Infection with Toxocara canis Inhibits the Production of IgE Antibodies to α-Gal in Humans: Towards a Conceptual Framework of the Hygiene Hypothesis?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Detection of Specific IgG Antibodies to Toxocara canis, Ascaris suum and Toxoplasma gondii in Sera of Patients with α-Gal Syndrome (AGS)
2.3. Detection of Serum IgG, IgM and IgE Antibodies to α-Gal in Patients Infected with Parasites and Parasitic Fungi
2.4. Determination of Antibody Levels to Ixodes ricinus Salivary Gland Proteins (SGP)
2.5. Detection of α-Gal Epitopes in Toxocara canis, Ascaris suum, Schistosoma mansoni, Toxoplasma gondii and Aspergillus fumigatus
2.5.1. Preparation of the Organisms for Immunoblot, Indirect ELISA, Immunofluorescence and Flow Cytometry
2.5.2. Western Blot
2.5.3. Indirect ELISA
2.5.4. Immunofluorescence and Flow Cytometry
2.6. Statistical Analyses
2.7. Ethics Statement
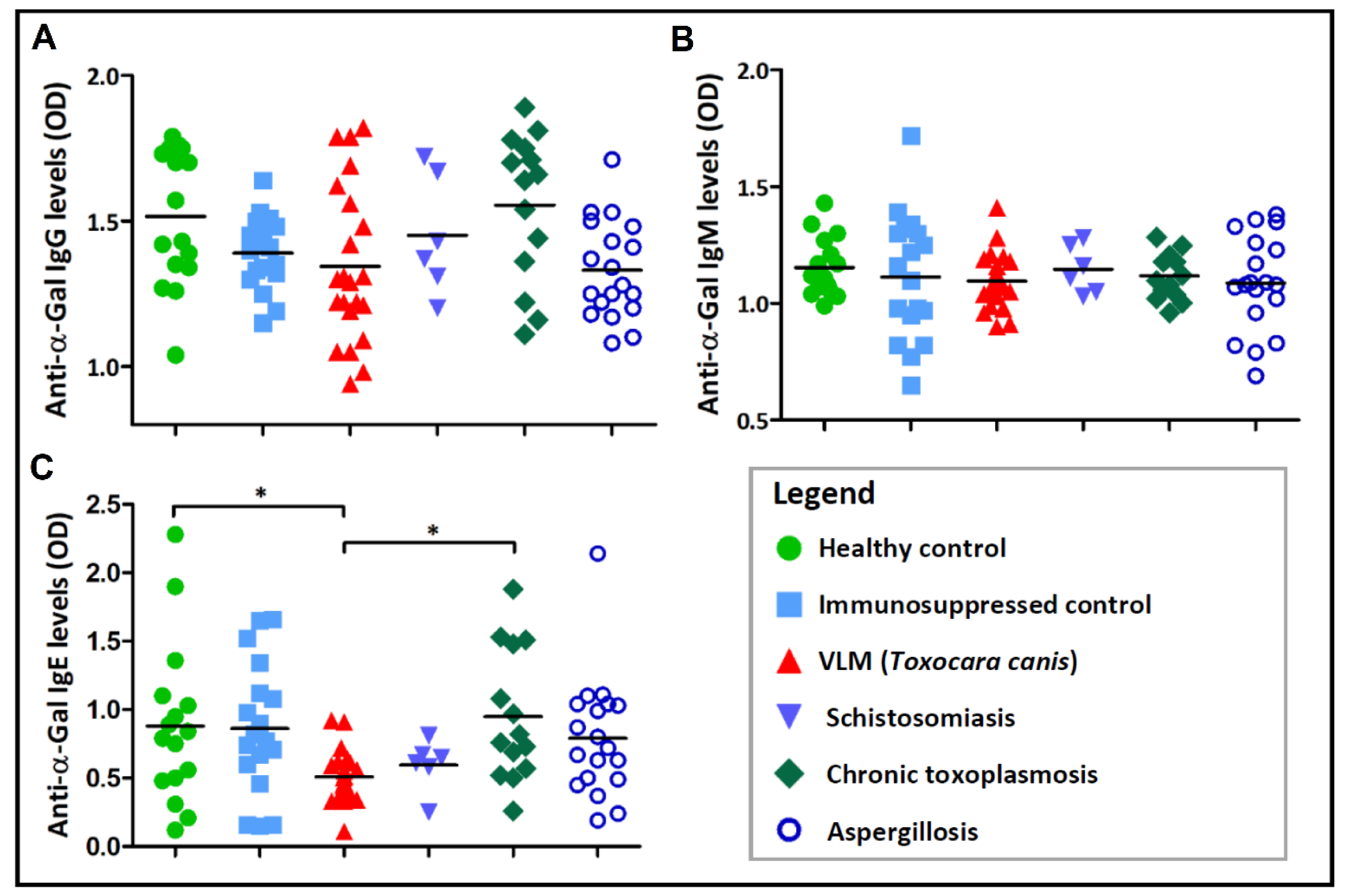
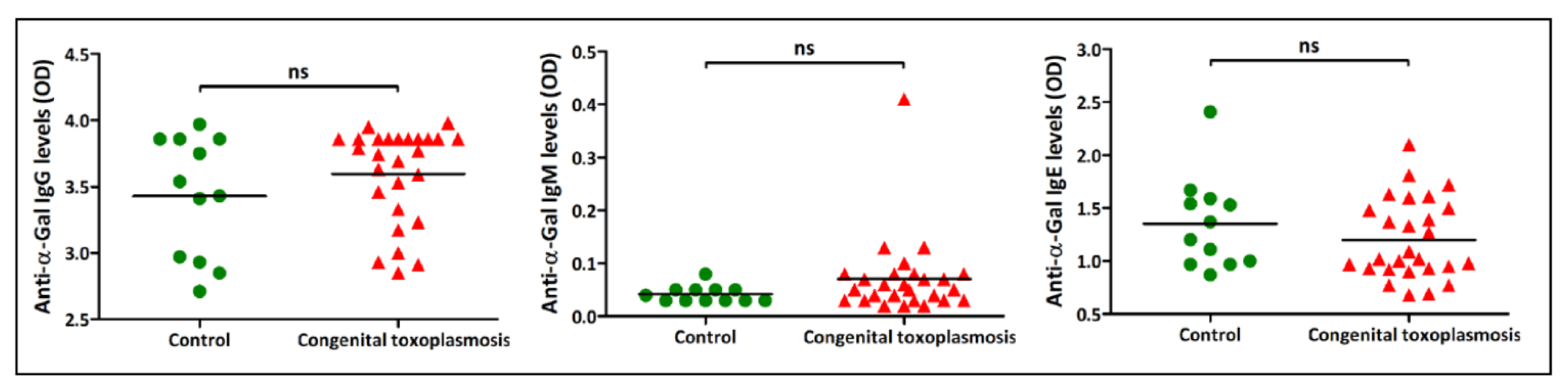
3. Results
3.1. Expression of Terminal α-Gal Moieties on Glycoproteins and the Surface of the Tested Helminths, Protozoa and Parasitic Fungi
3.2. Association between Immune Responses to α-Gal and Exposure to the Infectious Agents
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galili, U.; Shohet, S.B.; Kobrin, E.; Stults, C.L.; Macher, B.A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988, 263, 17755–17762. [Google Scholar] [PubMed]
- Blanken, W.M.; Van den Eijnden, D.H. Biosynthesis of terminal Galα1-3Galβ1-4GlcNAc-R oligosaccharide sequences on glycoconjugates. Purification and acceptor specificity of a UDP-Gal:N-acetyllactosaminide α1-3-galactosyltransferase from calf thymus. J. Biol. Chem. 1985, 260, 12927–12934. [Google Scholar] [PubMed]
- Galili, U.; Swanson, K. Gene sequences suggest inactivation of α-1,3-galactosyltransferase in catarrhines after the divergence of apes from monkeys. Proc. Natl. Acad. Sci. USA 1991, 88, 7401–7404. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. Evolution and pathophysiology of the human natural anti-α-galactosyl IgG (anti-Gal) antibody. Springer Semin. Immunopathol. 1993, 15, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Rachmilewitz, E.A.; Peleg, A.; Flechner, I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J. Exp. Med. 1984, 160, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Koike, C.; Uddin, M.; Wildman, D.E.; Gray, E.A.; Trucco, M.; Starzl, T.E.; Goodman, M. Functionally important glycosyltransferase gain and loss during catarrhine primate emergence. Proc. Natl. Acad. Sci. USA 2007, 104, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. Anti-Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013, 140, 1–11. [Google Scholar] [CrossRef]
- Yilmaz, B.; Portugal, S.; Tran, T.M.; Gozzelino, R.; Ramos, S.; Gomes, J.; Regalado, A.; Cowan, P.J.; d’Apice, A.J.; Chong, A.S.; et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell 2014, 159, 1277–1289. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; de la Fuente, J. Immunity to α-Gal: Toward a single-antigen pan-vaccine to control major infectious diseases. ACS Cent. Sci. 2017, 3, 1140–1142. [Google Scholar] [CrossRef]
- Iniguez, E.; Schocker, N.S.; Subramaniam, K.; Portillo, S.; Montoya, A.L.; Al-Salem, W.S.; Torres, C.L.; Rodriguez, F.; Moreira, O.C.; Acosta-Serrano, A.; et al. An α-Gal-containing neoglycoprotein-based vaccine partially protects against murine cutaneous leishmaniasis caused by Leishmania major. PLoS Negl. Trop. Dis. 2017, 11, e0006039. [Google Scholar] [CrossRef]
- Portillo, S.; Zepeda, B.G.; Iniguez, E.; Olivas, J.J.; Karimi, N.H.; Moreira, O.C.; Marques, A.F.; Michael, K.; Maldonado, R.A.; Almeida, I.C. A prophylactic α-Gal-based glycovaccine effectively protects against murine acute Chagas disease. NPJ Vaccines 2019, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; Satinover, S.M.; Hosen, J.; Mozena, J.; Borish, L.; Lewis, B.D.; Woodfolk, J.A.; Platts-Mills, T.A. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-α-1,3-galactose. J. Allergy Clin. Immunol. 2009, 123, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Schuyler, A.J.; Schroeder, N.; Platts-Mills, T.A. Galactose-α-1,3-Galactose: Atypical food allergen or model IgE hypersensitivity? Curr. Allergy Asthma Rep. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Hodžić, A.; Román-Carrasco, P.; Mateos-Hernández, L.; Duscher, G.G.; Sinha, D.K.; Hemmer, W.; Swoboda, I.; Estrada-Peña, A.; de la Fuente, J. Environmental and molecular drivers of the α-Gal syndrome. Front. Immunol. 2019, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Pacheco, I.; Villar, M.; Cabezas-Cruz, A. The α-Gal syndrome: New insights into the tick-host conflict and cooperation. Parasit. Vectors 2019, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Mateos-Hernández, L.; Pérez-Cruz, M.; Valdés, J.J.; Mera, I.G.F.; Villar, M.; de la Fuente, J. Regulation of the immune response to α-Gal and vector-borne diseases. Trends Parasitol. 2015, 31, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, T.A.; Schuyler, A.J.; Tripathi, A.; Commins, S.P. Anaphylaxis to the carbohydrate side Chain alpha-gal. Immunol. Allergy. Clin. N. Am. 2015, 35, 247–260. [Google Scholar] [CrossRef]
- Van Nunen, S.A.; O’Connor, K.S.; Fernando, S.L.; Clarke, L.R.; Boyle, R.X. An association between Ixodes holocyclus tick bite reactions and red meat allergy. Intern. Med. J. 2007, 37 (Suppl. S5), A132. [Google Scholar]
- Commins, S.P.; James, H.R.; Kelly, L.A.; Pochan, S.L.; Workman, L.J.; Perzanowski, M.S.; Kocan, K.M.; Fahy, J.V.; Nganga, L.W.; Ronmark, E.; et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J. Allergy Clin. Immunol. 2011, 127, 1286–1293.e6. [Google Scholar] [CrossRef]
- Araujo, R.N.; Franco, P.F.; Rodrigues, H.; Santos, L.C.B.; McKay, C.S.; Sanhueza, C.A.; Brito, C.R.N.; Azevedo, M.A.; Venuto, A.P.; Cowan, P.J.; et al. Amblyomma sculptum tick saliva: α-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int. J. Parasitol. 2016, 46, 213–220. [Google Scholar] [CrossRef]
- Chinuki, Y.; Ishiwata, K.; Yamaji, K.; Takahashi, H.; Morita, E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy 2016, 71, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Hernández, L.; Villar, M.; Moral, A.; Rodríguez, C.G.; Arias, T.A.; de la Osa, V.; Brito, F.F.; Fernández de Mera, I.G.; Alberdi, P.; Ruiz-Fons, F.; et al. Tick-host conflict: Immunoglobulin E antibodies to tick proteins in patients with anaphylaxis to tick bite. Oncotarget 2017, 8, 20630–20644. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Fujiyama, T.; Umayahara, T.; Kageyama, R.; Walls, A.F.; Satoh, T. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-α-1,3-galactose carbohydrate IgE antibody levels: A retrospective cohort study in a single institution. J. Am. Acad. Dermatol. 2018, 78, 1135–1141.e3. [Google Scholar] [CrossRef] [PubMed]
- Apostolović, D.; Mihailović, J.; Commins, S.P.; Wijnveld, M.; Kazimirova, M.; Starkhammar, M.; Stockinger, H.; Platts-Mills, T.A.E.; Ćirković Veličković, T.; Hamsten, C.; et al. Allergenomics of the tick Ixodes ricinus reveals important α-Gal-carrying IgE-binding proteins in red meat allergy. Allergy 2019, 75, 217. [Google Scholar] [CrossRef]
- Crispell, G.; Commins, S.P.; Archer-Hartman, S.A.; Choudhary, S.; Dharmarajan, G.; Azadi, P.; Karim, S. Discovery of alpha-Gal-containing antigens in North American tick species believed to induce red meat allergy. Front. Immunol. 2019, 10, 1056. [Google Scholar] [CrossRef]
- Hodžić, A.; Mateos-Hernández, L.; Leschnik, M.; Alberdi, P.; Rego, R.O.M.; Contreras, M.; Villar, M.; de la Fuente, J.; Cabezas-Cruz, A.; Duscher, G.G. Tick bites induce anti-α-Gal antibodies in dogs. Vaccines 2019, 7, 114. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Espinosa, P.J.; Alberdi, P.; Šimo, L.; Valdés, J.J.; Mateos-Hernández, L.; Contreras, M.; Rayo, M.V.; de la Fuente, J. Tick galactosyltransferases are involved in α-Gal synthesis and play a role during Anaplasma phagocytophilum infection and Ixodes scapularis tick vector development. Sci. Rep. 2018, 8, 14224. [Google Scholar] [CrossRef]
- Stoltz, L.P.; Cristiano, L.M.; Dowling, A.P.G.; Wilson, J.M.; Platts-Mills, T.A.E.; Traister, R.S. Could chiggers be contributing to the prevalence of galactose-alpha-1,3-galactose sensitization and mammalian meat allergy? J. Allergy Clin. Immunol. Pract. 2019, 7, 664–666. [Google Scholar] [CrossRef]
- Hosen, J.; Perzanowski, M.; Carter, M.C.; Odhiambo, J.; Nganga, L.; Ngari, P.; Satinover, S.; Platts-Mills, T.A.E. IgE antibodies to helminths and the cross-reactive oligosaccharide galactose-alpha-1,3-galactose (alphaGal) among children in a village in Africa. J. Allergy Clin. Immunol. 2008, 121, S140. [Google Scholar] [CrossRef]
- Arkestål, K.; Sibanda, E.; Thors, C.; Troye-Blomberg, M.; Mduluza, T.; Valenta, R.; Grönlund, H.; van Hage, M. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-α1,3-galactose. J. Allergy Clin. Immunol. 2011, 127, 1024–1028. [Google Scholar] [CrossRef]
- Commins, S.P.; Kelly, L.A.; Rönmark, E.; James, H.R.; Pochan, S.L.; Peters, E.J.; Lundbäck, B.; Nganga, L.W.; Cooper, P.J.; Hoskins, J.M.; et al. Galactose-α-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Dam Laursen, A.S.; Vidal, C.; Skaaby, T.; Gude, F.; Linneberg, A. IgE antibodies to alpha-gal in the general adult population: Relationship with tick bites, atopy, and cat ownership. Clin. Exp. Allergy 2014, 44, 1061–1068. [Google Scholar] [CrossRef]
- Khoo, K.H.; Nieto, A.; Morris, H.R.; Dell, A. Structural characterization of the N-glycans from Echinococcus granulosus hydatid cyst membrane and protoscoleces. Mol. Biochem. Parasitol. 1997, 86, 237–248. [Google Scholar] [CrossRef]
- Duffy, M.S.; Morris, H.R.; Dell, A.; Appleton, J.A.; Haslam, S.M. Protein glycosylation in Parelaphostrongylus tenuis—First description of the Galα1-3Gal sequence in a nematode. Glycobiology 2006, 16, 854–862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Stijn, C.M.V.; van den Broek, M.; Vervelde, L.; Alvarez, R.A.; Cummings, R.D.; Tefsen, B.; van Die, I. Vaccination-induced IgG response to Galα1-3GalNAc glycan epitopes in lambs protected against Haemonchus contortus challenge infection. Int. J. Parasitol. 2010, 40, 215. [Google Scholar] [CrossRef]
- French National Authority for Health (HAS). Updating of Medical Biology Acts Related to the Diagnosis of Schistosomiasis; HAS: Saint-Denis La Plaine, France, 2017. [Google Scholar]
- French National Authority for Health (HAS). Updating of Medical Biology Acts Related to the Serological Diagnosis of Toxocariasis (Visceral Larva Migrans); HAS: Saint-Denis La Plaine, France, 2017. [Google Scholar]
- French National Authority for Health (HAS). Biological Diagnosis of Acquired Toxoplasmosis in Immunocompetent Subjects (Including Pregnant Women); Congenital Toxoplasmosis (Pre- and Postnatal Diagnosis) and Ocular Toxoplasmosis; HAS: Saint-Denis La Plaine, France, 2017. [Google Scholar]
- Reischl, U.; Bretagne, S.; Krüger, D.; Ernault, P.; Costa, J.M. Comparison of two DNA targets for the diagnosis of toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect. Dis. 2003, 3, 7. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 2019, in press. [Google Scholar] [CrossRef]
- De Savigny, D.H.; Voller, A.; Woodruff, A.W. Toxocariasis: Serological diagnosis by enzyme immunoassay. J. Clin. Pathol. 1979, 32, 284–288. [Google Scholar] [CrossRef]
- Magnaval, J.F.; Fabre, R.; Maurieres, P.; Charlet, J.P.; de Larrard, B. Application of the western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitol. Res. 1991, 77, 697–702. [Google Scholar] [CrossRef]
- Yoshida, A.; Kikuchi, T.; Nakagaki, S.; Maruyama, H. Optimal ELISA antigen for the diagnosis of Ascaris suum infection in humans. Parasitol. Res. 2016, 115, 4701–4705. [Google Scholar] [CrossRef]
- Gazzinelli-Guimarães, A.C.; Gazzinelli-Guimarães, P.H.; Nogueira, D.S.; Oliveira, F.M.S.; Barbosa, F.S.; Amorim, C.C.O.; Cardoso, M.S.; Kraemer, L.; Caliari, M.V.; Akamatsu, M.A.; et al. IgG Induced by vaccination with Ascaris suum extracts is protective against infection. Front. Immunol. 2018, 9, 2535. [Google Scholar] [CrossRef] [PubMed]
- Apostolović, D.; Rodrigues, R.; Thomas, P.; Starkhammar, M.; Hamsten, C.; van Hage, M. Immunoprofile of α-Gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy 2018, 73, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Savigny, D.H. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J. Parasitol. 1975, 61, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Pinto, F.J.; Gazzinelli, G.; Howells, R.E.; Mota-Santos, T.A.; Figueiredo, E.A.; Pellegrino, J. Schistosoma mansoni: Defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp. Parasitol. 1974, 36, 360–372. [Google Scholar] [CrossRef]
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval Estimation for a proportion. Statist. Sci. 2001, 16, 101–133. [Google Scholar]
- Galili, U.; LaTemple, D.C.; Radic, M.Z. A sensitive assay for measuring alpha-Gal epitope expression on cells by a monoclonal anti-Gal antibody. Transplantation 1998, 65, 1129–1132. [Google Scholar] [CrossRef]
- Kirkeby, S.; Moe, D. Binding of Griffonia simplicifolia 1 isolectin B4 (GS1 B4) to α-galactose antigens. Immunol. Cell Biol. 2001, 79, 121–127. [Google Scholar] [CrossRef]
- Schneider, R.; Auer, H. Incidence of Ascaris suum-specific antibodies in Austrian patients with suspected larva migrans visceralis (VLM) syndrome. Parasitol. Res. 2016, 115, 1213–1219. [Google Scholar] [CrossRef]
- Hamsten, C.; Starkhammar, M.; Tran, T.A.; Johansson, M.; Bengtsson, U.; Ahlén, G.; Sällberg, M.; Grönlund, H.; van Hage, M. Identification of galactose-α-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus, possible relationship with red meat allergy. Allergy 2013, 68, 549–552. [Google Scholar] [CrossRef]
- Hamsten, C.; Tran, T.A.T.; Starkhammar, M.; Brauner, A.; Commins, S.P.; Platts-Mills, T.A.E.; van Hage, M. Red meat allergy in Sweden: Association with tick sensitization and B-negative blood groups. J. Allergy Clin. Immunol. 2013, 132, 1431–1434. [Google Scholar] [CrossRef]
- Hodžić, A.; Mateos-Hernández, L.; de la Fuente, J.; Cabezas-Cruz, A. Delayed hypersensitivity reaction to mammalian galactose-α-1,3-galactose (α-Gal) after repeated tick bites in a patient from France. Ticks Tick Borne Dis. 2019, 10, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Mateos-Hernández, L.; Chmelař, J.; Villar, M.; de la Fuente, J. Salivary prostaglandin E2: Role in tick-induced allergy to red meat. Trends Parasitol. 2017, 33, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Apostolović, D.; Biedermann, T.; Commins, S.P.; Iweala, O.I.; Platts-Mills, T.A.E.; Savi, E.; van Hage, M.; Wilson, J.M. Galactose α-1,3-galactose phenotypes: Lessons from various patient populations. Ann. Allergy Asthma Immunol. 2019, 122, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Poole, N.M.; Mamidanna, G.; Smith, R.A.; Coons, L.B.; Cole, J.A. Prostaglandin E(2) in tick saliva regulates macrophage cell migration and cytokine profile. Parasit. Vectors 2013, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Maizels, R.M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011, 11, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Versini, M.; Jeandel, P.Y.; Bashi, T.; Bizzaro, G.; Blank, M.; Shoenfeld, Y. Unraveling the hygiene hypothesis of helminthes and autoimmunity: Origins, pathophysiology, and clinical applications. BMC Med. 2015, 13, 81. [Google Scholar] [CrossRef]
- Aranzamendi, C.; Sofronić-Milosavljević, L.; Pinelli, E. Helminths: Immunoregulation and inflammatory diseases-which side are Trichinella spp. and Toxocara spp. on? J. Parasitol. Res. 2013, 2013, 329438. [Google Scholar] [CrossRef]
- McSorley, H.J.; Maizels, R.M. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 2012, 25, 585–608. [Google Scholar] [CrossRef]
- Maizels, R.; Yazdanbakhsh, M. T-cell regulation in helminth parasite infections: Implications for inflammatory diseases. Chem. Immunol. Allergy 2008, 94, 112–123. [Google Scholar]
- Taylor, M.D.; van der Werf, N.; Maizels, R.M. T cells in helminth infection: The regulators and the regulated. Trends Immunol. 2012, 33, 181–189. [Google Scholar] [CrossRef]
- Feary, J.R.; Venn, A.J.; Mortimer, K.; Brown, A.P.; Hooi, D.; Falcone, F.H.; Pritchard, D.I.; Britton, J.R. Experimental hookworm infection: A randomized placebo-controlled trial in asthma. Clin. Exp. Allergy 2010, 40, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Kapel, C.; Roepstorff, A.; Thamsborg, S.; Arnved, J.; Rønborg, S.; Kristensen, B.; Poulsen, L.K.; Wohlfahrt, J.; Melbye, M. Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo-controlled double-blind clinical trial. PLoS ONE 2011, 6, e22346. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; McSorley, H.J.; Smyth, D.J. Helminths in the hygiene hypothesis: Sooner or later? Clin. Exp. Immunol. 2014, 177, 38–46. [Google Scholar] [CrossRef]
- Araujo, M.I.; Lopes, A.A.; Medeiros, M.; Cruz, A.A.; Sousa-Atta, L.; Sole, D.; Carvalho, E.M. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 2000, 123, 145–148. [Google Scholar] [CrossRef]
- Van den Biggelaar, A.H.; van Ree, R.; Rodrigues, L.C.; Lell, B.; Deelder, A.M.; Kremsner, P.G.; Yazdanbakhsh, M. Decreased atopy in children infected with Schistosoma haematobium: A role for parasite-induced interleukin-10. Lancet 2000, 356, 1723–1727. [Google Scholar] [CrossRef]
- Cooper, P.J.; Chico, M.E.; Rodrigues, L.C.; Ordonez, M.; Strachan, D.; Griffin, G.E.; Nutman, T.B. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J. Allergy Clin. Immunol. 2003, 111, 995–1000. [Google Scholar] [CrossRef]
- Dold, S.; Heinrich, J.; Wichmann, H.E.; Wjst, M. Ascaris-specific IgE and allergic sensitization in a cohort of school children in the former East Germany. J. Allergy. Clin. Immunol. 1998, 102, 414–420. [Google Scholar] [CrossRef]
- Palmer, L.J.; Celedon, J.C.; Weiss, S.T.; Wang, B.; Fang, Z.; Xu, X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am. J. Respir. Crit. Care Med. 2002, 165, 1489–1493. [Google Scholar] [CrossRef]
- Choi, M.H.; Chang, Y.S.; Lim, M.K.; Bae, Y.M.; Hong, S.T.; Oh, J.K.; Yun, E.H.; Bae, M.J.; Kwon, H.S.; Lee, S.M.; et al. Clonorchis sinensis infection is positively associated with atopy in endemic area. Clin. Exp. Allergy 2011, 41, 697–705. [Google Scholar] [CrossRef]
- Yariktas, M.; Demirci, M.; Aynali, G.; Kaya, S.; Doner, F. Relationship between Toxocara seropositivity and allergic rhinitis. Am. J. Rhinol. 2007, 21, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.J. Toxocara canis infection: An important and neglected environmental risk factor for asthma? Clin. Exp. Allergy 2008, 38, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Santiago, H.C.; Nutman, T.B. Human helminths and allergic disease: The hygiene hypothesis and beyond. Am. J. Trop. Med. Hyg. 2016, 95, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.; Yazdanbakhsh, M.; van Ree, R.; Aalberse, R.; Selkirk, M.E.; Partono, F.; Maizels, R.M. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J. Immunol. 1993, 150, 3941–3950. [Google Scholar] [PubMed]
- Jeannin, P.; Lecoanet, S.; Delneste, Y.; Gauchat, J.F.; Bonnefoy, J.Y. IgE versus IgG4 production can be differentially regulated by IL-10. J. Immunol. 1998, 160, 3555–3561. [Google Scholar] [PubMed]
- Maizels, R.M. Toxocara canis: Molecular basis of immune recognition and evasion. Vet. Parasitol. 2013, 193, 365–374. [Google Scholar] [CrossRef]
- Długosz, E.; Wasyl, K.; Klockiewicz, M.; Wiśniewski, M. Toxocara canis mucins among other excretory-secretory antigens induce in vitro secretion of cytokines by mouse splenocytes. Parasitol. Res. 2015, 114, 3365–3371. [Google Scholar] [CrossRef]
- Kuroda, E.; Yoshida, Y.; En Shan, B.; Yamashita, U. Suppression of macrophage interleukin-12 and tumour necrosis factor-alpha production in mice infected with Toxocara canis. Parasite Immunol. 2001, 23, 305–311. [Google Scholar] [CrossRef]
- Ruiz-Manzano, R.A.; Hernández-Cervantes, R.; Del Río-Araiza, V.H.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Morales-Montor, J. Immune response to chronic Toxocara canis infection in a mice model. Parasite Immunol. 2019, 41, e12672. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Park, M.K.; Kang, S.A.; Choi, S.H.; Ahn, S.C.; Yu, H.S. Trichinella spiralis infection suppressed gut inflammation with CD4+CD25+Foxp3+ T cell recruitment. Korean J. Parasitol. 2012, 50, 385–390. [Google Scholar] [CrossRef]
- Titz, T.O.; de Araújo, C.A.A.; Enobe, C.S.; Rigato, P.O.; Oshiro, T.M.; de Macedo-Soares, M.F. Ascaris suum infection modulates inflammation: Implication of CD4+ CD25high Foxp3+ T cells and IL-10. Parasite Immunol. 2017, 39, e12453. [Google Scholar] [CrossRef] [PubMed]
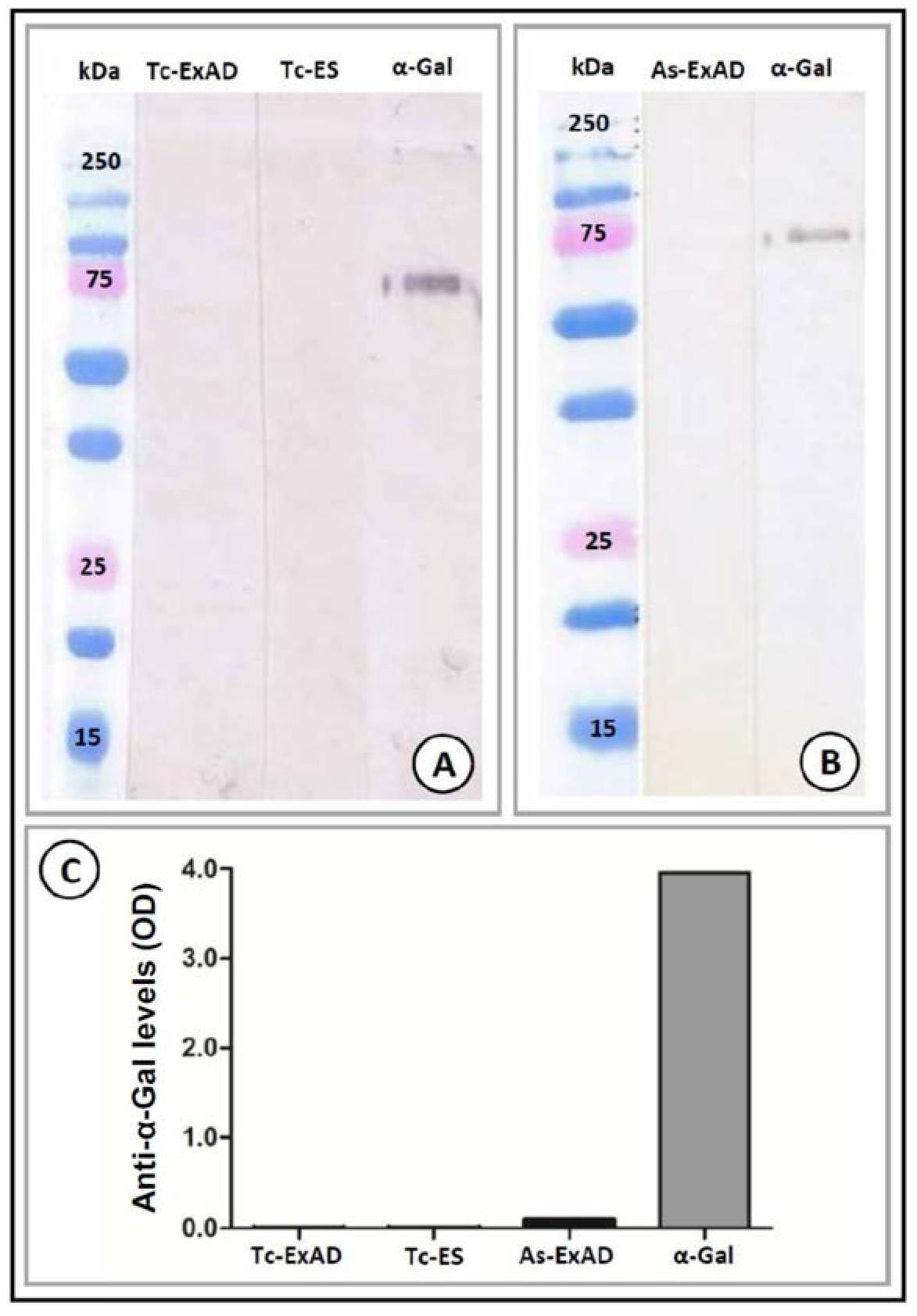
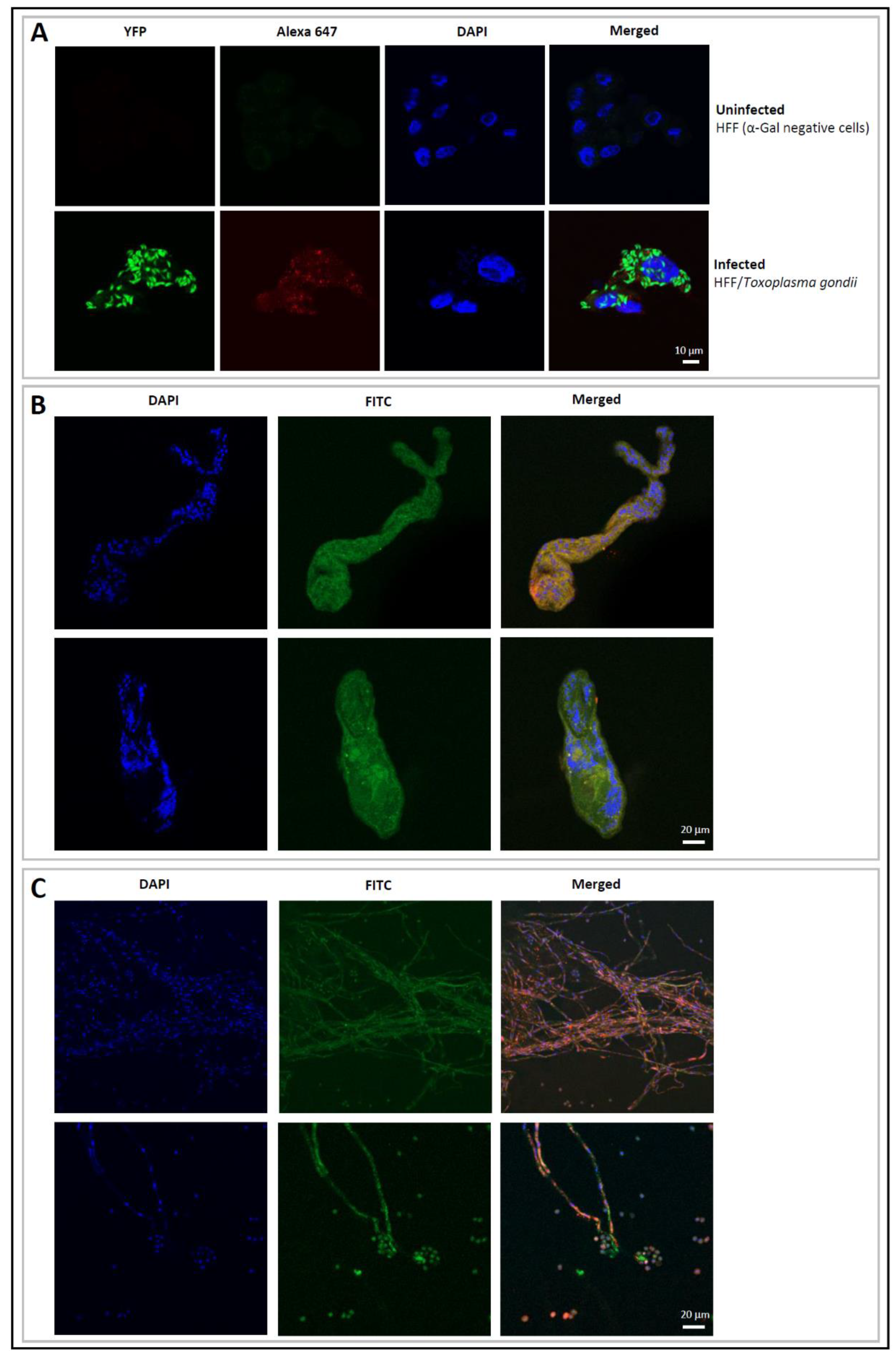
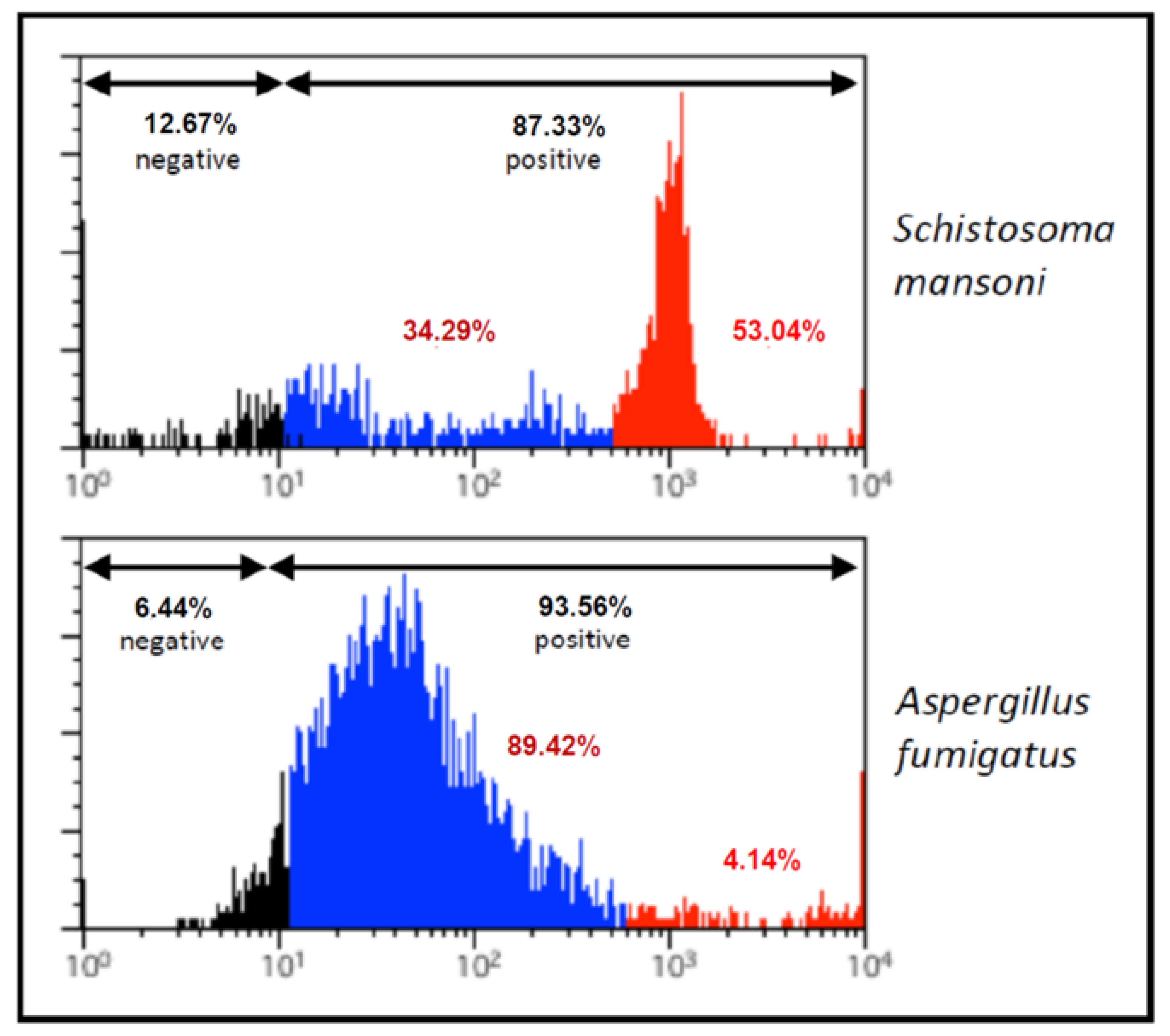
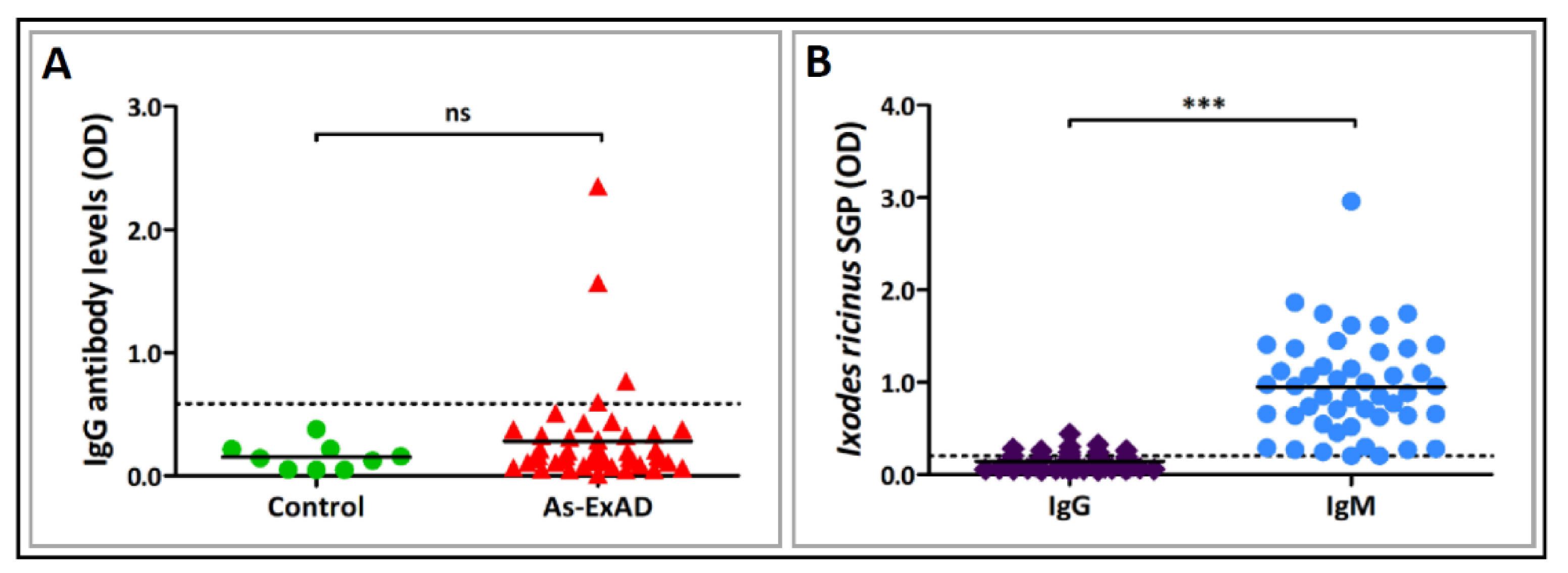
| Country | AGS | VLM (Toxocara canis) | VLM (Ascaris suum) | Schistosomiasis | Toxoplasmosis | Aspergillosis | AGS vs. Infection | |
|---|---|---|---|---|---|---|---|---|
| Congenital | Chronic | |||||||
| Austria * | 47 | 3 | 0 | n. t. | n/a | 0 | n. t. | 47/3 |
| France ** | 0 | 22 | n. t. | 6 | 27 | 14 | 19 | 0/88 |
| α-Gal epitopes | Toxocara canis | Ascaris suum | Schistosoma mansoni | Toxoplasma gondii | Aspergillus fumigatus | |||
| no | no | yes | no | yes | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodžić, A.; Mateos-Hernández, L.; Fréalle, E.; Román-Carrasco, P.; Alberdi, P.; Pichavant, M.; Risco-Castillo, V.; Le Roux, D.; Vicogne, J.; Hemmer, W.; et al. Infection with Toxocara canis Inhibits the Production of IgE Antibodies to α-Gal in Humans: Towards a Conceptual Framework of the Hygiene Hypothesis? Vaccines 2020, 8, 167. https://doi.org/10.3390/vaccines8020167
Hodžić A, Mateos-Hernández L, Fréalle E, Román-Carrasco P, Alberdi P, Pichavant M, Risco-Castillo V, Le Roux D, Vicogne J, Hemmer W, et al. Infection with Toxocara canis Inhibits the Production of IgE Antibodies to α-Gal in Humans: Towards a Conceptual Framework of the Hygiene Hypothesis? Vaccines. 2020; 8(2):167. https://doi.org/10.3390/vaccines8020167
Chicago/Turabian StyleHodžić, Adnan, Lourdes Mateos-Hernández, Emilie Fréalle, Patricia Román-Carrasco, Pilar Alberdi, Muriel Pichavant, Veronica Risco-Castillo, Delphine Le Roux, Jérôme Vicogne, Wolfgang Hemmer, and et al. 2020. "Infection with Toxocara canis Inhibits the Production of IgE Antibodies to α-Gal in Humans: Towards a Conceptual Framework of the Hygiene Hypothesis?" Vaccines 8, no. 2: 167. https://doi.org/10.3390/vaccines8020167
APA StyleHodžić, A., Mateos-Hernández, L., Fréalle, E., Román-Carrasco, P., Alberdi, P., Pichavant, M., Risco-Castillo, V., Le Roux, D., Vicogne, J., Hemmer, W., Auer, H., Swoboda, I., Duscher, G. G., de la Fuente, J., & Cabezas-Cruz, A. (2020). Infection with Toxocara canis Inhibits the Production of IgE Antibodies to α-Gal in Humans: Towards a Conceptual Framework of the Hygiene Hypothesis? Vaccines, 8(2), 167. https://doi.org/10.3390/vaccines8020167










