Liposomal Formulation of ChimeraT, a Multiple T-Cell Epitope-Containing Recombinant Protein, Is a Candidate Vaccine for Human Visceral Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Recombinant ChimeraT Protein
2.2. Preparation of Liposomes Incorporating ChimeraT
2.3. Mice and Parasites
2.4. Vaccination of BALB/c Mice and Infection with L. infantum
2.5. Evaluating Cellular Responses before and after L. infantum Infection
2.6. Humoral Response Generated before and after L. infantum Challenge
2.7. Evaluation of the Parasite Load by the Limiting Dilution Technique and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
2.8. Human Sera
2.9. Post-Therapy Serological Follow-up
2.10. Statistical Analysis
3. Results
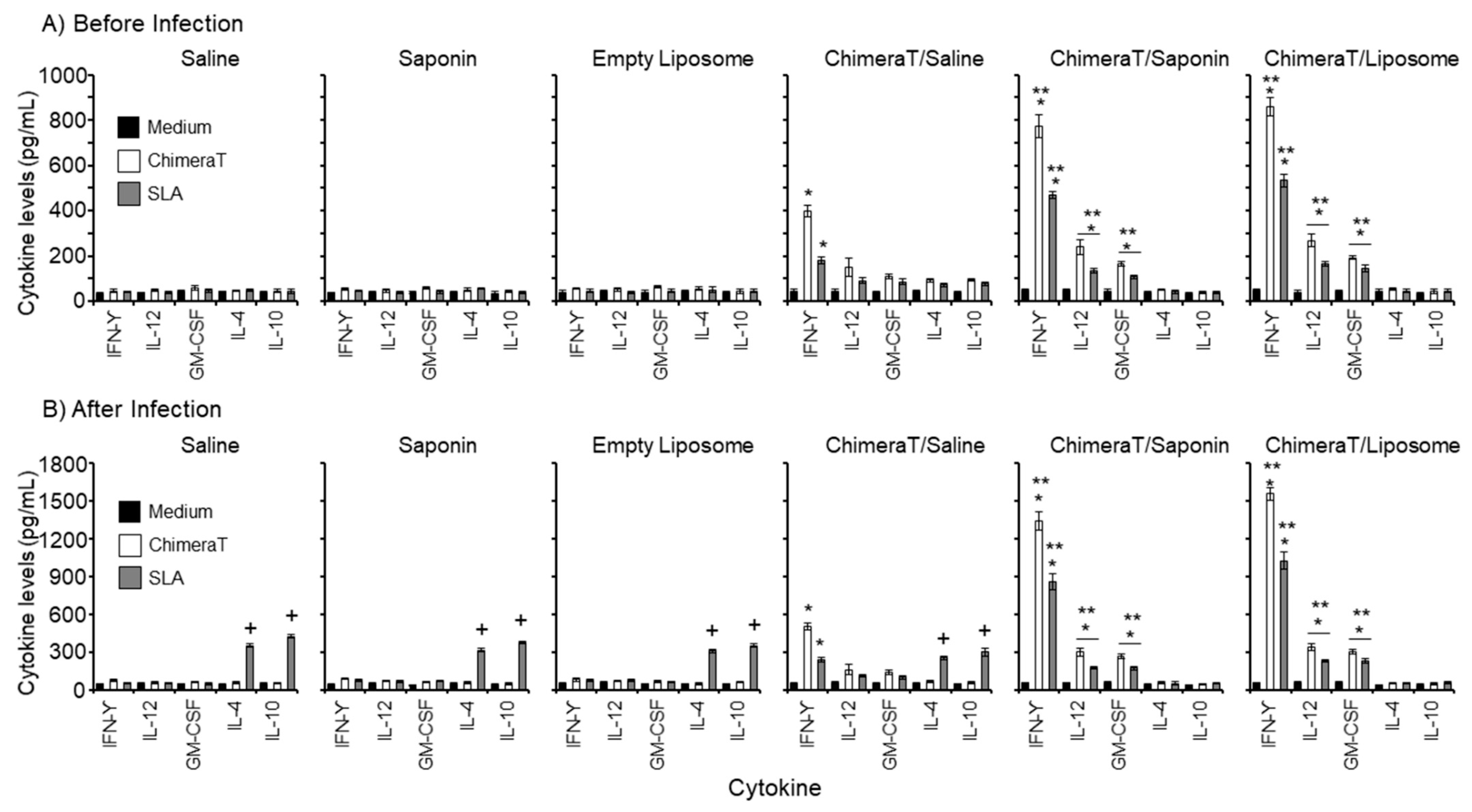
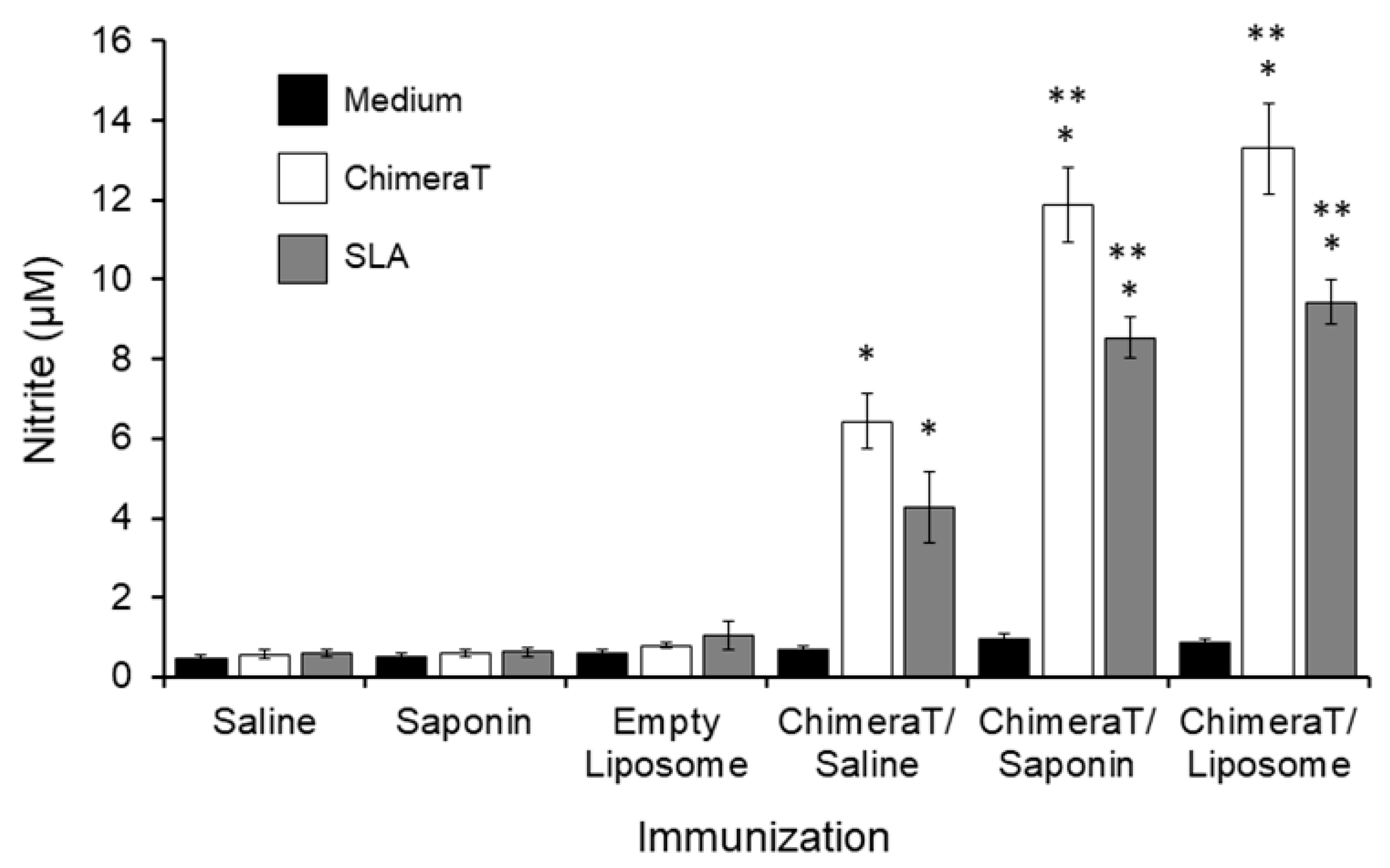
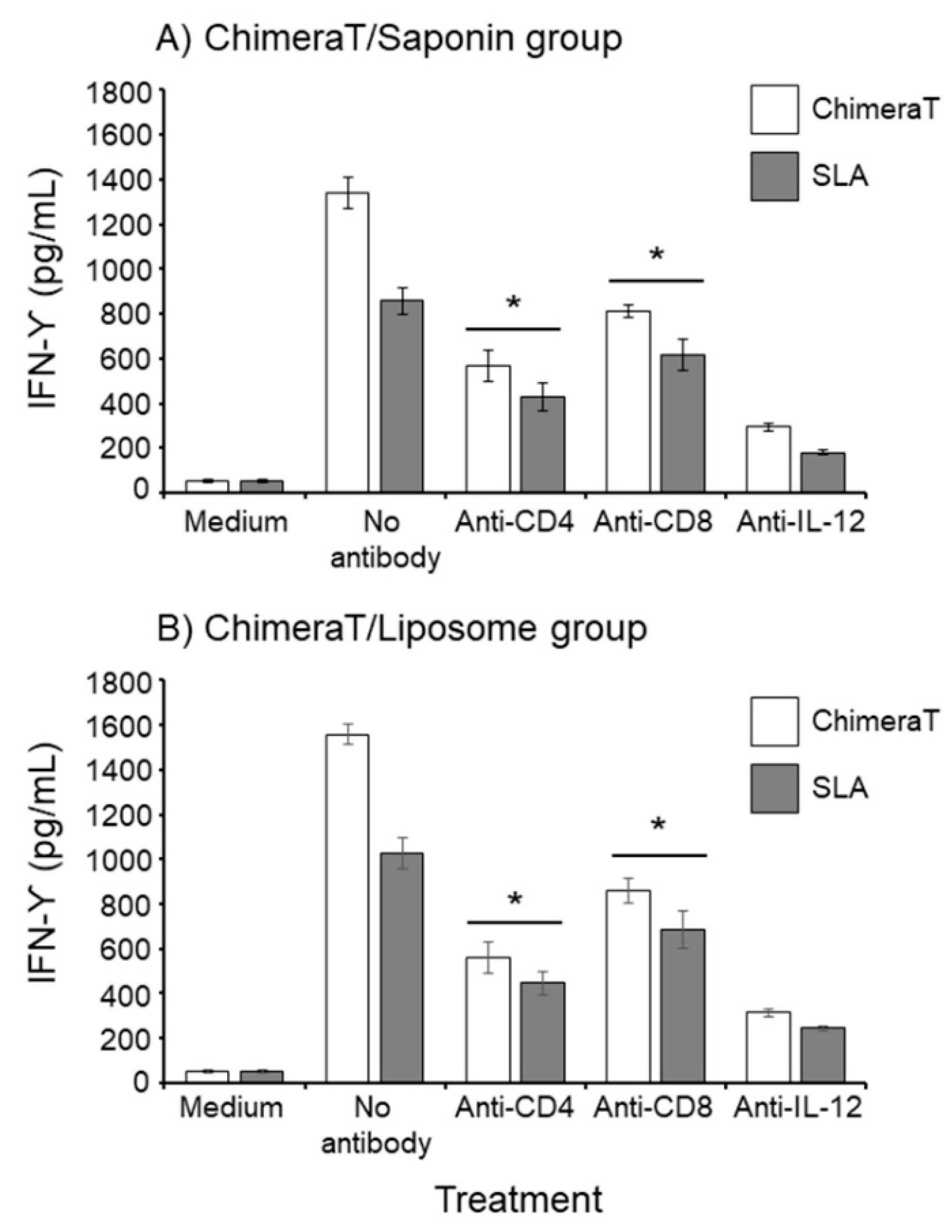
3.1. Cellular Responses in Vaccinated Mice before and after L. infantum Infection
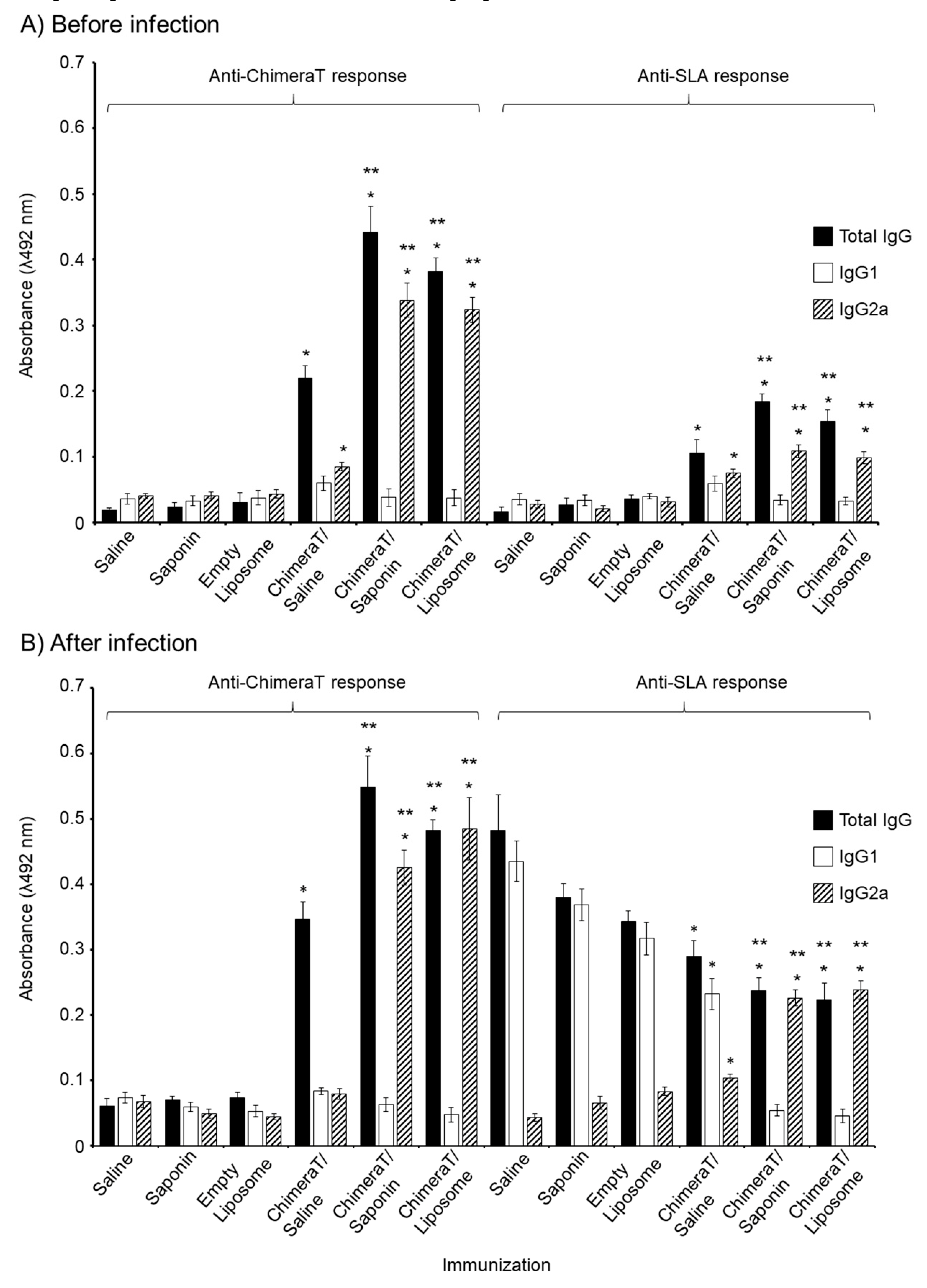
3.2. Total IgG, IgG1, and IgG2a Antibody Responses in Vaccinated Mice before and after L. infantum Infection
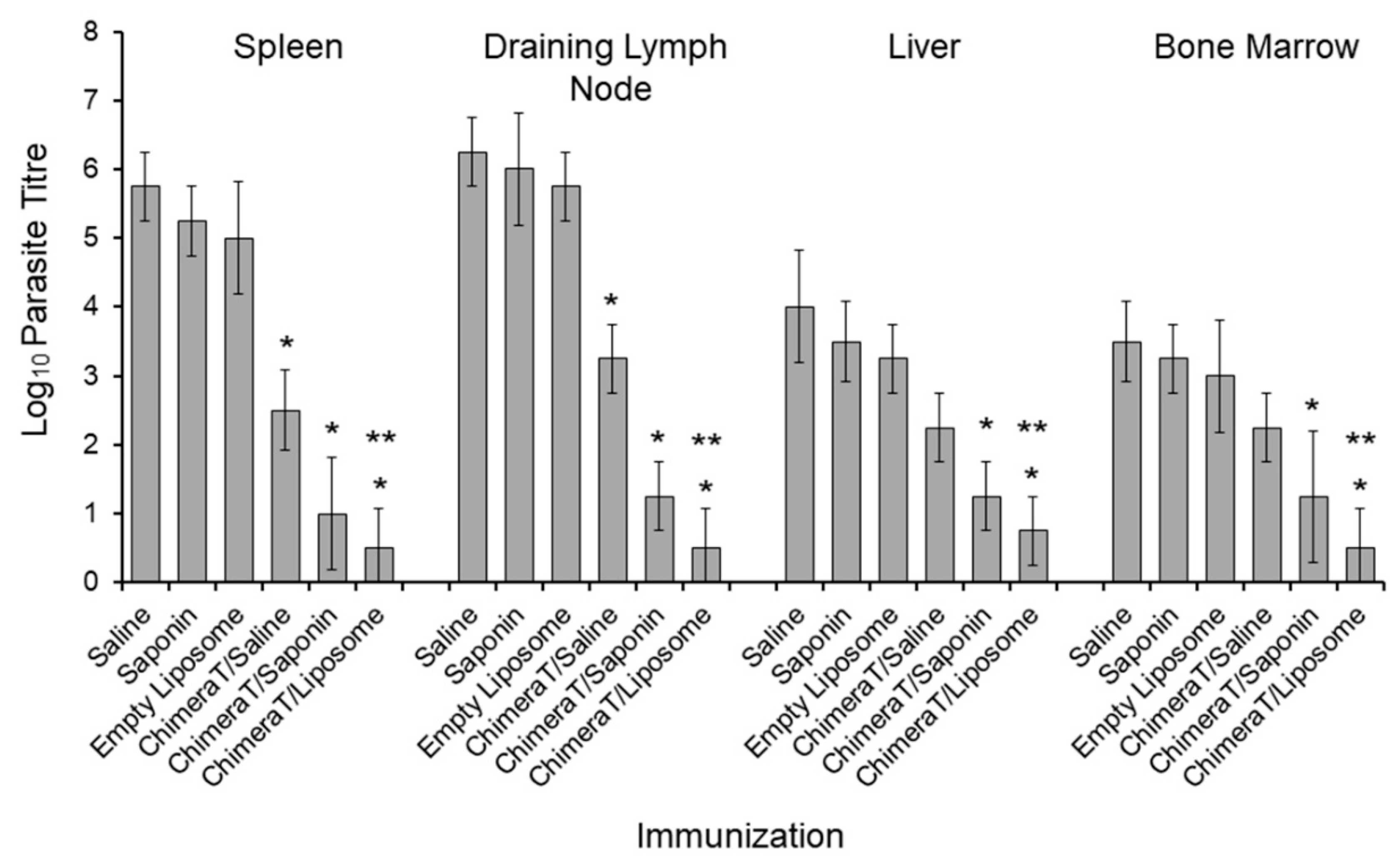
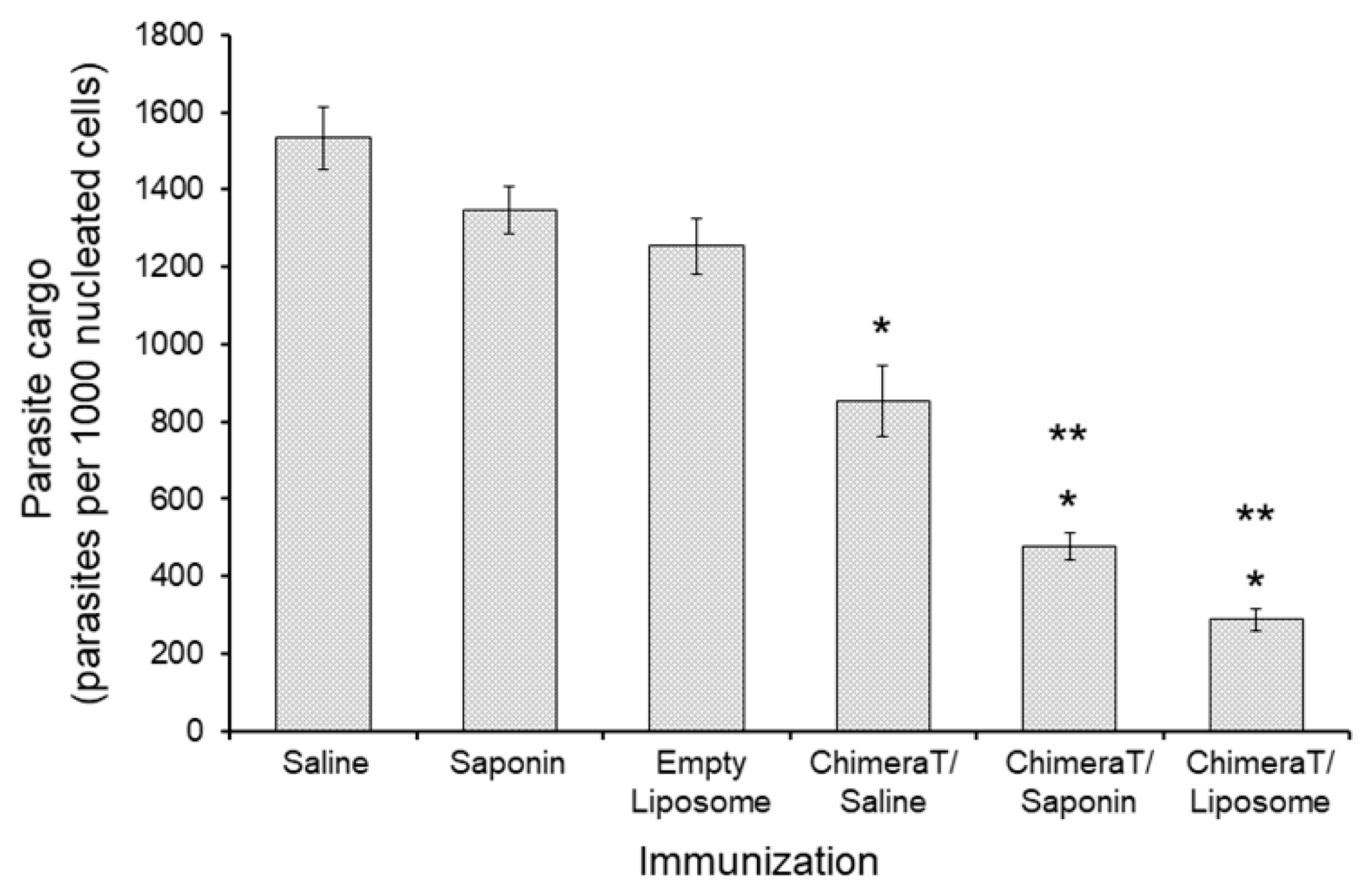
3.3. Estimation of Parasite Burden
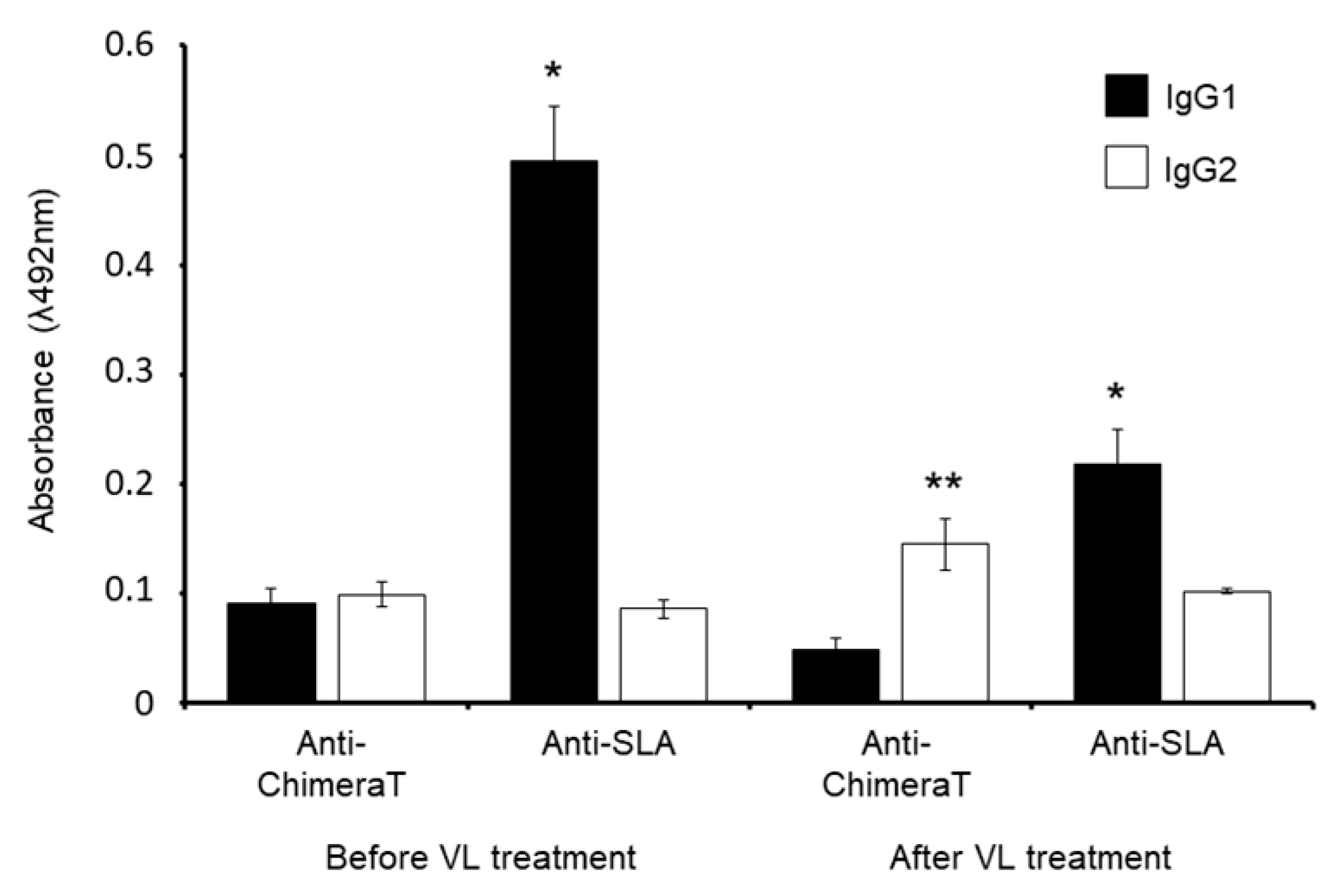
3.4. Post-Therapy Serological Follow-up
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World-Health-Organisation. Available online: http://www.who.int/topics/leishmaniasis/en/ (accessed on 30 March 2020).
- Grimaldi, G., Jr.; Tesh, R.B. Leishmaniases of the New World: Current concepts and implications for future research. Clin. Microbiol. Rev. 1993, 6, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, J.; Hasker, E.; Kansal, S.; Singh, O.P.; Malaviya, P.; Singh, A.K.; Chourasia, A.; Singh, T.; Sudarshan, M.; Singh, A.P.; et al. Determinants for progression from asymptomatic infection to symptomatic visceral leishmaniasis: A cohort study. PLoS Negl. Trop. Dis. 2019, 13, e0007216. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A. Recent developments and future prospects in the treatment of visceral leishmaniasis. Ther. Adv. Infect. Dis. 2016, 3, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A. Chemotherapeutics of visceral leishmaniasis: Present and future developments. Parasitology 2018, 145, 481–489. [Google Scholar] [CrossRef]
- Cunha, M.A.; Leao, A.C.Q.; Soler, R.D.; Lindoso, J.A.L. Efficacy and Safety of Liposomal Amphotericin B for the Treatment of Mucosal Leishmaniasis from the New World: A Retrospective Study. Am. J. Trop. Med. Hyg. 2015, 93, 1214–1218. [Google Scholar] [CrossRef]
- Duarte, M.C.; Lage, L.M.D.; Lage, D.P.; Martins, V.T.; Carvalho, A.; Roatt, B.M.; Menezes-Souza, D.; Tavares, C.A.P.; Alves, R.J.; Barichello, J.M.; et al. Treatment of murine visceral leishmaniasis using an 8-hydroxyquinoline-containing polymeric micelle system. Parasitol. Int. 2016, 65, 728–736. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef]
- Valenzuela-Oses, J.K.; Garcia, M.C.; Feitosa, V.A.; Pachioni-Vasconcelos, J.A.; Gomes-Filho, S.M.; Lourenco, F.R.; Cerize, N.N.P.; Basseres, D.S.; Rangel-Yagui, C.O. Development and characterization of miltefosine-loaded polymeric micelles for cancer treatment. Mat. Sci. Eng. C 2017, 81, 327–333. [Google Scholar] [CrossRef]
- Palic, S.; Bhairosing, P.; Beijnen, J.H.; Dorlo, T.P.C. Systematic Review of Host-Mediated Activity of Miltefosine in Leishmaniasis through Immunomodulation. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Pijpers, J.; den Boer, M.L.; Essink, D.R.; Ritmeijer, K. The safety and efficacy of miltefosine in the long-term treatment of post-kala-azar dermal leishmaniasis in South Asia-A review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Crouch, K.; Forrester, S.; Silva, V.C.; Carvalho, S.F.G.; Damasceno, J.D.; Brown, E.; Dickens, N.J.; Costa, D.L.; Costa, C.H.N.; et al. A Leishmania infantum genetic marker associated with miltefosine treatment failure for visceral leishmaniasis. Ebiomed 2018, 36, 83–91. [Google Scholar] [CrossRef]
- Mondelaers, A.; Hendrickx, S.; Van Bockstal, L.; Maes, L.; Caljon, G. Miltefosine-resistant Leishmania infantum strains with an impaired MT/ROS3 transporter complex retain amphotericin B susceptibility. J. Antimicrob. Chemother. 2018, 73, 392–394. [Google Scholar] [CrossRef]
- Kedzierski, L.; Evans, K.J. Immune responses during cutaneous and visceral leishmaniasis. Parasitology 2014, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, A.; Chandrasekaran, S.; Kuchipudi, S.V.; Kalangi, S.K. Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy. Front. Immunol. 2019, 10, 670. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Dasgupta, S.; Ghose, A.C. Immunoglobulin G subclass-specific antileishmanial antibody responses in Indian kala-azar and post-kala-azar dermal leishmaniasis. Clin. Diagn. Lab. Immunol. 1995, 2, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Anam, K.; Afrin, F.; Banerjee, D.; Pramanik, N.; Guha, S.K.; Goswami, R.P.; Gupta, P.N.; Saha, S.K.; Ali, N. Immunoglobulin subclass distribution and diagnostic value of Leishmania donovani antigen-specific immunoglobulin G3 in Indian kala-azar patients. Clin. Diagn. Lab. Immunol. 1999, 6, 231–235. [Google Scholar] [CrossRef]
- da Matta, V.L.; Hoshino-Shimizu, S.; Dietze, R.; Corbett, C.E. Detection of specific antibody isotypes and subtypes before and after treatment of American visceral leishmaniasis. J. Clin. Lab. Anal. 2000, 14, 5–12. [Google Scholar] [CrossRef]
- Ryan, J.R.; Smithyman, A.M.; Rajasekariah, G.H.; Hochberg, L.; Stiteler, J.M.; Martin, S.K. Enzyme-linked immunosorbent assay based on soluble promastigote antigen detects immunoglobulin M (IgM) and IgG antibodies in sera from cases of visceral and cutaneous leishmaniasis. J. Clin. Microbiol. 2002, 40, 1037–1043. [Google Scholar] [CrossRef]
- Caldas, A.; Favali, C.; Aquino, D.; Vinhas, V.; van Weyenbergh, J.; Brodskyn, C.; Costa, J.; Barral-Netto, M.; Barral, A. Balance of IL-10 and interferon-gamma plasma levels in human visceral leishmaniasis: Implications in the pathogenesis. BMC Infect. Dis. 2005, 5, 113. [Google Scholar] [CrossRef]
- Castellano, L.R.; Filho, D.C.; Argiro, L.; Dessein, H.; Prata, A.; Dessein, A.; Rodrigues, V. Th1/Th2 immune responses are associated with active cutaneous leishmaniasis and clinical cure is associated with strong interferon-gamma production. Hum. Immunol. 2009, 70, 383–390. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Jaafari, M.R.; Khamesipour, A.; Badiee, A. Liposomal adjuvant development for leishmaniasis vaccines. Ther. Adv. Vaccines 2017, 5, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Pirdel, L.; Farajnia, S. A Non-pathogenic Recombinant Leishmania Expressing Lipophosphoglycan 3 against Experimental Infection with Leishmania infantum Scand. J. Immunol. 2017, 86, 15–22. [Google Scholar] [CrossRef]
- Abbehusen, M.M.C.; Cunha, J.; Suarez, M.S.; Teixeira, C.; Almeida, V.D.A.; Pereira, L.D.S.; Bordoni, M.; Gil-Santana, L.; Solca, M.D.S.; Fraga, D.B.M.; et al. Immunization of Experimental Dogs With Salivary Proteins From Lutzomyia longipalpis, Using DNA and Recombinant Canarypox Virus Induces Immune Responses Consistent With Protection Against Leishmania infantum Front. Immunol. 2018, 9, 2558. [Google Scholar] [CrossRef]
- Yadav, N.K.; Joshi, S.; Ratnapriya, S.; Sahasrabuddhe, A.A.; Dube, A. Immunotherapeutic potential of Leishmania (Leishmania) donovani Th1 stimulatory proteins against experimental visceral leishmaniasis. Vaccine 2018, 36, 2293–2299. [Google Scholar] [CrossRef]
- Joshi, S.; Yadav, N.K.; Rawat, K.; Kumar, V.; Ali, R.; Sahasrabuddhe, A.A.; Siddiqi, M.I.; Haq, W.; Sundar, S.; Dube, A. Immunogenicity and Protective Efficacy of T-Cell Epitopes Derived From Potential Th1 Stimulatory Proteins of Leishmania (Leishmania) donovani. Front. Immunol. 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.S.; Ribeiro, P.A.F.; Martins, V.T.; Lage, D.P.; Costa, L.E.; Chavez-Fumagalli, M.A.; Ramos, F.F.; Santos, T.T.O.; Ludolf, F.; Oliveira, J.S.; et al. Vaccination with a CD4(+) and CD8(+) T-cell epitopes-based recombinant chimeric protein derived from Leishmania infantum proteins confers protective immunity against visceral leishmaniasis. Transl. Res. 2018, 200, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Margaroni, M.; Athanasiou, E.; Toubanaki, D.K.; Kontonikola, K.; Karidi, K.; Kammona, O.; Kiparissides, C.; Karagouni, E. Identification of BALB/c Immune Markers Correlated with a Partial Protection to Leishmania infantum after Vaccination with a Rationally Designed Multi-epitope Cysteine Protease A Peptide-Based Nanovaccine. PLoS Negl. Trop. Dis. 2017, 11, e0005311. [Google Scholar] [CrossRef]
- Alves-Silva, M.V.; Nico, D.; Morrot, A.; Palatnik, M.; Palatnik-de-Sousa, C.B. A Chimera Containing CD4+ and CD8+ T-Cell Epitopes of the Leishmania donovani Nucleoside Hydrolase (NH36) Optimizes Cross-Protection against Leishmania amazonesis Infection. Front. Immunol. 2017, 8, 100. [Google Scholar] [CrossRef]
- Martins, V.T.; Duarte, M.C.; Lage, D.P.; Costa, L.E.; Carvalho, A.M.; Mendes, T.A.; Roatt, B.M.; Menezes-Souza, D.; Soto, M.; Coelho, E.A. A recombinant chimeric protein composed of human and mice-specific CD4(+) and CD8(+) T-cell epitopes protects against visceral leishmaniasis. Parasite Immunol. 2017, 39. [Google Scholar] [CrossRef]
- Nagill, R.; Kaur, T.; Joshi, J.; Kaur, S. Immunogenicity and efficacy of recombinant 78 kDa antigen of Leishmania donovani formulated in various adjuvants against murine visceral leishmaniasis. Asian Pac. J. Trop. Med. 2015, 8, 513–519. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; De Gregorio, E. The path to a successful vaccine adjuvant—‘The long and winding road’. Drug Discov. Today 2009, 14, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.A.F.; Dias, D.S.; Novais, M.V.M.; Lage, D.P.; Tavares, G.S.V.; Mendonca, D.V.C.; Oliveira, J.S.; Chavez-Fumagalli, M.A.; Roatt, B.M.; Duarte, M.C.; et al. A Leishmania hypothetical protein-containing liposome-based formulation is highly immunogenic and induces protection against visceral leishmaniasis. Cytokine 2018, 111, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ratnapriya, S.; Sahasrabuddhe, A.A.; Dube, A. Visceral leishmaniasis: An overview of vaccine adjuvants and their applications. Vaccine 2019, 37, 3505–3519. [Google Scholar] [CrossRef] [PubMed]
- Rajput, Z.I.; Hu, S.H.; Xiao, C.W.; Arijo, A.G. Adjuvant effects of saponins on animal immune responses. J. Zhejiang Univ. Sci. B 2007, 8, 153–161. [Google Scholar] [CrossRef]
- Coelho, E.A.; Tavares, C.A.; Carvalho, F.A.; Chaves, K.F.; Teixeira, K.N.; Rodrigues, R.C.; Charest, H.; Matlashewski, G.; Gazzinelli, R.T.; Fernandes, A.P. Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect. Immun. 2003, 71, 3988–3994. [Google Scholar] [CrossRef]
- Kaur, H.; Thakur, A.; Kaur, S. Studies on cocktails of 31-kDa, 36-kDa and 51-kDa antigens of Leishmania donovani along with saponin against murine visceral leishmaniasis. Parasite Immunol. 2015, 37, 192–203. [Google Scholar] [CrossRef]
- Thakur, A.; Kaur, H.; Kaur, S. Studies on the protective efficacy of freeze thawed promastigote antigen of Leishmania donovani along with various adjuvants against visceral leishmaniasis infection in mice. Immunobiology 2015, 220, 1031–1038. [Google Scholar] [CrossRef]
- Santos, T.T.O.; Martins, V.T.; Lage, D.P.; Costa, L.E.; Salles, B.C.S.; Carvalho, A.; Dias, D.S.; Ribeiro, P.A.F.; Chavez-Fumagalli, M.A.; Machado-de-Avila, R.A.; et al. Probing the efficacy of a heterologous Leishmania/L. Viannia braziliensis recombinant enolase as a candidate vaccine to restrict the development of L. infantum in BALB/c mice. Acta. Trop. 2017, 171, 8–16. [Google Scholar] [CrossRef]
- Das, P.; Paik, D.; Naskar, K.; Chakraborti, T. Leishmania donovani serine protease encapsulated in liposome elicits protective immunity in experimental visceral leishmaniasis. Microbes Infect. 2018, 20, 37–47. [Google Scholar] [CrossRef]
- Koutsoni, O.S.; Barhoumi, M.; Guizani, I.; Dotsika, E. New Insights on the Adjuvant Properties of the Leishmania infantum Eukaryotic Initiation Factor. J. Immunol. Res. 2019, 2019, 9124326. [Google Scholar] [CrossRef]
- Noormehr, H.; Zavaran Hosseini, A.; Soudi, S.; Beyzay, F. Enhancement of Th1 immune response against Leishmania cysteine peptidase A, B by PLGA nanoparticle. Int. Immunopharmacol. 2018, 59, 97–105. [Google Scholar] [CrossRef]
- De Brito, R.C.F.; Cardoso, J.M.O.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.O.; Teixeira-Carvalho, A.; Roatt, B.M.; Correa-Oliveira, R.; Ruiz, J.C.; Resende, D.M.; et al. Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice. Vaccines 2019, 7, 162. [Google Scholar] [CrossRef]
- Badiee, A.; Heravi Shargh, V.; Khamesipour, A.; Jaafari, M.R. Micro/nanoparticle adjuvants for antileishmanial vaccines: Present and future trends. Vaccine 2013, 31, 735–749. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.; Dong, W.; Gao, Y.; Meng, Y.; Wang, H.; Li, L.; Jin, J.; Ji, M.; Xia, X.; et al. Drug-free mannosylated liposomes inhibit tumor growth by promoting the polarization of tumor-associated macrophages. Int. J. Nanomed. 2019, 14, 3203–3220. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Schumack, N.M.; Gariepy, C.L.; Eggleston, H.; Nunez, G.; Espinoza, N.; Nieto, M.; Castillo, R.; Rojas, J.; McCoy, A.J.; et al. Enhanced Immunogenicity and Protective Efficacy of a Campylobacter jejuni Conjugate Vaccine Coadministered with Liposomes Containing Monophosphoryl Lipid A and QS-21. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Rao, M.; Onkar, S.; Peachman, K.K.; White, Y.; Trinh, H.V.; Jobe, O.; Zhou, Y.; Dawson, P.; Eller, M.A.; Matyas, G.R.; et al. Liposome-Encapsulated Human Immunodeficiency Virus-1 gp120 Induces Potent V1V2-Specific Antibodies in Humans. J. Infect. Dis. 2018, 218, 1541–1550. [Google Scholar] [CrossRef]
- Jain, R.; Ghoshal, A.; Mandal, C.; Shaha, C. Leishmania cell surface prohibitin: Role in host-parasite interaction. Cell Microbiol. 2010, 12, 432–452. [Google Scholar] [CrossRef]
- Duarte, M.C.; Pimenta, D.C.; Menezes-Souza, D.; Magalhaes, R.D.; Diniz, J.L.; Costa, L.E.; Chavez-Fumagalli, M.A.; Lage, P.S.; Bartholomeu, D.C.; Alves, M.J.; et al. Proteins Selected in Leishmania (Viannia) braziliensis by an Immunoproteomic Approach with Potential Serodiagnosis Applications for Tegumentary Leishmaniasis. Clin. Vaccine Immunol. 2015, 22, 1187–1196. [Google Scholar] [CrossRef]
- Duarte, M.C.; Lage, D.P.; Martins, V.T.; Costa, L.E.; Lage, L.M.; Carvalho, A.M.; Ludolf, F.; Santos, T.T.; Roatt, B.M.; Menezes-Souza, D.; et al. A vaccine combining two Leishmania braziliensis proteins offers heterologous protection against Leishmania infantum infection. Mol. Immunol. 2016, 76, 70–79. [Google Scholar] [CrossRef]
- Dias, D.S.; Ribeiro, P.A.F.; Martins, V.T.; Lage, D.P.; Ramos, F.F.; Dias, A.L.T.; Rodrigues, M.R.; Portela, A.S.B.; Costa, L.E.; Caligiorne, R.B.; et al. Recombinant prohibitin protein of Leishmania infantum acts as a vaccine candidate and diagnostic marker against visceral leishmaniasis. Cell Immunol. 2018, 323, 59–69. [Google Scholar] [CrossRef]
- Lage, D.P.; Ludolf, F.; Silveira, P.C.; Machado, A.S.; Ramos, F.F.; Dias, D.S.; Ribeiro, P.A.F.; Costa, L.E.; Vale, D.L.; Tavares, G.S.V.; et al. Screening diagnostic candidates from Leishmania infantum proteins for human visceral leishmaniasis using an immunoproteomics approach. Parasitology 2019, 146, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.T.; Lage, D.P.; Duarte, M.C.; Carvalho, A.M.; Costa, L.E.; Mendes, T.A.; Vale, D.L.; Menezes-Souza, D.; Roatt, B.M.; Tavares, C.A.; et al. A recombinant fusion protein displaying murine and human MHC class I- and II-specific epitopes protects against Leishmania amazonensis infection. Cell Immunol. 2017, 313, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Reinherz, E.L. Prediction of peptide-MHC binding using profiles. Methods Mol. Biol. 2007, 409, 185–200. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Duarte, M.C.; Lage, D.P.; Martins, V.T.; Chavez-Fumagalli, M.A.; Roatt, B.M.; Menezes-Souza, D.; Goulart, L.R.; Soto, M.; Tavares, C.A.; Coelho, E.A. Recent updates and perspectives on approaches for the development of vaccines against visceral leishmaniasis. Rev. Soc. Bras. Med. Trop. 2016, 49, 398–407. [Google Scholar] [CrossRef]
- Agallou, M.; Pantazi, E.; Tsiftsaki, E.; Toubanaki, D.K.; Gaitanaki, C.; Smirlis, D.; Karagouni, E. Induction of protective cellular immune responses against experimental visceral leishmaniasis mediated by dendritic cells pulsed with the N-terminal domain of Leishmania infantum elongation factor-2 and CpG oligodeoxynucleotides. Mol. Immunol. 2018, 103, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.S.; Silva, M.V.T.; dos Santos, J.C.; van Linge, C.; Reis, J.M.; Teixeira, M.M.; Pinto, S.A.; Dorta, M.L.; Bai, X.Y.; Chan, E.D.; et al. Human Interleukin-32 gamma Plays a Protective Role in an Experimental Model of Visceral Leishmaniasis in Mice. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Sabur, A.; Bhowmick, S.; Chhajer, R.; Ejazi, S.A.; Didwania, N.; Asad, M.; Bhattacharyya, A.; Sinha, U.; Ali, N. Liposomal Elongation Factor-1 alpha Triggers Effector CD4 and CD8 T Cells for Induction of Long-Lasting Protective Immunity against Visceral Leishmaniasis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Costa, L.E.; Alves, P.T.; Carneiro, A.P.; Dias, A.C.S.; Fujimura, P.T.; Araujo, G.R.; Tavares, G.S.V.; Ramos, F.F.; Duarte, M.C.; Menezes-Souza, D.; et al. Leishmania infantum-Tubulin Identified by Reverse Engineering Technology through Phage Display Applied as Theranostic Marker for Human Visceral Leishmaniasis. Int. J. Mol. Sci. 2019, 20, 1812. [Google Scholar] [CrossRef]
- Vale, D.L.; Dias, D.S.; Machado, A.S.; Ribeiro, P.A.F.; Lage, D.P.; Costa, L.E.; Steiner, B.T.; Tavares, G.S.V.; Ramos, F.F.; Martinez-Rodrigo, A.; et al. Diagnostic evaluation of the amastin protein from Leishmania infantum in canine and human visceral leishmaniasis and immunogenicity in human cells derived from patients and healthy controls. Diagn. Microbiol. Infect. Dis. 2019, 95, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.A.F.; Vale, D.L.; Dias, D.S.; Lage, D.P.; Mendonca, D.V.C.; Ramos, F.F.; Carvalho, L.M.; Carvalho, A.; Steiner, B.T.; Roque, M.C.; et al. Leishmania infantum amastin protein incorporated in distinct adjuvant systems induces protection against visceral leishmaniasis. Cytokine 2020, 129. [Google Scholar] [CrossRef] [PubMed]
- Melby, P.C.; Yang, Y.Z.; Cheng, J.; Zhao, W.G. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect. Immun. 1998, 66, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Engwerda, C.R.; Kaye, P.M. Organ-specific immune responses associated with infectious disease. Immunol. Today 2000, 21, 73–78. [Google Scholar] [CrossRef]
- Carrion, J.; Nieto, A.; Iborra, S.; Iniesta, V.; Soto, M.; Folgueira, C.; Abanades, D.R.; Requena, J.M.; Alonso, C. Immunohistological features of visceral leishmaniasis in BALB/c mice. Parasite Immunol. 2006, 28, 173–183. [Google Scholar] [CrossRef]
- Olekhnovitch, R.; Bousso, P. Induction, Propagation, and Activity of Host Nitric Oxide: Lessons from Leishmania Infection. Trends Parasitol. 2015, 31, 653–664. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Korsholm, K.S.; Andersen, P.; Perrie, Y.; Christensen, D. Liposomal vaccine delivery systems. Expert Opin. Drug Deliv. 2011, 8, 505–519. [Google Scholar] [CrossRef]
- Badiee, A.; Khamesipour, A.; Samiei, A.; Soroush, D.; Shargh, V.H.; Kheiri, M.T.; Barkhordari, F.; Robert Mc Master, W.; Mahboudi, F.; Jaafari, M.R. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp. Parasitol. 2012, 132, 403–409. [Google Scholar] [CrossRef]
- Singh, O.P.; Stober, C.B.; Singh, A.K.; Blackwell, J.M.; Sundar, S. Cytokine responses to novel antigens in an Indian population living in an area endemic for visceral leishmaniasis. PLoS Negl. Trop. Dis. 2012, 6, e1874. [Google Scholar] [CrossRef]
- Portela, A.S.B.; Costa, L.E.; Salles, B.C.S.; Lima, M.P.; Santos, T.T.O.; Ramos, F.F.; Lage, D.P.; Martins, V.T.; Caligiorne, R.B.; Lessa, D.R.; et al. Identification of immune biomarkers related to disease progression and treatment efficacy in human visceral leishmaniasis. Immunobiology 2018, 223, 303–309. [Google Scholar] [CrossRef]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.T.; Duarte, M.C.; Chavez-Fumagalli, M.A.; Menezes-Souza, D.; Coelho, C.S.P.; de Magalhaes-Soares, D.F.; Fernandes, A.P.; Soto, M.; Tavares, C.A.P.; Coelho, E.A.F. A Leishmania-specific hypothetical protein expressed in both promastigote and amastigote stages of Leishmania infantum employed for the serodiagnosis of, and as a vaccine candidate against, visceral leishmaniasis. Parasites Vectors 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.T.; Lage, D.P.; Duarte, M.C.; Costa, L.E.; Garde, E.; Rodrigues, M.R.; Chavez-Fumagalli, M.A.; Menezes-Souza, D.; Roatt, B.M.; Tavares, C.A.P.; et al. A new Leishmania-specific hypothetical protein, LiHyT, used as a vaccine antigen against visceral leishmaniasis. Acta Trop. 2016, 154, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.S.; Martins, V.T.; Ribeiro, P.A.F.; Ramos, F.F.; Lage, D.P.; Tavares, G.S.V.; Mendonca, D.V.C.; Chavez-Fumagalli, M.A.; Oliveira, J.S.; Silva, E.S.; et al. Antigenicity, immunogenicity and protective efficacy of a conserved Leishmania hypothetical protein against visceral leishmaniasis. Parasitology 2018, 145, 740–751. [Google Scholar] [CrossRef]
- Ribeiro, P.A.F.; Dias, D.S.; Lage, D.P.; Martins, V.T.; Costa, L.E.; Santos, T.T.O.; Ramos, F.F.; Tavares, G.S.V.; Mendonca, D.V.C.; Ludolf, F.; et al. Immunogenicity and protective efficacy of a new Leishmania hypothetical protein applied as a DNA vaccine or in a recombinant form against Leishmania infantum infection. Mol. Immunol. 2019, 106, 108–118. [Google Scholar] [CrossRef]
- Saljoughian, N.; Taheri, T.; Zahedifard, F.; Taslimi, Y.; Doustdari, F.; Bolhassani, A.; Doroud, D.; Azizi, H.; Heidari, K.; Vasei, M.; et al. Development of Novel Prime-Boost Strategies Based on a Tri-Gene Fusion Recombinant L. tarentolae. Vaccine against Experimental Murine Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef]
- Mortazavidehkordi, N.; Farjadfar, A.; Khanahmad, H.; Najafabadi, Z.G.; Hashemi, N.; Fallah, A.; Najafi, A.; Kia, V.; Hejazi, S.H. Evaluation of a novel lentiviral vaccine expressing KMP11-HASPB fusion protein against Leishmania infantum in BALB/c mice. Parasite Immunol. 2016, 38, 670–677. [Google Scholar] [CrossRef]
- Wang, Q.; Barry, M.A.; Seid, C.A.; Hudspeth, E.M.; McAtee, C.P.; Heffernan, M.J. 3M-052 as an adjuvant for a PLGA microparticle-based Leishmania donovani recombinant protein vaccine. J. Biomed. Mat. Res. Part B 2018, 106, 1587–1594. [Google Scholar] [CrossRef]
- Vasconcelos, E.J.; Terrao, M.C.; Ruiz, J.C.; Vencio, R.Z.; Cruz, A.K. In silico identification of conserved intercoding sequences in Leishmania genomes: Unraveling putative cis-regulatory elements. Mol. Biochem. Parasitol 2012, 183, 140–150. [Google Scholar] [CrossRef]
| Immunization | Ratio IFN-γ/IL-10 in Spleen Cells Stimulated with | |||||
|---|---|---|---|---|---|---|
| Before Infection | After Infection | |||||
| Medium | ChimeraT | SLA | Medium | ChimeraT | SLA | |
| Saline | 0.8 | 1.0 | 0.96 | 0.95 | 1.38 | 0.13 |
| Saponin | 1.03 | 1.23 | 1.15 | 1.04 | 1.80 | 0.20 |
| Empty Liposome | 1.03 | 1.29 | 1.01 | 1.10 | 1.43 | 0.21 |
| ChimeraT/Saline | 1.18 | 4.28 * | 2.31 | 1.05 | 8.47 * | 0.79 |
| ChimeraT/Saponin | 1.42 | 19.96 * | 12.21 * | 1.42 | 30.12 * | 17.31 * |
| ChimeraT/Liposome | 1.41 | 20.70 * | 12.03 * | 1.37 | 32.78 * | 19.33 * |
| Immunization | (A) Before Infection | (B) After Infection | ||||||
|---|---|---|---|---|---|---|---|---|
| Anti-ChimeraT | Anti-SLA | Anti-ChimeraT | Anti-SLA | |||||
| IgG2a/ IgG1 | IgG1/ IgG2a | IgG2a/ IgG1 | IgG1/ IgG2a | IgG2a/ IgG1 | IgG1/ IgG2a | IgG2a/ IgG1 | IgG1/ IgG2a | |
| Saline | 1.12 | 0.89 | 0.79 | 1.27 | 0.92 | 1.09 | 0.10 | 10.10 |
| Saponin | 1.25 | 0.80 | 0.63 | 1.59 | 0.82 | 1.22 | 0.18 | 5.60 |
| Empty Liposome | 1.15 | 0.89 | 0.78 | 1.28 | 0.83 | 1.20 | 0.26 | 3.85 |
| ChimeraT/Saline | 1.42 | 0.7 | 1.26 | 0.79 | 0.95 | 1.05 | 0.45 | 2.22 |
| ChimeraT/Saponin | 8.84 * | 0.11 | 3.14 * | 0.32 | 6.81 * | 0.15 | 4.19 * | 0.24 |
| ChimeraT/Liposome | 8.58 * | 0.12 | 3.02 * | 0.33 | 10.27 * | 0.10 | 5.29 * | 0.19 |
| (A) Before VL Treatment | (B) After VL Treatment | |||
|---|---|---|---|---|
| Antigen | IgG2/IgG1 | IgG1/IgG2 | IgG2/IgG1 | IgG1/IgG2 |
| ChimeraT | 1.08 | 0.93 | 2.99 | 0.33 |
| SLA | 0.17 | 5.75 | 0.47 | 2.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lage, D.P.; Ribeiro, P.A.F.; Dias, D.S.; Mendonça, D.V.C.; Ramos, F.F.; Carvalho, L.M.; Steiner, B.T.; Tavares, G.S.V.; Martins, V.T.; Machado, A.S.; et al. Liposomal Formulation of ChimeraT, a Multiple T-Cell Epitope-Containing Recombinant Protein, Is a Candidate Vaccine for Human Visceral Leishmaniasis. Vaccines 2020, 8, 289. https://doi.org/10.3390/vaccines8020289
Lage DP, Ribeiro PAF, Dias DS, Mendonça DVC, Ramos FF, Carvalho LM, Steiner BT, Tavares GSV, Martins VT, Machado AS, et al. Liposomal Formulation of ChimeraT, a Multiple T-Cell Epitope-Containing Recombinant Protein, Is a Candidate Vaccine for Human Visceral Leishmaniasis. Vaccines. 2020; 8(2):289. https://doi.org/10.3390/vaccines8020289
Chicago/Turabian StyleLage, Daniela P., Patrícia A.F. Ribeiro, Daniel S. Dias, Débora V.C. Mendonça, Fernanda F. Ramos, Lívia M. Carvalho, Bethina T. Steiner, Grasiele S.V. Tavares, Vívian T. Martins, Amanda S. Machado, and et al. 2020. "Liposomal Formulation of ChimeraT, a Multiple T-Cell Epitope-Containing Recombinant Protein, Is a Candidate Vaccine for Human Visceral Leishmaniasis" Vaccines 8, no. 2: 289. https://doi.org/10.3390/vaccines8020289
APA StyleLage, D. P., Ribeiro, P. A. F., Dias, D. S., Mendonça, D. V. C., Ramos, F. F., Carvalho, L. M., Steiner, B. T., Tavares, G. S. V., Martins, V. T., Machado, A. S., Oliveira-da-Silva, J. A., Santos, T. T. O., Freitas, C. S., Oliveira, J. S., Roatt, B. M., Machado-de-Ávila, R. A., Humbert, M. V., Christodoulides, M., & Coelho, E. A. F. (2020). Liposomal Formulation of ChimeraT, a Multiple T-Cell Epitope-Containing Recombinant Protein, Is a Candidate Vaccine for Human Visceral Leishmaniasis. Vaccines, 8(2), 289. https://doi.org/10.3390/vaccines8020289





