The Tat Protein of HIV-1 Prevents the Loss of HSV-Specific Memory Adaptive Responses and Favors the Control of Viral Reactivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptides and Antibodies
2.2. Herpes Simplex Virus Type 1 and Mice
2.3. Determination of Cellular and Humoral Responses
2.4. Statistics
3. Results and Discussion
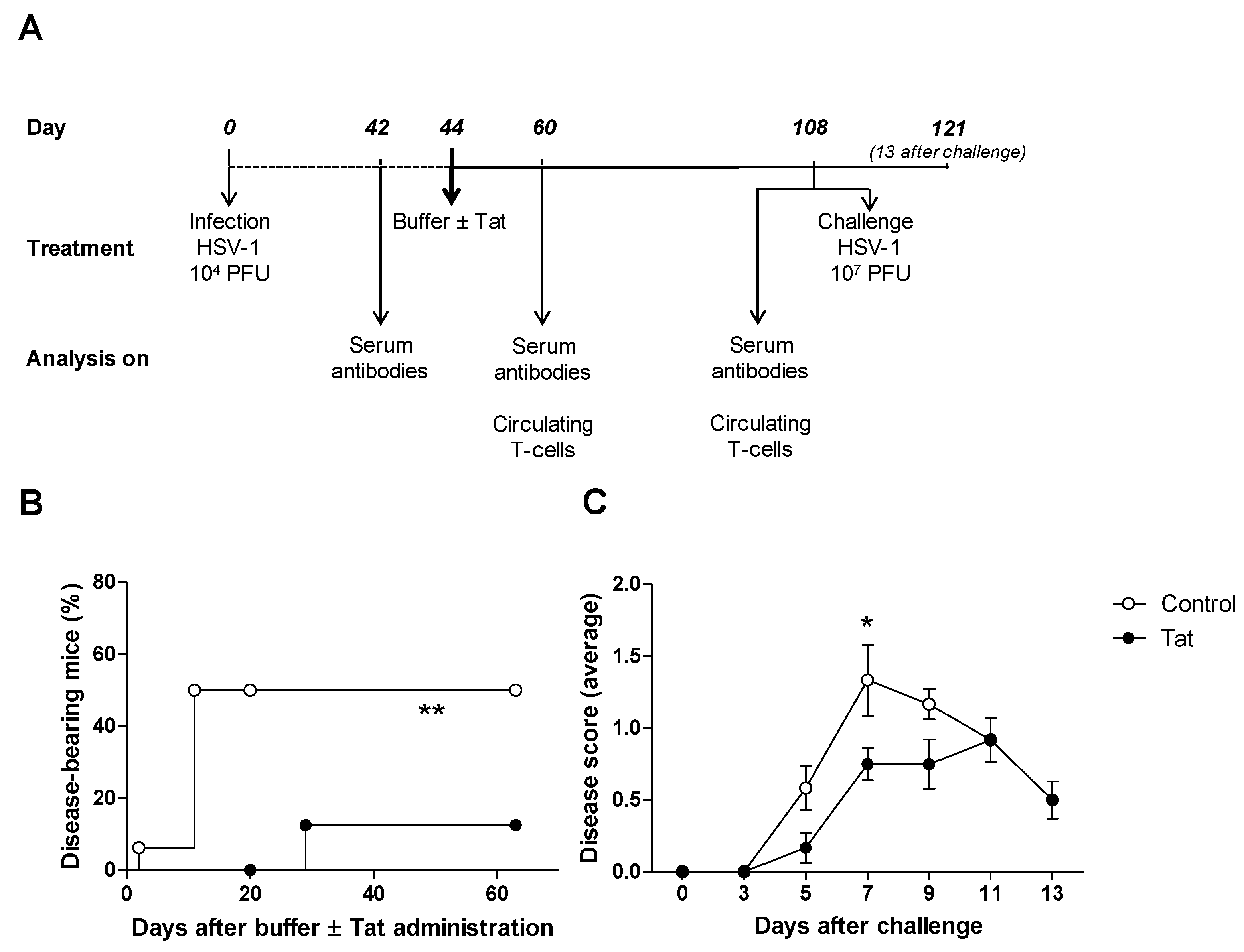
3.1. The HIV-1 Tat Protein Has a Therapeutic Effect in Mice Infected with HSV-1
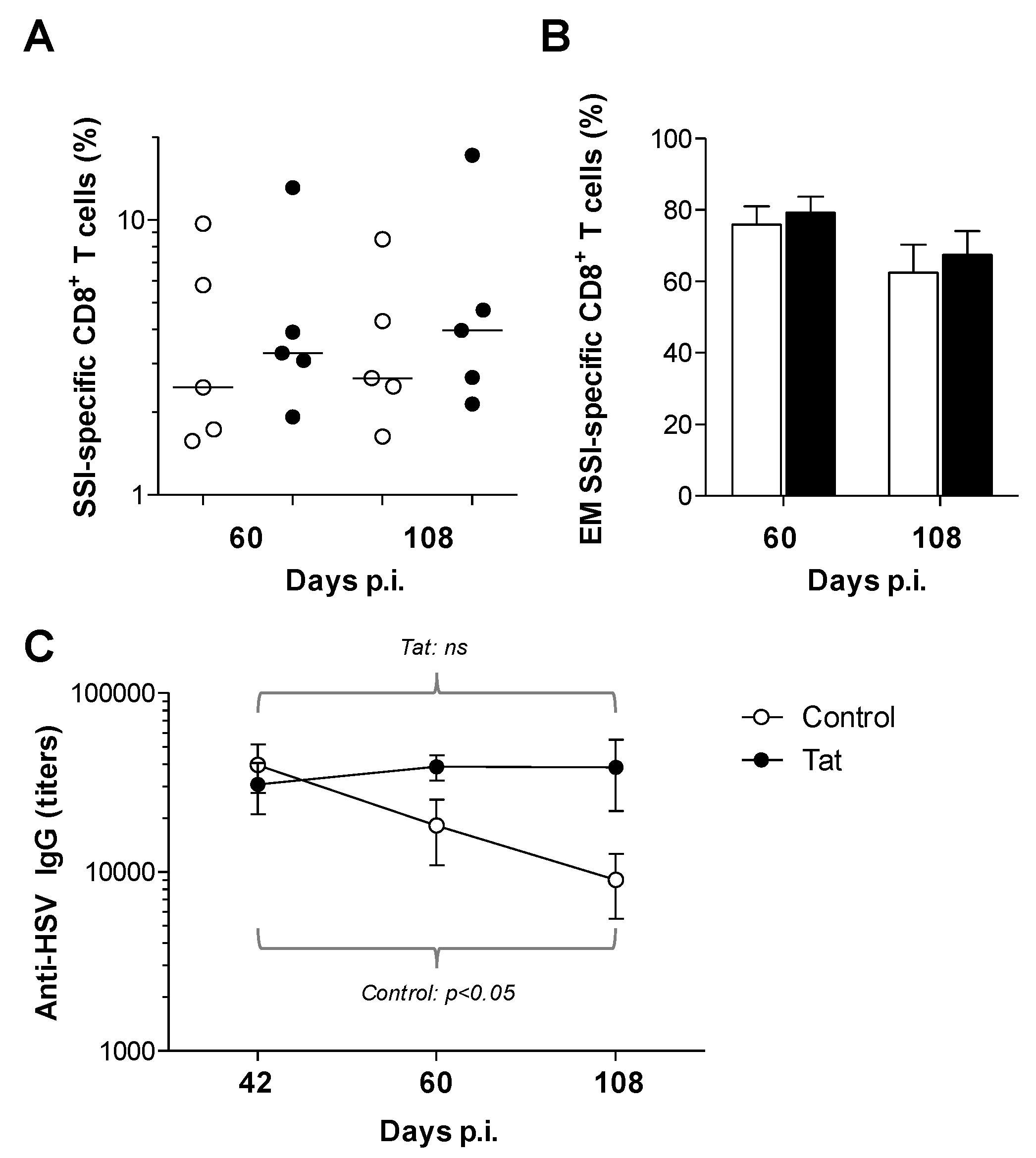
3.2. Administration of the HIV-1 Tat Protein Prevents the Time-Dependent Reduction in Antigen-Specific Adaptive Humoral Responses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Looker, K.J.; Garnett, G.P. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex. Transm. Infect. 2005, 81, 103–107. [Google Scholar] [CrossRef]
- Mahant, S.; Hall, M.; Schondelmeyer, A.C.; Berry, J.G.; Kimberlin, D.W.; Shah, S.S.; for the Pediatric Research in Inpatient Settings Network and the Collaborative Antiviral Study Group; Pediatric Research in Inpatient Settings Network and the Collaborative Antiviral Study Group. Neonatal Herpes Simplex Virus Infection Among Medicaid-Enrolled Children: 2009–2015. Pediatrics 2019, 143, e20183233. [Google Scholar] [CrossRef]
- George, B.P.; Schneider, E.B.; Venkatesan, A. Encephalitis Hospitalization Rates and Inpatient Mortality in the United States, 2000–2010. PLoS ONE 2014, 9, e104169. [Google Scholar] [CrossRef]
- Saylor, D.; Thakur, K.; Venkatesan, A. Acute encephalitis in the immunocompromised individual. Curr. Opin. Infect. Dis. 2015, 28, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Sicurella, M.; Agostini, S.; Marconi, P.; Clerici, M. Herpes simplex virus type 1 and Alzheimer’s disease: Link and potential impact on treatment. Expert Rev. Anti Infect. Ther. 2019, 17, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Sun, D.-D.; Wang, Y.; Liu, W.; Yang, J. Herpes Simplex Virus Type 1 and Type 2 Infection Increases Atherosclerosis Risk: Evidence Based on a Meta-Analysis. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; E Turner, K.M.; Vickerman, P.; Newman, L.M.; Gottlieb, S.L. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health 2017, 5, e300–e309. [Google Scholar] [CrossRef]
- Sivarajah, V.; Venus, K.; Yudin, M.H.; E Murphy, K.; A Morrison, S.; Tan, D.H.S. Does maternal HSV-2 coinfection increase mother-to-child transmission of HIV? A systematic review. Sex. Transm. Infect. 2017, 93, 535–542. [Google Scholar] [CrossRef]
- Esber, A.; Miguel, R.D.V.; Cherpes, T.L.; Klebanoff, M.A.; Gallo, M.F.; Turner, A.N. Risk of Bacterial Vaginosis among Women with Herpes Simplex Virus Type 2 Infection: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2015, 212, 8–17. [Google Scholar] [CrossRef]
- Field, H.J.; Thackray, A.M. Early Therapy with Valaciclovir or Famciclovir Reduces But Does Not Abrogate Herpes Simplex Virus Neuronal Latency. Nucleotides Nucleic Acids 2000, 19, 461–470. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob. Agents Chemother. 2010, 55, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.L.; Giersing, B.K.; Hickling, J.; Jones, R.; Deal, C.; Kaslow, D.C. Meeting report: Initial World Health Organization consultation on herpes simplex virus (HSV) vaccine preferred product characteristics, March 2017. Vaccine 2019, 37, 7408–7418. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Li, Q. Characteristics of herpes simplex virus infection and pathogenesis suggest a strategy for vaccine development. Rev. Med. Virol. 2019, 29, e2054. [Google Scholar] [CrossRef]
- Shin, H.; Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012, 491, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tang, Q.; Hendricks, R.L. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 1996, 70, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Gallerani, E.; Skarlis, C.; Sicurella, M.; Cafaro, A.; Ensoli, B.; Caputo, A.; Marconi, P.; Gavioli, R. Systemic immunodominant CD8 responses with an effector-like phenotype are induced by intravaginal immunization with attenuated HSV vectors expressing HIV Tat and mediate protection against HSV infection. Vaccine 2016, 34, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Srivastava, R.; Lopes, P.P.; Wang, C.; Pham, T.T.; Cochrane, J.; Thai, N.T.U.; Gutierrez, L.; Benmohamed, L. Asymptomatic memory CD8+ T cells: From development and regulation to consideration for human vaccines and immunotherapeutics. Hum. Vaccines Immunother. 2014, 10, 945–963. [Google Scholar] [CrossRef]
- Khan, A.A.; Srivastava, R.; Spencer, D.; Garg, S.; Fremgen, D.; Vahed, H.; Lopes, P.P.; Pham, T.T.; Hewett, C.; Kuang, J.; et al. Phenotypic and Functional Characterization of Herpes Simplex Virus Glycoprotein B Epitope-Specific Effector and Memory CD8+T Cells from Symptomatic and Asymptomatic Individuals with Ocular Herpes. J. Virol. 2015, 89, 3776–3792. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Khan, A.A.; Spencer, R.; Vahed, H.; Lopes, P.P.; Thai, N.T.U.; Wang, C.; Pham, T.T.; Huang, J.; Scarfone, V.M.; et al. HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes. J. Immunol. 2015, 194, 2232–2248. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Khanna, K.M.; Chen, X.; Fink, D.J.; Hendricks, R. Cd8+ T Cells Can Block Herpes Simplex Virus Type 1 (HSV-1) Reactivation from Latency in Sensory Neurons. J. Exp. Med. 2000, 191, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Nagafuchi, S.; Oda, H.; Mori, R.; Taniguchi, T. Mechanism of Acquired Resistance to Herpes Simplex Virus Infection as Studied in Nude Mice. J. Gen. Virol. 1979, 44, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.A.; Zhu, L.; Thebeau, L.G. Vaccine-Induced Serum Immunoglobin Contributes to Protection from Herpes Simplex Virus Type 2 Genital Infection in the Presence of Immune T Cells. J. Virol. 2001, 75, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Sicurella, M.; Nicoli, F.; Gallerani, E.; Volpi, I.; Berto, E.; Finessi, V.; Destro, F.; Manservigi, R.; Cafaro, A.; Ensoli, B.; et al. An Attenuated Herpes Simplex Virus Type 1 (HSV1) Encoding the HIV-1 Tat Protein Protects Mice from a Deadly Mucosal HSV1 Challenge. PLoS ONE 2014, 9, e100844. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr. Opin. Immunol. 2012, 24, 494–500. [Google Scholar] [CrossRef]
- Levin, M.J.; Smith, J.G.; Kaufhold, R.M.; Barber, D.; Hayward, A.R.; Chan, C.Y.; Chan, I.S.F.; Li, D.J.J.; Wang, W.; Keller, P.M.; et al. Decline in Varicella-Zoster Virus (VZV)–Specific Cell-Mediated Immunity with Increasing Age and Boosting with a High-Dose VZV Vaccine. J. Infect. Dis. 2003, 188, 1336–1344. [Google Scholar] [CrossRef]
- Weinberger, B. Vaccines for the elderly: Current use and future challenges. Immun. Ageing 2018, 15, 3. [Google Scholar] [CrossRef]
- Rocca, S.; Santilli, V.; Cotugno, N.; Concato, C.; Manno, E.C.; Nocentini, G.; Macchiarulo, G.; Cancrini, C.; Finocchi, A.; Guzzo, I.; et al. Waning of vaccine-induced immunity to measles in kidney transplanted children. Medicine 2016, 95, e4738. [Google Scholar] [CrossRef]
- Cotugno, N.; Finocchi, A.; Cagigi, A.; Di Matteo, G.; Chiriaco, M.; Di Cesare, S.; Rossi, P.; Aiuti, A.; Palma, P.; Douagi, I. Defective B-cell proliferation and maintenance of long-term memory in patients with chronic granulomatous disease. J. Allergy Clin. Immunol. 2015, 135, 753–761. [Google Scholar] [CrossRef]
- Laksono, B.M.; De Vries, R.D.; Verburgh, R.J.; Visser, E.G.; De Jong, A.; Fraaij, P.L.A.; Ruijs, W.L.M.; Nieuwenhuijse, D.F.; Ham, H.-J.V.D.; Koopmans, M.P.G.; et al. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat. Commun. 2018, 9, 4944. [Google Scholar] [CrossRef]
- Appay, V.; Van Lier, R.; Sallusto, F.; Roederer, M. Phenotype and function of human T lymphocyte subsets: Consensus and issues. Cytom. Part A 2008, 73, 975–983. [Google Scholar] [CrossRef]
- Nicoli, F.; Papagno, L.; Frere, J.J.; Cabral-Piccin, M.P.; Clave, E.; Gostick, E.; Toubert, A.; Price, D.A.; Caputo, A.; Appay, V. Naïve CD8+ T-Cells Engage a Versatile Metabolic Program Upon Activation in Humans and Differ Energetically From Memory CD8+ T-Cells. Front. Immunol. 2018, 9, 2736. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. Successful and Maladaptive T Cell Aging. Immunity 2017, 46, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, A.; Bennett, M.S.; Park, B.; Uhrlaub, J.; Martinez, C.; Pulko, V.; Currier, N.L.; Nikolich-Žugich, A.; Kaye, J.A.; Nikolich-Žugich, J. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J. Immunol. 2014, 192, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Yassai, M.B.; Naumov, Y.N.; Selin, L.K. Narrowing of Human Influenza A Virus-Specific T Cell Receptor α and β Repertoires with Increasing Age. J. Virol. 2015, 89, 4102–4116. [Google Scholar] [CrossRef] [PubMed]
- Papagno, L.; A Spina, C.; Marchant, A.; Salio, M.; Rufer, N.; Little, S.; Dong, T.; Chesney, G.; Waters, A.; Easterbrook, P.; et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004, 2, E20. [Google Scholar] [CrossRef] [PubMed]
- Pensieroso, S.; Cagigi, A.; Palma, P.; Nilsson, A.; Capponi, C.; Freda, E.; Bernardi, S.; Thorstensson, R.; Chiodi, F.; Rossi, P. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc. Natl. Acad. Sci. USA 2009, 106, 7939–7944. [Google Scholar] [CrossRef]
- Kwon, H.S.; Brent, M.M.; Getachew, R.; Jayakumar, P.; Chen, L.F.; Schnolzer, M.; McBurney, M.W.; Marmorstein, R.; Greene, W.C.; Ott, M. Human Immunodeficiency Virus Type 1 Tat Protein Inhibits the SIRT1 Deacetylase and Induces T Cell Hyperactivation. Cell Host Microbe 2008, 3, 158–167. [Google Scholar] [CrossRef]
- Nicoli, F.; Finessi, V.; Sicurella, M.; Rizzotto, L.; Gallerani, E.; Destro, F.; Cafaro, A.; Marconi, P.; Caputo, A.; Ensoli, B.; et al. The HIV-1 Tat Protein Induces the Activation of CD8+ T Cells and Affects In Vivo the Magnitude and Kinetics of Antiviral Responses. PLoS ONE 2013, 8, e77746. [Google Scholar] [CrossRef][Green Version]
- Bellino, S.; Francavilla, V.; Longo, O.; Tripiciano, A.; Paniccia, G.; Arancio, A.; Fiorelli, V.; Scoglio, A.; Collacchi, B.; Campagna, M.; et al. Parallel Conduction of the Phase I Preventive and Therapeutic Trials Based on the Tat Vaccine Candidate. Rev. Recent Clin. Trials 2009, 4, 195–204. [Google Scholar] [CrossRef]
- Sforza, F.; Nicoli, F.; Gallerani, E.; Finessi, V.; Reali, E.; Cafaro, A.; Caputo, A.; Ensoli, B.; Gavioli, R. HIV-1 tat affects the programming and functionality of human CD8+ T cells by modulating the expression of T-box transcription factors. AIDS 2014, 28, 1729–1738. [Google Scholar] [CrossRef]
- Nicoli, F.; Sforza, F.; Gavioli, R. Different expression of Blimp-1 in HIV infection may be used to monitor disease progression and provide a clue to reduce immune activation and viral reservoirs. AIDS 2015, 29, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Bultmann, H.; Brandt, C.R. Peptides Containing Membrane-transiting Motifs Inhibit Virus Entry. J. Boil. Chem. 2002, 277, 36018–36023. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.G.; Larsen, I.V.; Gauger, J.; Carballo, E.; Stern, R.; Brummel, R.; Brandt, C.R. A Cationic Peptide, TAT-Cd0, Inhibits Herpes Simplex Virus Type 1 Ocular Infection In Vivo. Investig. Opthalmology Vis. Sci. 2013, 54, 1070–1079. [Google Scholar] [CrossRef][Green Version]
- Bellino, S.; Tripiciano, A.; Picconi, O.; Francavilla, V.; Longo, O.; Sgadari, C.; Paniccia, G.; Arancio, A.; Angarano, G.; Ladisa, N.; et al. The presence of anti-Tat antibodies in HIV-infected individuals is associated with containment of CD4+ T-cell decay and viral load, and with delay of disease progression: Results of a 3-year cohort study. Retrovirology 2014, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Chachage, M.; Clowes, P.; Bauer, A.; Kowour, D.; Ensoli, B.; Cafaro, A.; Maboko, L.; Hoelscher, M.; Gavioli, R.; et al. Association between different anti-Tat antibody isotypes and HIV disease progression: Data from an African cohort. BMC Infect. Dis. 2016, 16, 344. [Google Scholar] [CrossRef]
- Ensoli, B.; Bellino, S.; Tripiciano, A.; Longo, O.; Francavilla, V.; Marcotullio, S.; Cafaro, A.; Picconi, O.; Paniccia, G.; Scoglio, A.; et al. Therapeutic Immunization with HIV-1 tat Reduces Immune Activation and Loss of Regulatory T-Cells and Improves Immune Function in Subjects on HAART. PLoS ONE 2010, 5, e13540. [Google Scholar] [CrossRef]
- Loret, E.P.; Darque, A.; Jouve, E.; Loret, E.A.; Nicolino-Brunet, C.; Morange, S.; Castanier, E.; Casanova, J.; Caloustian, C.; Bornet, C.; et al. Intradermal Injection of a Tat Oyi-based Therapeutic HIV Vaccine Reduces of 1.5 Log copies/mL the HIV RNA Rebound Median and No HIV DNA Rebound Following cART Interruption in a Phase I/II Randomized Controlled Clinical Trial. Retrovirology 2016, 13, 21. [Google Scholar] [CrossRef]
- Jin, H.; Li, D.; Lin, M.H.; Li, L.; Harrich, D. Tat-Based Therapies as an Adjuvant for an HIV-1 Functional Cure. Viruses 2020, 12, 415. [Google Scholar] [CrossRef]
- Alipour, S.; Mahdavi, A. Boosting Tat DNA Vaccine With Tat Protein Stimulates Strong Cellular and Humoral Immune Responses in Mice. Biotechnol. Lett. 2020, 42, 505–517. [Google Scholar] [CrossRef]
- Caputo, A.; Gavioli, R.; Bellino, S.; Longo, O.; Tripiciano, A.; Francavilla, V.; Sgadari, C.; Paniccia, G.; Titti, F.; Cafaro, A.; et al. HIV-1 Tat-Based Vaccines: An Overview and Perspectives in the Field of HIV/AIDS Vaccine Development. Int. Rev. Immunol. 2009, 28, 285–334. [Google Scholar] [CrossRef]
- Finessi, V.; Nicoli, F.; Gallerani, E.; Sforza, F.; Sicurella, M.; Cafaro, A.; Caputo, A.; Ensoli, B.; Gavioli, R. Effects of different routes of administration on the immunogenicity of the Tat protein and a Tat-derived peptide. Hum. Vaccines Immunother. 2015, 11, 1489–1493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicoli, F.; Gallerani, E.; Sforza, F.; Finessi, V.; Chachage, M.; Geldmacher, C.; Cafaro, A.; Ensoli, B.; Caputo, A.; Gavioli, R. The HIV-1 Tat protein affects human CD4+ T-cell programing and activation, and favors the differentiation of naïve CD4+ T cells. AIDS 2018, 32, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nordström, I.; Sun, J.-B.; Eriksson, K. Protective immunity to genital herpes simplex [correction of simpex] virus type 2 infection is mediated by T-bet. J. Immunol. 2005, 174, 6266–6273. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, C.J.; Pardee, A.B. Human Immunodeficiency Virus Type 1 TAT Protein Activates B Lymphocytes. Biochem. Biophys. Res. Commun. 1997, 237, 461–464. [Google Scholar] [CrossRef]
- Germini, D.; Tsfasman, T.; Klibi, M.; El-Amine, R.; Pichugin, A.; Iarovaia, O.V.; Bilhou-Nabera, C.; Subra, F.; Saada, Y.B.; Sukhanova, A.; et al. HIV Tat induces a prolonged MYC relocalization next to IGH in circulating B-cells. Leukemia 2017, 31, 2515–2522. [Google Scholar] [CrossRef]
- El-Amine, R.; Germini, D.; Zakharova, V.V.; Tsfasman, T.; Sheval, E.V.; Louzada, R.A.; Dupuy, C.; Bilhou-Nabera, C.; Hamade, A.; Najjar, F.; et al. HIV-1 Tat protein induces DNA damage in human peripheral blood B-lymphocytes via mitochondrial ROS production. Redox Biol. 2018, 15, 97–108. [Google Scholar] [CrossRef]
- Ben Haij, N.; Planès, R.; Leghmari, K.; Serrero, M.; Delobel, P.; Izopet, J.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat Protein Induces Production of Proinflammatory Cytokines by Human Dendritic Cells and Monocytes/Macrophages through Engagement of TLR4-MD2-CD14 Complex and Activation of NF-κB Pathway. PLoS ONE 2015, 10, e0129425. [Google Scholar] [CrossRef]
- Fanales-Belasio, E.; Moretti, S.; Fiorelli, V.; Tripiciano, A.; Cossut, M.R.P.; Scoglio, A.; Collacchi, B.; Nappi, F.; Macchia, I.; Bellino, S.; et al. HIV-1 Tat Addresses Dendritic Cells to Induce a Predominant Th1-Type Adaptive Immune Response That Appears Prevalent in the Asymptomatic Stage of Infection. J. Immunol. 2009, 182, 2888–2897. [Google Scholar] [CrossRef]
- Gibellini, D.; Re, M.C.; Ponti, C.; Maldini, C.; Celeghini, C.; Cappellini, A.; La Placa, M.; Zauli, G. HIV-1 Tat Protects CD4+ Jurkat T Lymphoblastoid Cells from Apoptosis Mediated by TNF-Related Apoptosis-Inducing Ligand. Cell. Immunol. 2001, 207, 89–99. [Google Scholar] [CrossRef]
- Mischiati, C.; Pironi, F.; Milani, D.; Giacca, M.; Mirandola, P.; Capitani, S.; Zauli, G. Extracellular HIV-1 Tat protein differentially activates the JNK and ERK/MAPK pathways in CD4 T cells. AIDS 1999, 13, 1637–1645. [Google Scholar] [CrossRef]
- Zauli, G.; Gibellini, D.; Celeghini, C.; Mischiati, C.; Bassini, A.; La Placa, M.; Capitani, S. Pleiotropic effects of immobilized versus soluble recombinant HIV-1 Tat protein on CD3-mediated activation, induction of apoptosis, and HIV-1 long terminal repeat transactivation in purified CD4+ T lymphocytes. J. Immunol. 1996, 157, 2216–2224. [Google Scholar] [PubMed]
- Zauli, G.; Gibellini, D.; Milani, D.; Mazzoni, M.; Borgatti, P.; La Placa, M.; Capitani, S. Human immunodeficiency virus type 1 Tat protein protects lymphoid, epithelial, and neuronal cell lines from death by apoptosis. Cancer Res. 1993, 53, 4481–4485. [Google Scholar]
- Murera, D.; Arbogast, F.; Arnold, J.; Bouis, D.; Muller, S.; Gros, F. CD4 T cell autophagy is integral to memory maintenance. Sci. Rep. 2018, 8, 5951. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.; Clambey, E.T.; Kappler, J.W.; Marrack, P. CD4 memory T cells: What are they and what can they do? Semin. Immunol. 2009, 21, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.; Rajewsky, K. Persistence of memory B cells in mice deprived of T cell help. Int. Immunol. 1990, 2, 487–494. [Google Scholar] [CrossRef]
- Sarkander, J.; Hojyo, S.; Tokoyoda, K. Vaccination to gain humoral immune memory. Clin. Transl. Immunol. 2016, 5, e120. [Google Scholar] [CrossRef]
- Gutjahr, A.; Papagno, L.; Nicoli, F.; Kanuma, T.; Kuse, N.; Cabral-Piccin, M.P.; Rochereau, N.; Gostick, E.; Lioux, T.; Perouzel, E.; et al. The STING ligand cGAMP potentiates the efficacy of vaccine-induced CD8+ T cells. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Gutjahr, A.; Tiraby, G.; Perouzel, E.; Verrier, B.; Paul, S. Triggering Intracellular Receptors for Vaccine Adjuvantation. Trends Immunol. 2016, 37, 716. [Google Scholar] [CrossRef]
- Gibellini, D.; Zauli, G.; Re, M.C.; Milani, D.; Furlini, G.; Caramelli, E.; Capitani, S.; La Placa, M. Recombinant human immunodeficiency virus type-1 (HIV-1) Tat protein sequentially up-regulates IL-6 and TGF-beta 1 mRNA expression and protein synthesis in peripheral blood monocytes. Br. J. Haematol. 1994, 88, 261–267. [Google Scholar] [CrossRef]
- Zauli, G.; Furlini, G.; Re, M.C.; Milani, D.; Capitani, S.; La Placa, M. Human immunodeficiency virus type 1 (HIV-1) tat-protein stimulates the production of interleukin-6 (IL-6) by peripheral blood monocytes. New Microbiol. 1993, 16, 115–120. [Google Scholar]
- Maeda, H.; Mehta, H.; Drevets, D.A.; Coggeshall, K.M. IL-6 Increases B-cell IgG Production in a Feed-Forward Proinflammatory Mechanism to Skew Hematopoiesis and Elevate Myeloid Production. Blood 2010, 115, 4699–4706. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Eaton, S.M.; Bond, J.P.; Neveu, W.; Moquin, D.; Noubade, R.; Briso, E.M.; Charland, C.; Leonard, W.J.; Ciliberto, G.; et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009, 206, 69–78. [Google Scholar] [CrossRef]
- Ben Haij, N.; Leghmari, K.; Planès, R.; Thieblemont, N.; Bahraoui, E. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-α and IL-10. Retrovirology 2013, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell. Mol. Immunol. 2012, 10, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Paul, S.; Appay, V. Harnessing the Induction of CD8+ T-Cell Responses Through Metabolic Regulation by Pathogen-Recognition-Receptor Triggering in Antigen Presenting Cells. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Ganley-Leal, L.M.; Liang, Y.; Jagannathan-Bogdan, M.; Farraye, F.; Nikolajczyk, B.S. Differential regulation of TLR4 expression in human B cells and monocytes. Mol. Immunol. 2010, 48, 82–88. [Google Scholar] [CrossRef]
- Nicoli, F.; Mantelli, B.; Gallerani, E.; Telatin, V.; Bonazzi, I.; Marconi, P.; Gavioli, R.; Gabrielli, L.; Lazzarotto, T.; Barzon, L.; et al. HPV-Specific Systemic Antibody Responses and Memory B Cells are Independently Maintained up to 6 Years and in a Vaccine-Specific Manner Following Immunization with Cervarix and Gardasil in Adolescent and Young Adult Women in Vaccination Programs in Italy. Vaccines 2020, 8, 26. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Van Crevel, R.; Curtis, N.; Shann, F.; Levy, O.; Network, O. Heterologous (“nonspecific”) and sex-differential effects of vaccines: Epidemiology, clinical trials, and emerging immunologic mechanisms. Clin. Infect. Dis. 2013, 57, 283–289. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.B.; Jacobs, C.; Van Loenhout, J.; Xavier, R.J.; Aaby, P.; Van Der Meer, J.W.M.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2013, 6, 152–158. [Google Scholar] [CrossRef]
- Nicoli, F.; Appay, V. Immunological considerations regarding parental concerns on pediatric immunizations. Vaccine 2017, 35, 3012–3019. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoli, F.; Gallerani, E.; Sicurella, M.; Pacifico, S.; Cafaro, A.; Ensoli, B.; Marconi, P.; Caputo, A.; Gavioli, R. The Tat Protein of HIV-1 Prevents the Loss of HSV-Specific Memory Adaptive Responses and Favors the Control of Viral Reactivation. Vaccines 2020, 8, 274. https://doi.org/10.3390/vaccines8020274
Nicoli F, Gallerani E, Sicurella M, Pacifico S, Cafaro A, Ensoli B, Marconi P, Caputo A, Gavioli R. The Tat Protein of HIV-1 Prevents the Loss of HSV-Specific Memory Adaptive Responses and Favors the Control of Viral Reactivation. Vaccines. 2020; 8(2):274. https://doi.org/10.3390/vaccines8020274
Chicago/Turabian StyleNicoli, Francesco, Eleonora Gallerani, Mariaconcetta Sicurella, Salvatore Pacifico, Aurelio Cafaro, Barbara Ensoli, Peggy Marconi, Antonella Caputo, and Riccardo Gavioli. 2020. "The Tat Protein of HIV-1 Prevents the Loss of HSV-Specific Memory Adaptive Responses and Favors the Control of Viral Reactivation" Vaccines 8, no. 2: 274. https://doi.org/10.3390/vaccines8020274
APA StyleNicoli, F., Gallerani, E., Sicurella, M., Pacifico, S., Cafaro, A., Ensoli, B., Marconi, P., Caputo, A., & Gavioli, R. (2020). The Tat Protein of HIV-1 Prevents the Loss of HSV-Specific Memory Adaptive Responses and Favors the Control of Viral Reactivation. Vaccines, 8(2), 274. https://doi.org/10.3390/vaccines8020274







