Cross-Reactivity Antibody Response after Vaccination with Modified Live and Killed Bovine Viral Diarrhoea Virus (BVD) Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Vaccination Design
- (a)
- Bovilis® BVD-MD (Msd Animal Health, Madison, NJ, USA)
- (b)
- Rispoval® D-BVD (Pfizer, New York, NY, USA)
- (c)
- Mucosiffa® (Merial, Lyon, France)
- (d)
- Bovela® (Boehringer Ingelheim, Ingelheim, Germany)
2.2. Virus Neutralisation Test (VNT)
2.3. Indirect ELISA Tests
2.4. Statistical Analysis
3. Results
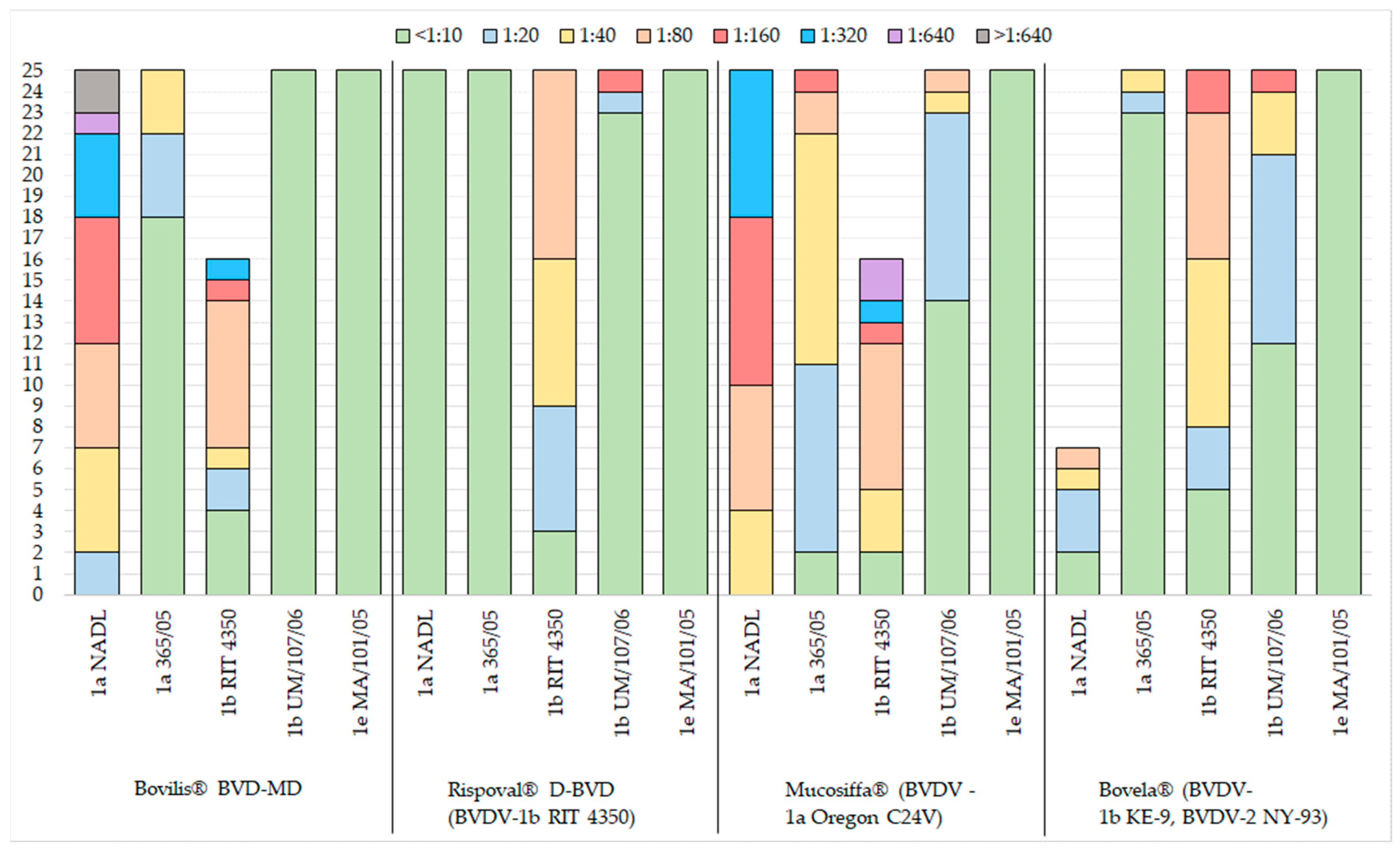
3.1. Virus Neutralisation Antibody Titres
- -
- Bovilis® BVD-MD (BVDV-1a) induced titres ≥1/20 against BVDV-1a NADL (25/25) and in most animals, also against the heterologous subgenotype BVDV-1b, RIT 4350 (21/25), but elicited a poor immunity versus the homologous genotype BVDV-1a 365/05;
- -
- Rispoval® D-BVD (BVDV-1b) induced consistent neutralising antibodies only against the homologous strain (22/25);
- -
- Mucosiffa® (BVDV-1a) induced neutralising antibodies with titres ≥1/20 against the two strains of the homologous subgenotype BVDV-1a NADL (25/25) and BVDV-1a 365/05 (23/25), but also against the heterologous subgenotype strain BVDV-1b RIT 4350 (23/25);
- -
- Bovela® (BVDV-1b and BVDV-2) induced neutralising antibodies with titres ≥1/20 against the two strains of the homologous subgenotype BVDV-1b RIT 4350 (20/25) and BVDV-1b UM/107/06 (13/25), with poor cross-reaction against the two strains of the heterologous subgenotype BVDV-1a.
3.2. Indirect ELISA Tests
- -
- Bovilis® BVD-MD (BVDV-1a): 23 animals out of 25 (23/25) developed antibodies against structural E2 protein and only 4 against NS3 (4/25);
- -
- Rispoval® D-BVD (BVDV-1b): 3 animals out of 25 (3/25) developed antibodies against structural E2 protein and only 4 against NS3 (4/25);
- -
- Mucosiffa® (BVDV-1a): all animals (25/25) developed antibodies against both structural E2 and NS3 proteins;
- -
- Bovela® (BVDV-1b and BVDV-2): 19 animals of 25 (19/25) developed antibodies against both structural E2 and NS3 proteins.
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Scott Muerhoff, A.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Jenckel, M.; Hoper, D.; Schirrmeier, H.; Reimann, I.; Goller, K.V.; Hoff-mann, B.; Beer, M. Mixed triple: Allied viruses in unique recent isolates of highly virulent type 2 bovine viral diarrhea virus detected by deep sequencing. J. Virol. 2014, 88, 6983–6992. [Google Scholar] [CrossRef]
- Yesilbag, K.; Alpay, G.; Becher, P. Variability and global distribution of subgenotypes of Bovine Viral Diarrhea Virus. Viruses 2017, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Luzzago, C.; Lauzi, S.; Ebranati, E.; Giammarioli, M.; Moreno, A.; Cannella, V.; Masoero, L.; Canelli, E.; Guercio, A.; Caruso, C.; et al. Extended genetic diversity of bovine viral diarrhea virus and frequency of genotypes and subtypes in cattle in Italy between 1995 and 2013. Biomed. Res. Int. 2014, 2014, 147145. [Google Scholar] [CrossRef] [PubMed]
- Luzzago, C.; Ebranati, E.; Sassera, D.; Lo Presti, A.; Lauzi, S.; Gabanelli, E.; Ciccozzi, M.; Zehender, G. Spatial and temporal reconstruction of bovine viral diarrhea virus genotype 1 dispersion in Italy. Infect. Genet. Evol. 2012, 12, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Lanave, G.; Lucente, M.S.; Mari, V.; Varello, K.; Losurdo, M.; Larocca, V.; Bozzetta, E.; Cavaliere, N.; Martella, V.; et al. Mucosal disease-like syndrome in a calf persistently infected by Hobi-like pestivirus. J. Clin. Microbiol. 2014, 52, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V.; Houe, H.; Lindberg, A. BVD control in Europe: Current status and perspectives. Anim. Health Res. Rev. 2005, 6, 63–74. [Google Scholar] [CrossRef]
- Graham, D.A.; German, A.; Mawhinney, K.; Goodall, E.A. Antibody responses of naive cattle to two inactivated bovine viral diarrhoea virus vaccines, measured by indirect and blocking ELISAs and virus neutralisation. Vet. Rec. 2003, 152, 795–800. [Google Scholar] [CrossRef]
- Makoschey, B.; Sonnemans, D.; Bielsa, J.M.; Franken, P.; Mars, M.; Santos, L.; Alvarez, M. Evaluation of the induction of NS3 specific BVDV antibodies using a commercial inactivated BVDV vaccine in immunization and challenge trials. Vaccine 2007, 25, 6140–6145. [Google Scholar] [CrossRef]
- Raue, R.; Harmeyer, S.S.; Nanjiani, I.A. Antibody responses to inactivated vaccines and natural infection in cattle using bovine viral diarrhoea virus ELISA kits: Assessment of potential to differentiate infected and vaccinated animals. Vet. J. 2011, 187, 330–334. [Google Scholar] [CrossRef]
- Houe, H.; Baker, J.C.; Maes, R.K.; Wuryastuti, H.; Wasito, R.; Ruegg, P.L.; Lloyd, J.W. Prevalence of cattle persistently infected with bovine viral diarrhea virus in 20 dairy herds in two counties in central Michigan and comparison of prevalence of antibody-positive cattle among herds with different infection and vaccination status. J. Vet. Diagn. Investig. 1995, 7, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, M.C.; Muñoz-Zanzi, C.A.; Hietala, S.K. Effect of calfhood vaccination on transmission of bovine viral diarrhea virus under typical drylot dairy conditions. J. Am. Vet. Med. Assoc. 2001, 219, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.E.; Neill, J.D.; Endsley, J.; Roth, J.A. Effect of passive immunity on the development of a protective immune response against bovine viral diarrhea virus in calves. Am. J. Vet. Res. 2003, 64, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, G.M.; Wentink, G.H.; Bruschke, C.; Westenbrink, F.J.; Brinkhof, J.; de Goey, I. Failure of foetal protection after vaccination against an experimental infection with bovine virus diarrhea virus. Vet Microbiol. 2002, 8, 255–265. [Google Scholar] [CrossRef]
- Grooms, D.L.; Bolin, S.R.; Coe, P.H.; Borges, R.J.; Coutu, C.E. Fetal protection against continual exposure to bovine viral diarrhea virus following administration of a vaccine containing an inactivated bovine viral diarrhea virus fraction to cattle. Am. J. Vet. Res. 2007, 68, 1417–1422. [Google Scholar] [CrossRef]
- OIE Manual for Terrestrial Animals 2015 cap 3.4.7 par B2.1: Bovine Viral Diarrhea—Diagnostic Techniques—Serological Tests—Virus Neutralization Test. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.07_BVD.pdf (accessed on 15 March 2020).
- Pezzoni, G.; Stercoli, L.; Cordioli, P.; Brocchi, E. Recombinant NS3 and monoclonal antibodies for serodiagnosis of pestiviruses infection by competitive ELISA. In Proceedings of the 8th International Congress of Veterinary Virology, Budapest, Hungary, 23–26 August 2009; p. 74. [Google Scholar]
- Brocchi, E.; Cordioli, P.; Berlinzani, A.; Gamba, D.; De Simone, F. Development of a panel of anti-pestivirus monoclonal antibodies useful for virus identification and antibody assessment. In Proceedings of the Second Symposium on Pestiviruses, Veyrier-du-Lac, France, 1–3 October 1992; pp. 215–218. [Google Scholar]
- Ridpath, J.F.; Neill, J.D.; Frey, M.; Landgraf, J.G. Phylogenetic, antigenic and clinical characterization of type 2 BVDV from North America. Vet. Microbiol. 2000, 77, 145–155. [Google Scholar] [CrossRef]
- Becher, P.; Ramirez, R.A.; Orlich, M.; Rosales, S.C.; Ko¨ nig, M.; Schweizer, M.; Stalder, H.; Schirrmeier, H.; Theil, H.J. Genetic and antigenic characterization of novel pestivirus genotypes: Implications for classification. Virology 2003, 311, 96–104. [Google Scholar] [CrossRef]
- Fulton, R.W.; Briggs, R.E.; Ridpath, J.F.; Saliki, J.T.; Confer, A.W.; Payton, M.E.; Duff, G.C.; Step, D.L.; Walker, D.A. Transmission of bovine viral diarrhea virus 1b to susceptible and vaccinated calves by exposure to persistently infected calves. Can. J. Vet. Res. 2005, 69, 161–169. [Google Scholar]
- Gripshover, E.M.; Givens, M.D.; Ridpath, J.F.; Brock, K.V.; Whitley, E.M.; Sartin, E.A. Variation in Erns viral glycoprotein associated with failure of immunohistochemistry and commercial antigen capture ELISA to detect a field strain of bovine viral diarrhea virus. Vet. Microbiol. 2007, 125, 11–21. [Google Scholar] [CrossRef]
- Ridpath, J. Practical significance of heterogeneity among BVDV strains: Impact of biotype and genotype on U.S. control programs. Prev. Vet. Med. 2005, 72, 17–30. [Google Scholar] [CrossRef]
- Alpay, G.; Yeşilbağ, K. Serological relationships among subgroups in bovine viral diarrhea virus genotype 1 (BVDV-1). Vet. Microbiol. 2015, 175, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bachofen, C.; Stalder, H.; Braun, U.; Hilbe, M.; Ehrensperger, F.; Peterhans, E. Co-existence of genetically and antigenically diverse bovine viral diarrhoea viruses in an endemic situation. Vet. Microbiol. 2008, 131, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Hehnen, H.R.; Wolfmeyer, A.; Poll, G.; Kaaden, O.R.; Wolf, G. A new inactivated BVDV genotype I and II vaccine. An immunization and challenge study with BVDV genotype I. Vet. Microbiol. 2000, 77, 195–208. [Google Scholar] [CrossRef]
- Bolin, S.R.; Ridpath, J.F. Assessment of protection from systemic infection or disease afforded by low to intermediate titers of passively acquired neutralizing antibody against bovine viral diarrhea virus in calves. Am. J. Vet. Res. 1995, 56, 755–759. [Google Scholar]
- Harmes, C.; Di Valentin, E.; Lecomte, C.; Lambot, M.; Joris, E.; Genicot, B.; Pastoret, P.P. Virus neutralising antibodies against 22 bovine viral diarrhoea virus isolates in vaccinated calves. Vet. J. 2002, 163, 61–67. [Google Scholar]
- Minami, F.; Nagai, M.; Ito, M.; Matsuda, T.; Takai, H.; Jinkawa, Y.; Shimano, T.; Hayashi, M.; Seki, Y.; Sakoda, Y.; et al. Reactivity and prevalence of neutralizing antibodies against Japanese strains of bovine viral diarrhea virus subgenotypes. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 35–39. [Google Scholar] [CrossRef]
- Ridpath, J.F. Bovine viral diarrhea virus: Global status. Vet. Clin. North. Am. Food. Anim. Pract. 2010, 26, 105–121. [Google Scholar] [CrossRef]
- Fulton, R.W.; Ridpath, J.F.; Confer, A.W.; Saliki, J.T.; Burge, L.J.; Payton, M.E. Bovine viral diarrhoea virus antigenic diversity: Impact on disease and vaccination programmes. Biologicals 2003, 31, 89–95. [Google Scholar] [CrossRef]
- Arias, P.; Orlich, M.; Prieto, M.; Cedillo Rosales, S.; Thiel, H.J.; Alvarez, M.; Becher, P. Genetic heterogeneity of bovine viral diarrhoea viruses from Spain. Vet. Microbiol. 2003, 96, 327–336. [Google Scholar] [CrossRef]

| Vaccine | Strain | Subgenotype | Inactivated/Live Modified | Vaccination Schedule |
|---|---|---|---|---|
| Bovilis® BVD-MD | C86 | 1a | Inactivated | Twice at an interval of 1 month |
| Rispoval® D-BVD | RIT4350 | 1b | Live modified | Twice at an interval of 1 month |
| Mucosiffa® | C24V | 1a | Live modified | Once |
| Bovela® | KE-9 | 1b | Live modified | Once |
| Strain | Genotype | Subgenotype | Biotype |
|---|---|---|---|
| NADL | BVDV-1 | 1a | Cp * |
| 365/05 | BVDV-1 | 1a | Cp |
| RIT4350 | BVDV-1 | 1b | Cp |
| UM/107/06 | BVDV-1 | 1b | Cp |
| MA/101/05 | BVDV-1 | 1e | Cp |
| Vaccine | Reference Strain | BVDV Strains Used for the SN Test/p-Value (*) | |||
|---|---|---|---|---|---|
| Bovilis® BVD-MD | 1a 365/05 | 1b RIT 4350 | 1b UM/107/06 | 1e MA/101/05 | |
| 1a NADL | <0.0001 (*) | 0.0011 | <0.0001 | <0.0001 | |
| Rispoval® D-BVD | 1a NADL | 1a 365/05 | 1b UM/107/06 | 1e MA/101/05 | |
| 1b RIT 4350 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Mucosiffa® | 1a 365/05 | 1b RIT 4350 | 1b UM/107/06 | 1e MA/101/05 | |
| 1a NADL | <0.0001 | 0.3545 | <0.0001 | <0.0001 | |
| Bovela® | 1a NADL | 1a 365/05 | 1b UM/107/06 | 1e MA/101/05 | |
| 1b RIT 4350 | <0.0001 | <0.0001 | 0.0004 | <0.0001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozzi, E.; Righi, C.; Boldini, M.; Bazzucchi, M.; Pezzoni, G.; Gradassi, M.; Petrini, S.; Lelli, D.; Ventura, G.; Pierini, I.; et al. Cross-Reactivity Antibody Response after Vaccination with Modified Live and Killed Bovine Viral Diarrhoea Virus (BVD) Vaccines. Vaccines 2020, 8, 374. https://doi.org/10.3390/vaccines8030374
Sozzi E, Righi C, Boldini M, Bazzucchi M, Pezzoni G, Gradassi M, Petrini S, Lelli D, Ventura G, Pierini I, et al. Cross-Reactivity Antibody Response after Vaccination with Modified Live and Killed Bovine Viral Diarrhoea Virus (BVD) Vaccines. Vaccines. 2020; 8(3):374. https://doi.org/10.3390/vaccines8030374
Chicago/Turabian StyleSozzi, Enrica, Cecilia Righi, Massimo Boldini, Moira Bazzucchi, Giulia Pezzoni, Matteo Gradassi, Stefano Petrini, Davide Lelli, Giordano Ventura, Ilaria Pierini, and et al. 2020. "Cross-Reactivity Antibody Response after Vaccination with Modified Live and Killed Bovine Viral Diarrhoea Virus (BVD) Vaccines" Vaccines 8, no. 3: 374. https://doi.org/10.3390/vaccines8030374
APA StyleSozzi, E., Righi, C., Boldini, M., Bazzucchi, M., Pezzoni, G., Gradassi, M., Petrini, S., Lelli, D., Ventura, G., Pierini, I., Moreno, A., Brocchi, E., Lavazza, A., & De Mia, G. M. (2020). Cross-Reactivity Antibody Response after Vaccination with Modified Live and Killed Bovine Viral Diarrhoea Virus (BVD) Vaccines. Vaccines, 8(3), 374. https://doi.org/10.3390/vaccines8030374








