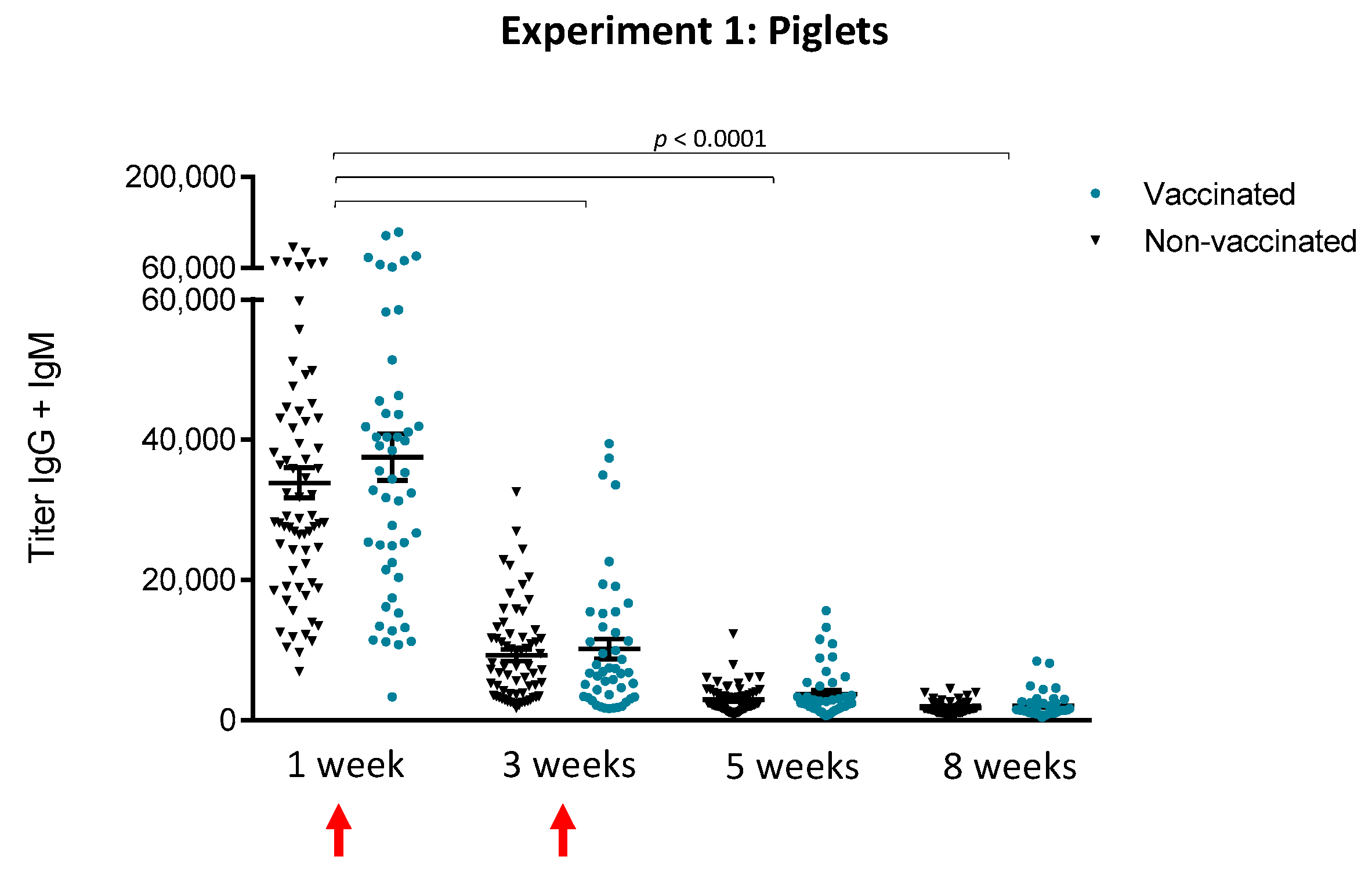
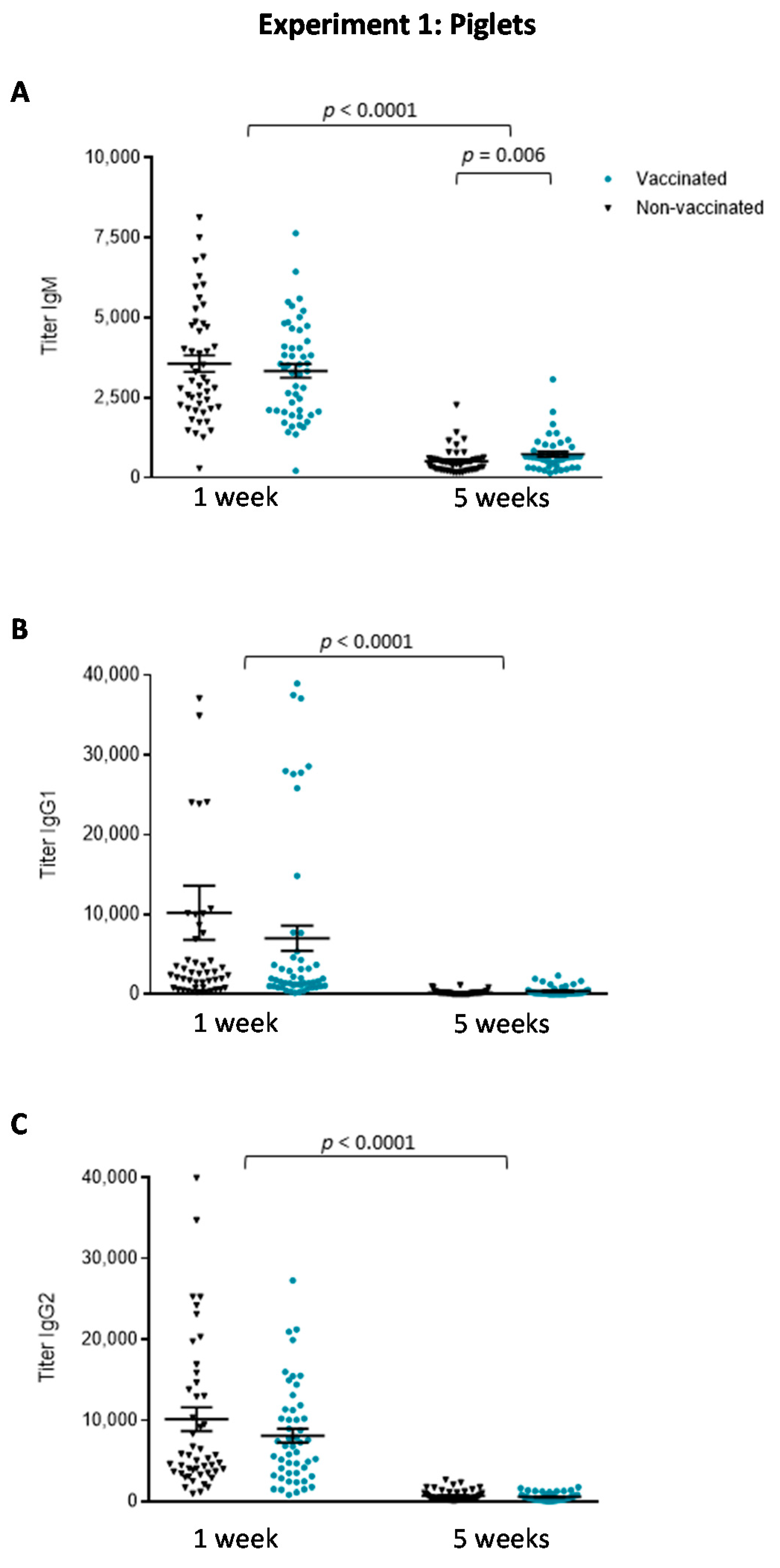
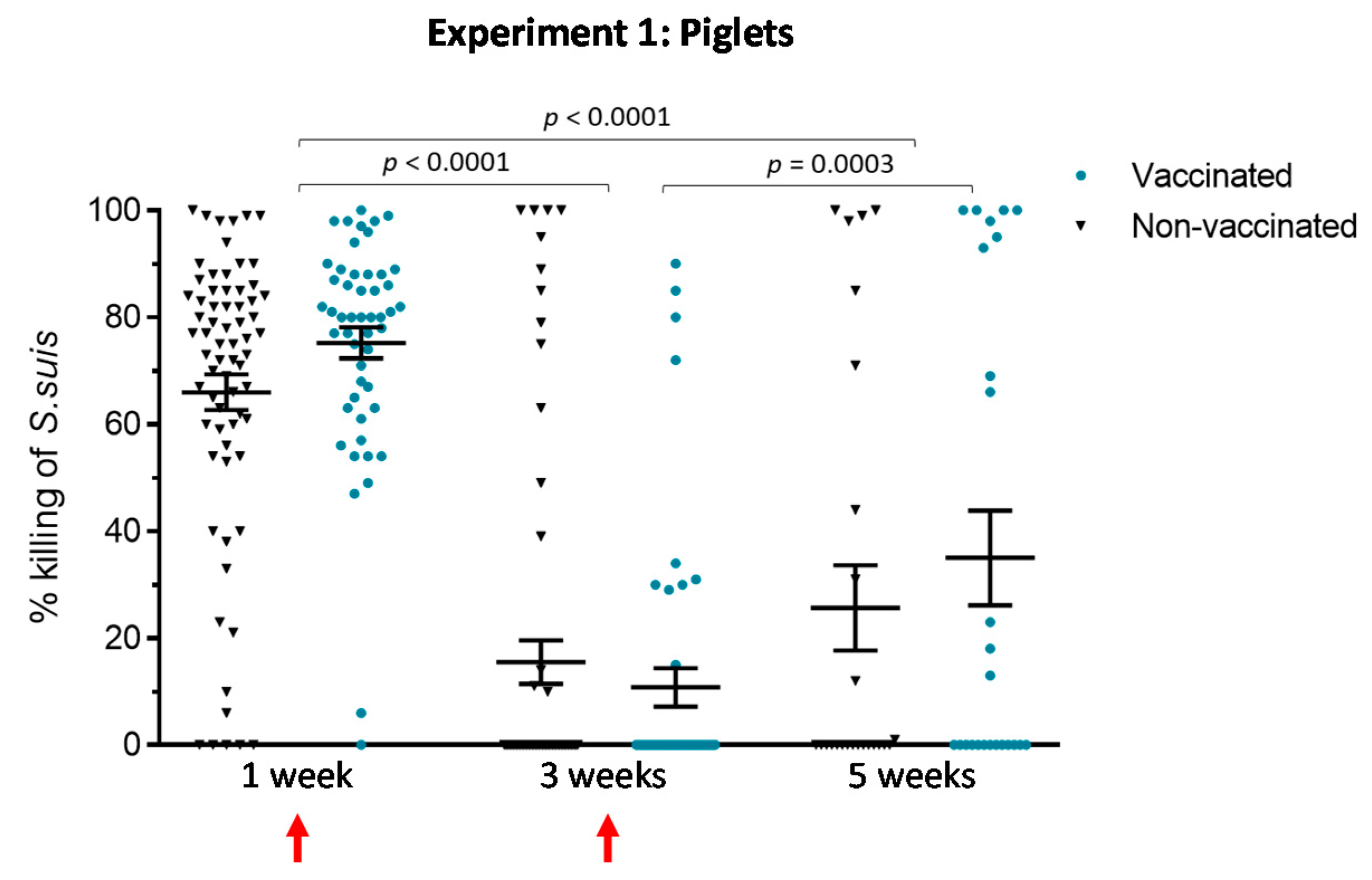
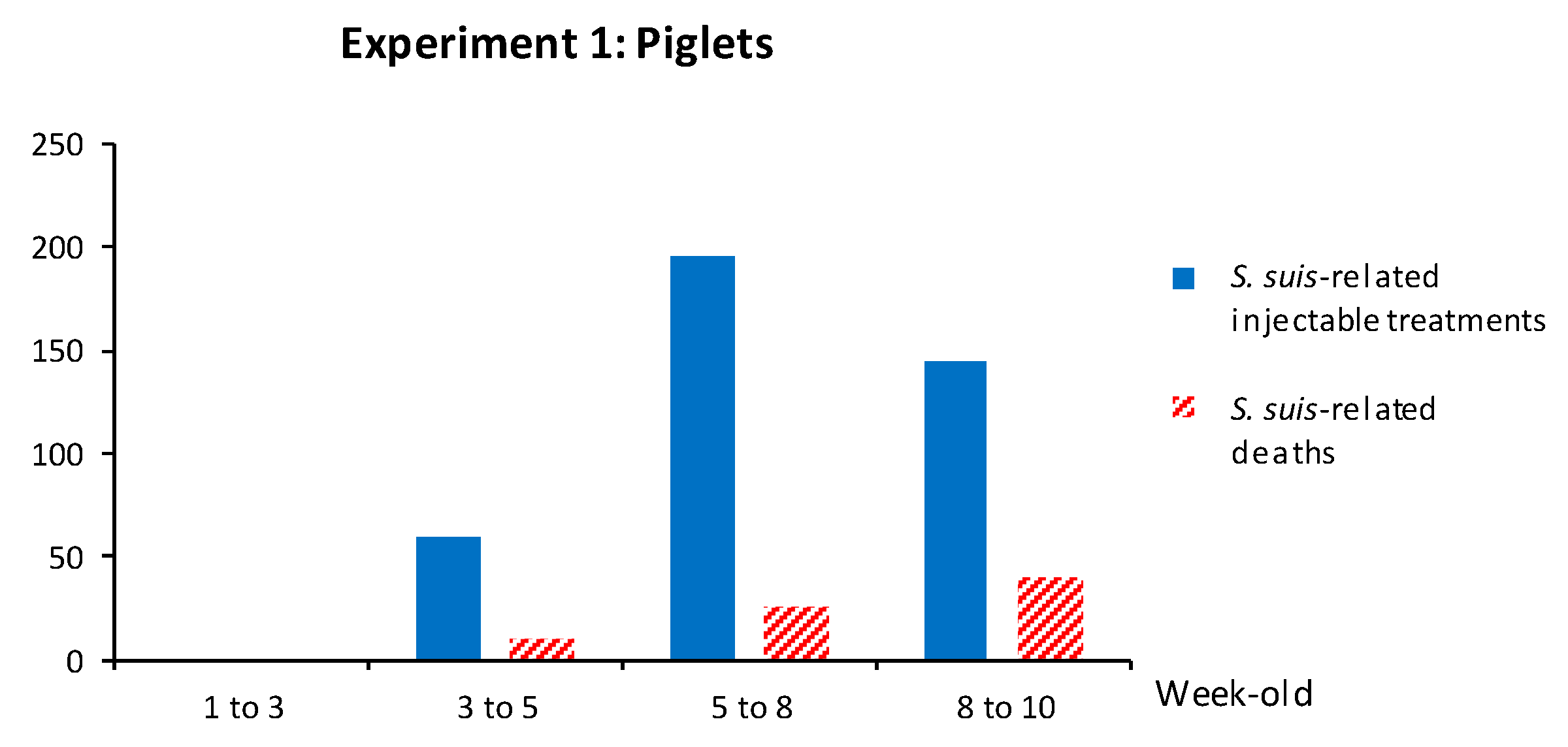
3.1. Experiment 1: Piglet Vaccination
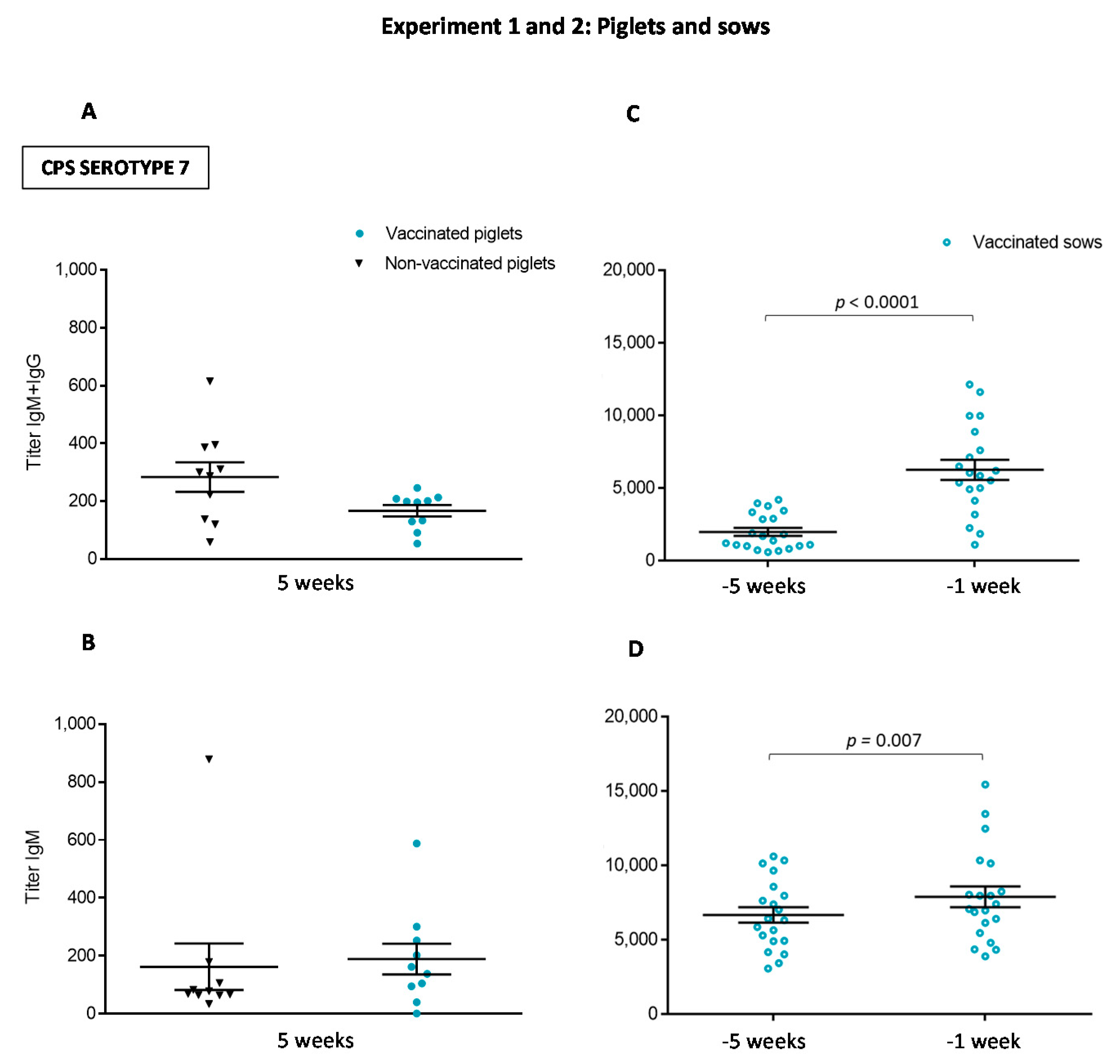
3.1.1. An Active Vaccination Program of Piglets with an Autogenous Bacterin Fails to Increase Antibody Levels Post-Weaning
3.1.2. The Isotype Profile of the Antibody Response in Piglets Was Unchanged by Vaccination with the Autogenous Bacterin
3.1.3. An Active Vaccination Program of Piglets with an Autogenous Bacterin Fails to Improve the Killing Capacity of Antibodies
3.1.4. Clinical Outcome Was Not Improved by an Active Vaccination Program of Piglets with an Autogenous Bacterin
3.2. Experiment 2: Sow Vaccination
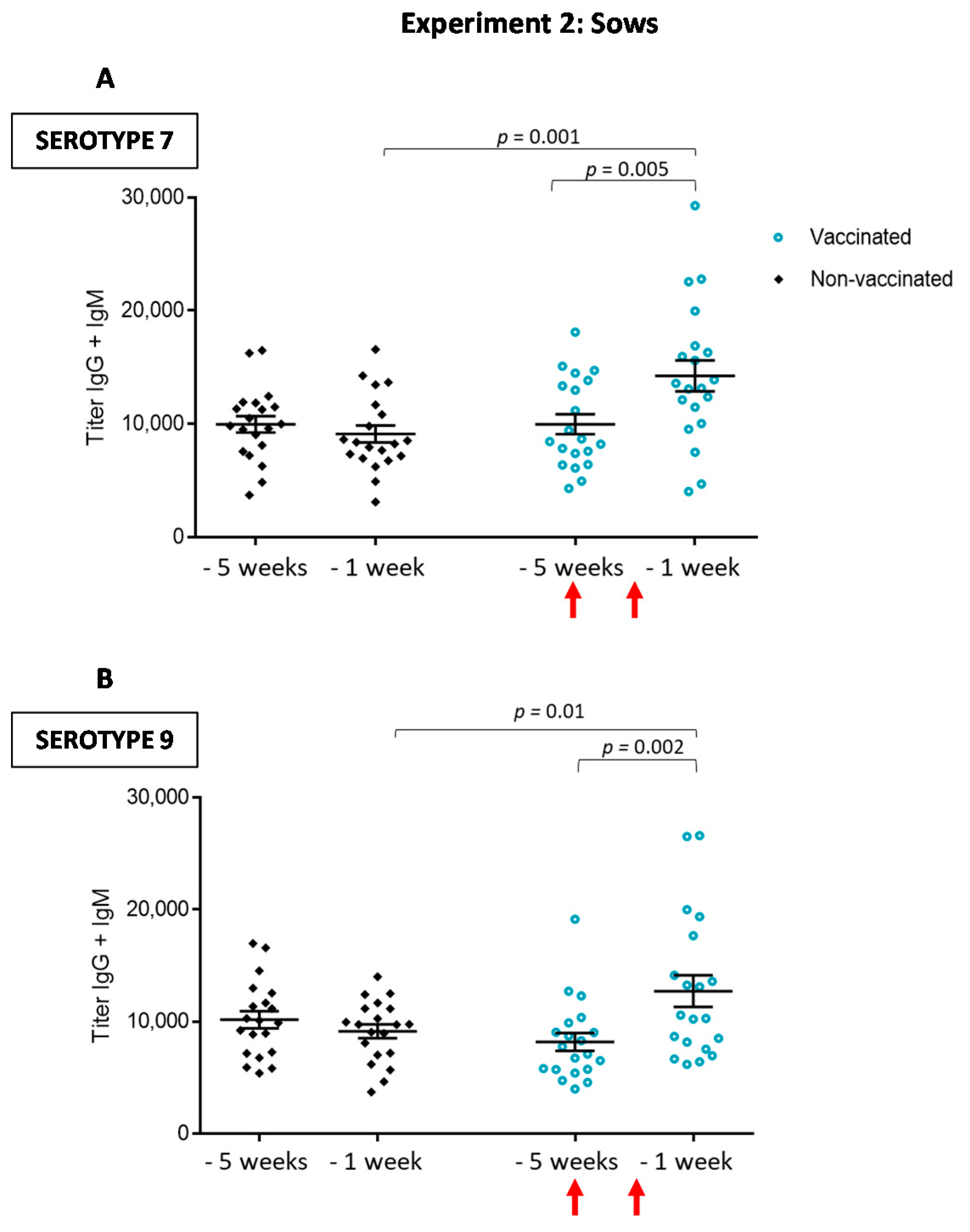
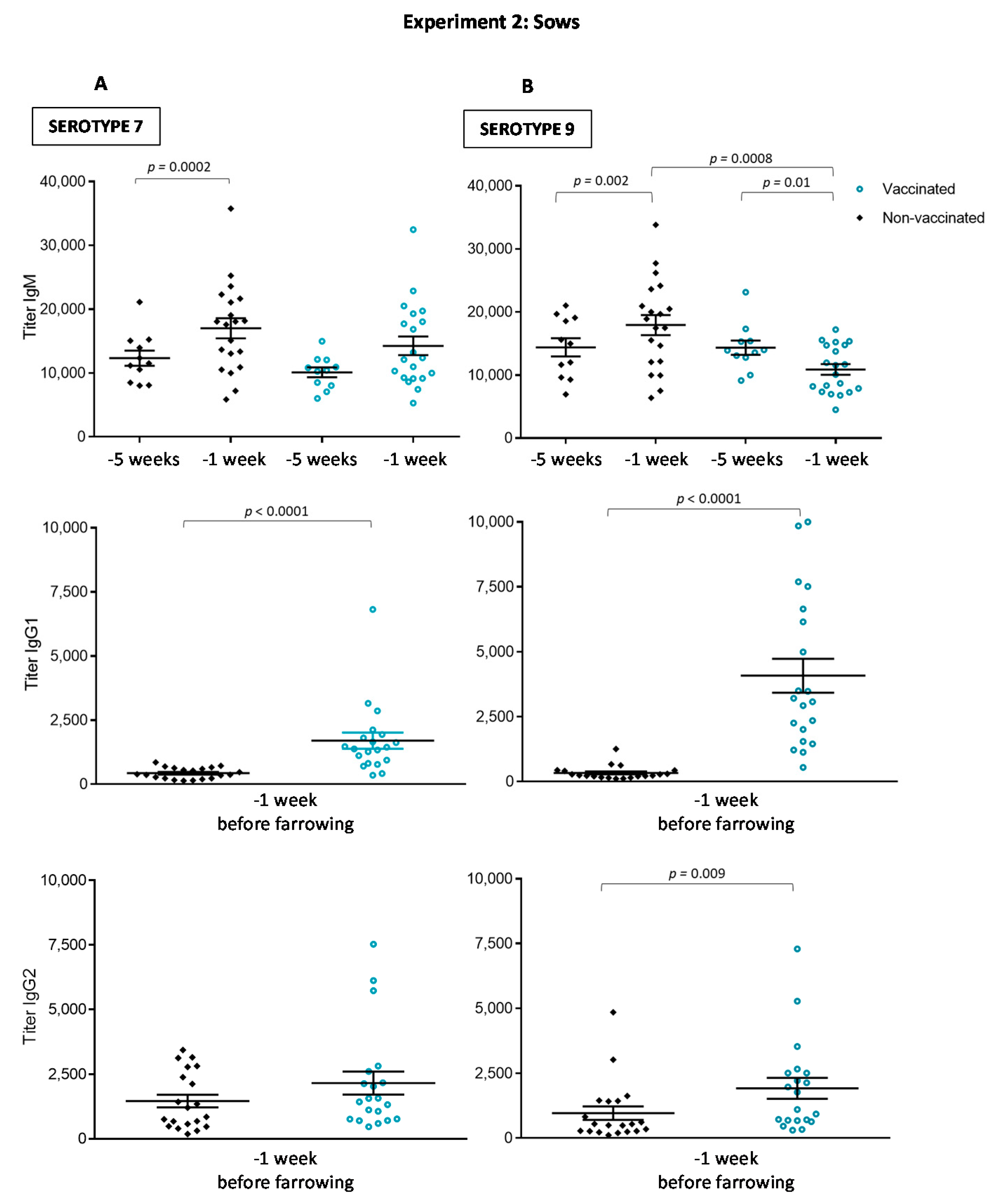
3.2.1. Antibody Levels against the Autogenous Vaccine Are Increased in Vaccinated Sows, yet the Ig Isotype Profile Differs between the Serotypes
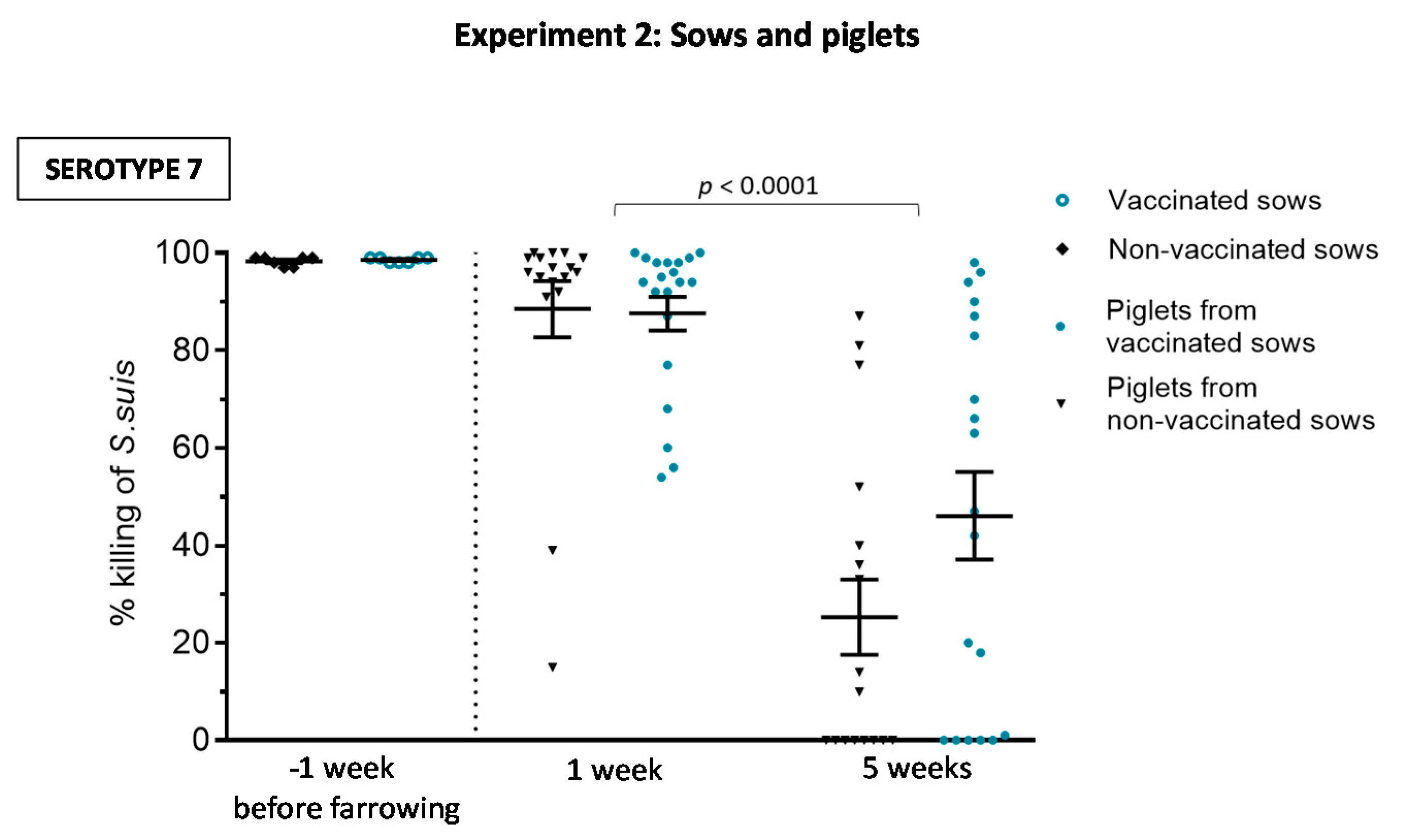
3.2.2. Maternal Antibody Transfer to Piglets Is Not Increased after Sow Vaccination
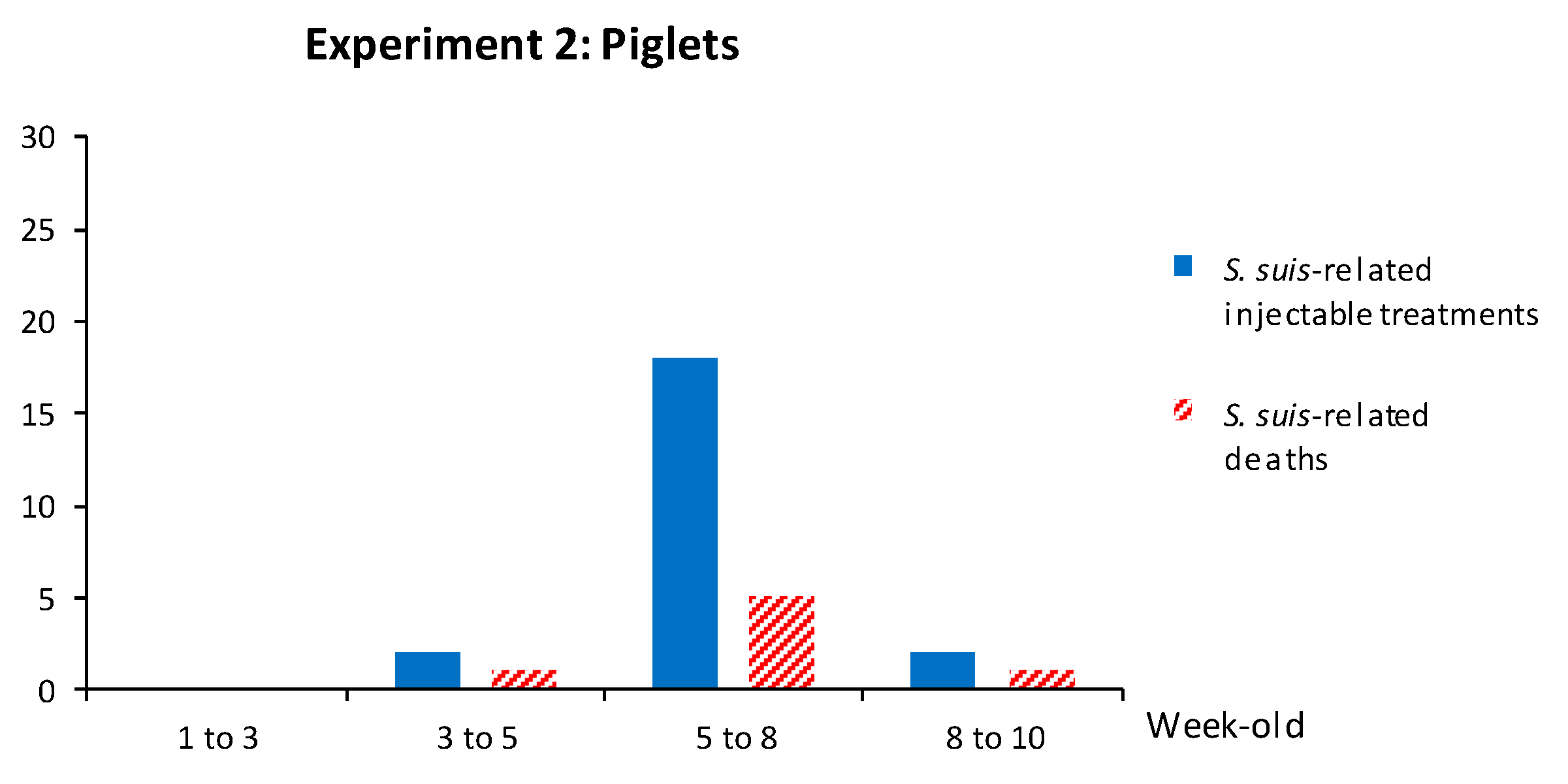
3.2.3. Weaned Piglets Are Not Clinically Protected after a Sow Vaccination Program with Two Doses of the S. suis Autogenous Bacterin
3.3. The Autogenous Vaccine Induces the Production of Anti-CPS Antibodies in Sows but Not in Piglets after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gottschalk, M.; Segura, M. Streptococcosis. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: West Sussex, UK, 2019; pp. 934–951. [Google Scholar]
- Nomoto, R.; Maruyama, F.; Ishida, S.; Tohya, M.; Sekizaki, T.; Osawa, R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22 and 26: Streptococcus parasuis sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 5, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—An update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes. Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Prüfer, T.L.; Rohde, J.; Verspohl, J.; Rohde, M.; de Greeff, A.; Willenborg, J.; Valentin-Weigand, P. Molecular typing of Streptococcus suis strains isolated from diseased and healthy pigs between 1996–2016. PLoS ONE 2019, 14, e0210801. [Google Scholar] [CrossRef]
- Gottschalk, M.; Lacouture, S. Canada: Distribution of Streptococcus suis (from 2012 to 2014) and Actinobacillus pleuropneumoniae (from 2011 to 2014) serotypes isolated from diseased pigs. Can. Vet. J. 2015, 56, 1093–1094. [Google Scholar]
- Estrada, A.A.; Gottschalk, M.; Rossow, S.; Rendahl, A.; Gebhart, C.; Marthaler, D.G. Serotype and genotype (Multilocus Sequence Type) of Streptococcus suis isolates from the United States serve as predictors of pathotype. J. Clin. Microbiol. 2019, 57, e00377-19. [Google Scholar] [CrossRef] [PubMed]
- Segura, M. Streptococcus suis vaccines: Candidate antigens and progress. Expert Rev. Vaccines 2015, 14, 1587–1608. [Google Scholar] [CrossRef]
- Seitz, M.; Valentin-Weigand, P.; Willenborg, J. Use of antibiotics and antimicrobial resistance in veterinary medicine as exemplified by the swine pathogen Streptococcus suis. Curr. Top. Microbiol. Immunol. 2016, 398, 103–121. [Google Scholar]
- Huang, J.; Ma, J.; Shang, K.; Hu, X.; Liang, Y.; Li, D.; Wu, Z.; Dai, L.; Chen, L.; Wang, L. Evolution and diversity of the antimicrobial resistance associated mobilome in Streptococcus suis: A probable mobile genetic elements reservoir for other Streptococci. Front. Cell. Infect. Microbiol. 2016, 6, e118. [Google Scholar] [CrossRef]
- Segura, M.; Aragon, V.; Brockmeier, S.L.; Gebhart, C.; de Greeff, A.; Kerdsin, A.; O’Dea, M.; Okura, M.; Saléry, M.; Schultsz, C.; et al. Update on Streptococcus suis research and prevention in the era of antimicrobial restriction: 4th international workshop on S. suis. Pathogens 2020, 9, 374. [Google Scholar] [CrossRef]
- Rieckmann, K.; Pendzialek, S.M.; Vahlenkamp, T.; Baums, C.G. A critical review speculating on the protective efficacies of autogenous Streptococcus suis bacterins as used in Europe. Porcine Health Manag. 2020, 6, e12. [Google Scholar] [CrossRef]
- Hopkins, D.; Poljak, Z.; Farzan, A.; Friendship, R. Field studies evaluating the direct, indirect, total, and overall efficacy of Streptococcus suis autogenous vaccine in nursery pigs. Can. Vet. J. 2019, 60, 386–390. [Google Scholar] [PubMed]
- Torremorell, M.; Pijoan, C.; Trigo, E. Vaccination against Streptococcus suis: Effect on nursery mortality. Swine Health Prod. 1997, 5, 139–143. [Google Scholar]
- Baums, C.G.; Bruggemann, C.; Kock, C.; Beineke, A.; Waldmann, K.H.; Valentin-Weigand, P. Immunogenicity of an autogenous Streptococcus suis bacterin in preparturient sows and their piglets in relation to protection after weaning. Clin. Vaccine Immunol. 2010, 17, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, D.J.; Polson, D.D.; Torremorell, M.; Morrison, B.; Classen, D.M.; Becton, L.; Henry, S.; Rodibaugh, M.T.; Rowland, R.R.; Snelson, H.; et al. Terminology for classifying the porcine reproductive and respiratory syndrome virus (PRRSV) status of swine herds. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2011, 39, 101–112. [Google Scholar] [PubMed]
- Gottschalk, M.; Higgins, R.; Boudreau, M. Use of polyvalent coagglutination reagents for serotyping of Streptococcus suis. J. Clin. Microbiol. 1993, 31, 2192–2194. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Calzas, C.; Shiao, T.C.; Neubauer, A.; Kempker, J.; Roy, R.; Gottschalk, M.; Segura, M. Protection against Streptococcus suis serotype 2 infection using a capsular polysaccharide glycoconjugate vaccine. Infect. Immun. 2016, 84, 2059–2075. [Google Scholar] [CrossRef] [PubMed]
- Calzas, C.; Lemire, P.; Auray, G.; Gerdts, V.; Gottschalk, M.; Segura, M. Antibody response specific to the capsular polysaccharide is impaired in Streptococcus suis serotype 2-infected animals. Infect. Immun. 2015, 83, 441–453. [Google Scholar] [CrossRef]
- Goyette-Desjardins, G.; Vinogradov, E.; Okura, M.; Takamatsu, D.; Gottschalk, M.; Segura, M. Structure determination of Streptococcus suis serotypes 7 and 8 capsular polysaccharides and assignment of functions of the cps locus genes involved in their biosynthesis. Carbohydr. Res. 2019, 473, 36–45. [Google Scholar] [CrossRef]
- Ishida, S.; Tien le, H.T.; Osawa, R.; Tohya, M.; Nomoto, R.; Kawamura, Y.; Takahashi, T.; Kikuchi, N.; Kikuchi, K.; Sekizaki, T. Development of an appropriate PCR system for the reclassification of Streptococcus suis. J. Microbiol. Methods 2014, 107, 66–70. [Google Scholar] [CrossRef]
- Okura, M.; Lachance, C.; Osaki, M.; Sekizaki, T.; Maruyama, F.; Nozawa, T.; Nakagawa, I.; Hamada, S.; Rossignol, C.; Gottschalk, M.; et al. Development of a two-step multiplex PCR assay for typing of capsular polysaccharide synthesis gene clusters of Streptococcus suis. J. Clin. Microbiol. 2014, 52, 1714–1719. [Google Scholar] [CrossRef]
- Wisselink, H.J.; Stockhofe-Zurwieden, N.; Hilgers, L.A.; Smith, H.E. Assessment of protective efficacy of live and killed vaccines based on a non-encapsulated mutant of Streptococcus suis serotype 2. Vet. Microbiol. 2002, 84, 155–168. [Google Scholar] [CrossRef]
- Smith, H.E.; Damman, M.; Van der Velde, J.; Wagenaar, F.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Stockhofe-Zurwieden, N.; Smits, M.A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999, 67, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Auger, J.P.; Segura, M.; Fittipaldi, N.; Takamatsu, D.; Okura, M.; Gottschalk, M. Role of the capsular polysaccharide as a virulence factor for Streptococcus suis serotype 14. Can. J. Vet. Res. 2015, 79, 141–146. [Google Scholar] [PubMed]
- Houde, M.; Gottschalk, M.; Gagnon, F.; Van Calsteren, M.R.; Segura, M. Streptococcus suis capsular polysaccharide inhibits phagocytosis through destabilization of lipid microdomains and prevents lactosylceramide-dependent recognition. Infect. Immun. 2012, 80, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Zhao, Y.; Sinkora, M.; Wertz, N.; Kacskovics, I. Immunoglobulins, antibody repertoire and B cell development. Dev. Comp. Immunol. 2009, 33, 321–333. [Google Scholar] [CrossRef]
- Lapointe, L.; D’Allaire, S.; Lebrun, A.; Lacouture, S.; Gottschalk, M. Antibody response to an autogenous vaccine and serologic profile for Streptococcus suis capsular type 1/2. Can. J. Vet. Res. 2002, 66, 8–14. [Google Scholar]
- Li, Y.; Gottschalk, M.; Esgleas, M.; Lacouture, S.; Dubreuil, J.D.; Willson, P.; Harel, J. Immunization with recombinant Sao protein confers protection against Streptococcus suis infection. Clin. Vaccine Immunol. 2007, 14, 937–943. [Google Scholar] [CrossRef]
- Schreiber, J.R.; Cooper, L.J.; Diehn, S.; Dahlhauser, P.A.; Tosi, M.F.; Glass, D.D.; Patawaran, M.; Greenspan, N.S. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosa lipopolysaccharide O-specific side chain function differently. J. Infect. Dis. 1993, 167, 221–226. [Google Scholar] [CrossRef]
- Martelet, L.; Lacouture, S.; Goyette-Desjardins, G.; Beauchamp, G.; Surprenant, C.; Gottschalk, M.; Segura, M. Porcine dendritic cells as an in vitro model to assess the immunological behaviour of Streptococcus suis subunit vaccine formulations and the polarizing effect of adjuvants. Pathogens 2017, 6, 13. [Google Scholar] [CrossRef]
- Sela-Culang, I.; Kunik, V.; Ofran, Y. The structural basis of antibody-antigen recognition. Front. Immunol. 2013, 4, e302. [Google Scholar] [CrossRef]
- Mond, J.J.; Lees, A.; Snapper, C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef] [PubMed]
- Baums, C.G.; Kock, C.; Beineke, A.; Bennecke, K.; Goethe, R.; Schroder, C.; Waldmann, K.H.; Valentin-Weigand, P. Streptococcus suis bacterin and subunit vaccine immunogenicities and protective efficacies against serotypes 2 and 9. Clin. Vaccine Immunol. 2009, 16, 200–208. [Google Scholar] [CrossRef] [PubMed]


| Experiment 1 1 | All Deaths | S. suis-Related Deaths | Alive | Total Pigs | ||
|---|---|---|---|---|---|---|
| Vaccinated | 31 | 5.32% | 28 | 4.80% | 552 | 583 |
| Non-vaccinated | 59 | 6.48% | 50 | 5.50% | 852 | 911 |
| Total pigs | 90 | 6.02% | 78 | 5.22% | 1404 | 1494 |
| Experiment 1 1 | Number of Injectable Treatments | Time in Pig Weeks (No. Pigs in Each Group × No. Days in Nursery) | Incidence Rate of Treatments |
|---|---|---|---|
| Vaccinated | 208 | 28,567 | 0.73% |
| Non-vaccinated | 398 | 44,639 | 0.89% |
| Total treatments | 606 | 73,206 | 0.83% |
| Experiment 2 1 | S. suis-Related Deaths | Alive | Total Pigs | |
|---|---|---|---|---|
| Vaccinated | 7 | 3.38% | 200 | 207 |
| Non-vaccinated | 6 | 3.28% | 177 | 183 |
| Total pigs | 13 | 3.33% | 377 | 390 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsaut, L.; Misener, M.; Canning, P.; Beauchamp, G.; Gottschalk, M.; Segura, M. Field Study on the Immunological Response and Protective Effect of a Licensed Autogenous Vaccine to Control Streptococcus suis Infections in Post-Weaned Piglets. Vaccines 2020, 8, 384. https://doi.org/10.3390/vaccines8030384
Corsaut L, Misener M, Canning P, Beauchamp G, Gottschalk M, Segura M. Field Study on the Immunological Response and Protective Effect of a Licensed Autogenous Vaccine to Control Streptococcus suis Infections in Post-Weaned Piglets. Vaccines. 2020; 8(3):384. https://doi.org/10.3390/vaccines8030384
Chicago/Turabian StyleCorsaut, Lorelei, Marty Misener, Paisley Canning, Guy Beauchamp, Marcelo Gottschalk, and Mariela Segura. 2020. "Field Study on the Immunological Response and Protective Effect of a Licensed Autogenous Vaccine to Control Streptococcus suis Infections in Post-Weaned Piglets" Vaccines 8, no. 3: 384. https://doi.org/10.3390/vaccines8030384
APA StyleCorsaut, L., Misener, M., Canning, P., Beauchamp, G., Gottschalk, M., & Segura, M. (2020). Field Study on the Immunological Response and Protective Effect of a Licensed Autogenous Vaccine to Control Streptococcus suis Infections in Post-Weaned Piglets. Vaccines, 8(3), 384. https://doi.org/10.3390/vaccines8030384






