Impact of Mixed Equine Influenza Vaccination on Correlate of Protection in Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vaccines
2.3. Vaccination Protocol
2.4. Collection of Samples
2.5. Serology
2.6. Statistical Analysis
3. Results
3.1. Maternally Derived Antibodies (MDA)
3.2. SRH Antibody Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paillot, R. A systematic review of recent advances in equine influenza vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef] [PubMed]
- Sovinova, O.; Tumova, B.; Pouska, F.; Nemec, J. Isolation of a virus causing respiratory disease in horses. Acta Virol. 1958, 2, 52–61. [Google Scholar] [PubMed]
- Equine Influenza (Infection with Equine Influenza Virus). Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.05.07_EQ_INF.pdf (accessed on 9 September 2018).
- Wadell, G.; Teigland, M.; Sigel, M. A new influenza virus associated with equine respiratory disease. J. Am. Vet. Med. Assoc. 1963, 143, 587–590. [Google Scholar]
- Daly, J.M.; Lai, A.C.K.; Binns, M.M.; Chambers, T.M.; Barrandeguy, M.; Mumford, J.A. Antigenic and genetic evolution of equine H3N8 influenza A. viruses. J. Gen. Virol. 1996, 77, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.C.K.; Chambers, T.M.; Holland, R.E.; Morley, P.S.; Haines, D.M.; Townsend, H.G.G.; Barrandeguy, M. Diverged evolution of recent equine-2 influenza (H3N8) viruses in the Western Hemisphere. Arch. Virol. 2001, 146, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Rash, A.S.; Woodward, A.L.; Medcalf, E.; Helwegen, M.; Wohlfender, F.; Cruz, F.; Herrmann, C.; Borchers, K.; Tiwari, A.; et al. Isolation and characterisation of equine influenza viruses (H3N8) from Europe and North America from 2008 to 2009. Vet. Microbiol. 2011, 147, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Pitel, C.; D’Ablon, X.; Pronost, S. Equine Vaccines: How, When and Why? Report of the vaccinology session, french equine veterinarians association, 2016, Reims. Vaccines 2017, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Timoney, P.J. Equine influenza. Comp. Immunol. Microbiol. Infect. Dis. 1996, 19, 205–211. [Google Scholar] [CrossRef]
- Cullinane, A.; Newton, J.R. Equine influenza-A global perspective. Vet. Microbiol. 2013, 167, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Kane, J.; Carrick, J.; Axon, J.E.; Wilkie, I.; Begg, A.P. The pathology of bronchointerstitial pneumonia in young foals associated with the first outbreak of equine influenza in Australia. Equine Vet. J. 2008, 40, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; El-Hage, C. The use of a recombinant canarypox-based equine influenza vaccine during the 2007 australian outbreak: A systematic review and summary. Pathogens 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Daniels, P.; Kirkland, P.; Carroll, A.; Jeggo, M. The 2007 outbreak of equine influenza in Australia: Lessons learned for international trade in horses. Rev. Sci. Tech. 2011, 30, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Fougerolle, S.; Legrand, L.; Garrett, D.; Birand, I.; Foursin, M.; D’Ablon, X.; Bayssat, P.; Newton, R.J.; Pronost, S.; Paillot, R. Influential factors inducing suboptimal humoral response to vector-based influenza immunisation in Thoroughbred foals. Vaccine 2016, 34, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Xie, Y.; Ye, Y.P. ISCOMs and ISCOMATRIXTM. Vaccine 2009, 27, 4388–4401. [Google Scholar] [CrossRef] [PubMed]
- Pearse, M.J.; Drane, D. ISCOMATRIX® adjuvant for antigen delivery. Adv. Drug Deliv. Rev. 2005, 57, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Sjölander, A.; Cox, J.C.; Barr, I.G. ISCOMs: An adjuvant with multiple functions. J. Leukoc. Biol. 1998, 64, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Prowse, L. ISCOM-matrix-based equine influenza (EIV) vaccine stimulates cell-mediated immunity in the horse. Vet. Immunol. Immunopathol. 2012, 145, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E. Animal Influneza, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Edlund Toulemonde, C.; Daly, J.; Sindle, T.; Guigal, P.M.; Audonnet, J.C.; Minke, J.M. Efficacy of a recombinant equine influenza vaccine against challenge with an American lineage H3N8 influenza virus responsible for the 2003 outbreak in the United Kingdom. Vet. Rec. 2005, 156, 367–371. [Google Scholar] [CrossRef] [PubMed]
- El-Hage, C.M.; Savage, C.J.; Minke, J.M.; Ficorilli, N.P.; Watson, J.; Gilkerson, J.R. Accelerated vaccination schedule provides protective levels of antibody and complete herd immunity to equine influenza. Equine Vet. J. 2013, 45, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Kydd, J.H.; Sindle, T.; Hannant, D.; Edlund Toulemonde, C.; Audonnet, J.C.; Minke, J.M.; Daly, J.M. Antibody and IFN-γ responses induced by a recombinant canarypox vaccine and challenge infection with equine influenza virus. Vet. Immunol. Immunopathol. 2006, 112, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Fraser, S.; Prowse-Davis, L.; Rash, N.; Montesso, F.; Slootmans, N.; Thomas, A.; Besognet, B.; Meinert, T.; Ons, E.; et al. ISCOM-based equine influenza vaccine: Duration of immunity and randomised clinical trials to assess an accelerated schedule of immunisation and efficacy. Trials Vaccinol. 2015, 4, 61–70. [Google Scholar] [CrossRef]
- Paillot, R.; Rash, N.; Garrett, D.; Prowse-Davis, L.; Montesso, F.; Cullinane, A.; Lemaitre, L.; Thibault, J.-C.; Wittreck, S.; Dancer, A. How to meet the last oie expert surveillance panel recommendations on equine influenza (ei) vaccine composition: A review of the process required for the recombinant canarypox-based EI vaccine. Pathogens 2016, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. A comparison of antibody responses to commercial equine influenza vaccines following primary vaccination of Thoroughbred weanlings—A randomised blind study. Vaccine 2011, 29, 9214–9223. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Arkins, S.; Cullinane, A. A comparative antibody study of the potential susceptibility of Thoroughbred and non-Thoroughbred horse populations in Ireland to equine influenza virus. Influenza Respir. Viruses 2010, 4, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Quinlivan, M.; Murphy, B.A.; Cullinane, A. Humoral response and antiviral cytokine expression following vaccination of thoroughbred weanlings-A blinded comparison of commercially available vaccines. Vaccine 2013, 31, 5216–5222. [Google Scholar] [CrossRef] [PubMed]
- Tabynov, K.; Kydyrbayev, Z.; Ryskeldinova, S.; Assanzhanova, N.; Sansyzbay, A. Duration of the protective immune response after prime and booster vaccination of yearlings with a live modified cold-adapted viral vaccine against equine influenza. Vaccine 2014, 32, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Mumford, J.; Wood, J. Establishing an acceptability threshold for equine influenza vaccines. Dev. Biol. Stand. 1992, 79, 137–146. [Google Scholar] [PubMed]
- Newton, J.; Townsend, H.; Wood, J.; Sinclair, R.; Hannant, D.; Mumford, J. Immunity to equine influenza: Relationship of vaccine-induced antibody in young thoroughbred racehorses to protection against field infection with influenza A/equine-2 viruses (H3N8). Equine Vet. J. 2000, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Gildea, S.; Walsh, C.; Cullinane, A. The impact of different equine influenza vaccine products and other factors on equine influenza antibody levels in Thoroughbred racehorses. Equine Vet. J. 2015, 47, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Equine influenza vaccine (inactivated) monograph 0249. In European Pharmacopoeia, 8th ed.; Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2013; pp. 968–970. [Google Scholar]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Jüni, P.; Altman, D.; Egger, M.; Chan, A.; Altman, D.; Glasziou, P.; Meats, E.; et al. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Fougerolle, S.; Legrand, L.; Lecouturier, F.; Sailleau, C.; Paillot, R.; Hans, A.; Pronost, S. Genetic evolution of equine influenza virus strains (H3N8) isolated in France from 1967 to 2015 and the implications of several potential pathogenic factors. Virology 2017, 505, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Laabassi, F.; Lecouturier, F.; Amelot, G.; Gaudaire, D.; Mamache, B.; Laugier, C.; Legrand, L.; Zientara, S.; Hans, A. Epidemiology and Genetic Characterization of H3N8 Equine Influenza Virus Responsible for Clinical Disease in Algeria in 2011. Transbound. Emerg. Dis. 2014, 62, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Garrett, D.; Lopez-Alvarez, M.; Birand, I.; Montesso, F.; Horspool, L. The Immunity Gap Challenge: Protection against a Recent Florida Clade 2 Equine Influenza Strain. Vaccines 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Mumford, J.A. Vaccines and viral antigenic diversity. Rev. Sci. Tech. 2007, 26, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Prowse, L.; Montesso, F.; Huang, C.M.; Barnes, H.; Escala, J. Whole inactivated equine influenza vaccine: Efficacy against a representative clade 2 equine influenza virus, IFNgamma synthesis and duration of humoral immunity. Vet. Microbiol. 2013, 162, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Mumford, J.; Jessett, D.; Rollinson, E.; Hannant, D.; Draper, M. Duration of protective efficacy of equine influenza immunostimulating complex/tetanus vaccines. Vet. Rec. 1994, 134, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Mumford, J.A. Control of influenza from an international perspective. Equine Infect. In Proceedings of the Eighth International Conference on Equine Infectious Diseases, Dubai, UAE, 23–26 March 1998; Newmarket. pp. 11–24. [Google Scholar]
- Paillot, R.; Grimmett, H.; Elton, D.; Daly, J.; Paillot, R.; Grimmett, H.; Elton, D.; Protection, J.D.; Daly, J.M. Protection, systemic IFN γ, and antibody responses induced by an ISCOM-based vaccine against a recent equine influenza virus in its natural host. Veterinary Res. 2008. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Paillot, R.; Rash, A.S.; Medcalf, E.; Montesso, F.; Ross, J.; Watson, J.; Jeggo, M.; Lewis, N.S.; Newton, J.R.; et al. Comparison of two modern vaccines and previous influenza infection against challenge with an equine influenza virus from the Australian 2007 outbreak. Vet. Res. 2010, 41. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.A.; Townsend, H.G.G.; Kohler, A.K.; Hussey, S.; Breathnach, C.; Barnett, C.; Holland, R.; Lunn, D.P. Immune responses to commercial equine vaccines against equine herpesvirus-1, equine influenza virus, eastern equine encephalomyelitis, and tetanus. Vet. Immunol. Immunopathol. 2006, 111, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.; Weld, J.; Osborne, M.; Nelly, M.; Mcbride, C.; Walsh, C. Field studies on equine influenza vaccination regimes in Thoroughbred foals and yearlings. Vet. J. 2001, 161, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Heldens, J.; Van De Wouw, J.; Van Loon, A. An updated equine influenza vaccine and an equine influenza-herpesvirus combination vaccine containing an immunostim adjuvant provoke equal antibody levels in young foals throughout the primary vaccination course. Vet. J. 2002, 164, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Mumford, J.A.; Jessett, D.; Dunleavy, U.; Wood, J.; Hannant, D.; Sundquist, B.; Cook, R.F. Antigenicity and immunogenicity of experimental equine influenza ISCOM vaccines. Vaccine 1994, 12, 857–863. [Google Scholar] [CrossRef]
- Heldens, J.G.M.; Pouwels, H.G.W.; Derks, C.G.G.; Van de Zande, S.M.A.; Hoeijmakers, M.J.H. Duration of immunity induced by an equine influenza and tetanus combination vaccine formulation adjuvanted with ISCOM-Matrix. Vaccine 2010, 28, 6989–6996. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Kydd, J.H.; MacRae, S.; Minke, J.M.; Hannant, D.; Daly, J.M. New assays to measure equine influenza virus-specific Type 1 immunity in horses. Vaccine 2007, 25, 7385–7398. [Google Scholar] [CrossRef] [PubMed]
- Barquero, N.; Daly, J.M.; Newton, J.R. Risk factors for influenza infection in vaccinated racehorses: Lessons from an outbreak in Newmarket, UK in 2003. Vaccine 2007, 25, 7520–7529. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Horspool, L. Large scale sero-epidemiological investigation of Equine Influenza vaccination in Hong Kong. J. Equine Vet. Sci. 2016, 39. [Google Scholar] [CrossRef]
- Prime Boost Vaccine for the Protection of Equines Against Equine Influenza. Available online: http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2007051763&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCT+Biblio (accessed on 18 September 2014).
| Technology | Study | Vaccine/Manufacturer | Nature | Adjuvant | Compositions |
|---|---|---|---|---|---|
| Whole inactivated/Sub-unit ISCOM/ISCOM-Matrix | Used | Equilis® Prequenza-TE (MSD Animal Health) | Whole inactivated | ISCOMatrix, chol., P. saponin, Phos. choline. | -A/Equi-2/South Africa/4/03 -A/Equi-2/Newmarket/2/93 -Anatoxine tétanique |
| Used | Calvenza-03 EIV/EHV® (Boehringer Ingelheim) | Whole inactivated | Carbimmune | -A/Equi-2/Newmarket/2/1993 -A/Equi-2/Kentucky/1995 -A/Equi-2/Ohio/2003 -EHV 1 souche KyA | |
| Not used | Duvaxyn IE-T® (Elanco Animal Health) | Whole inactivated | Carbomer, Alum. Hydr. | -A/Equi-1/Prague/56 (H7N7) -A/Equi-2/Newmarket 1/93 -A/Equi-2/Suffolk/89 -Anatoxine tétanique | |
| Not used | Equip-FT®(Pfizer) | Subunit | ISCOM, Quillaic Acid derivative, Aluminium phosphate | -A/Equi-1/Newmarket 77 -A/Equi-2/Borlange 91 -A/Equi-2/Kentucky 98 -Anatoxine tétanique | |
| Viral-vector based | Used | Proteqflu-TE® (Boehringer Ingelheim) | Recominant canarypox | Carbomer | -A/Eq/Ohio/03 -A/Eq/Richmond/1/07 -Anatoxine tétanique |
| Modified live EIV | Not Used | Flu Avert® I.N. (MSD Animal Health) | whole virus | Not applicable | Attenuated, cold adapted EIV: Kentucky/91 (H3N8) |
| Weanling Groups | Number of Weanlings | Vaccination Protocol |
|---|---|---|
| Group #1 | 7 | V1: Prequenza-TE® V2: Prequenza-TE® V3: Prequenza-TE® |
| Group #2 | 7 | V1: Proteqflu-TE® V2: Proteqflu-TE® V3: Proteqflu-TE® |
| Group #3 | 7 | V1: Calvenza-03® V2: Calvenza-03® V3: Calvenza-03® |
| Group #4 | 7 | V1: Prequenza-TE® V2: Proteqflu-TE® V3: Calvenza-03® |
| Group #5 | 7 | V1: Proteqflu-TE® V2: Prequenza-TE® V3: Calvenza-03® |
| Group #6 | 7 | V1: Calvenza-03® V2: Prequenza-TE® V3: Proteqflu-TE® |
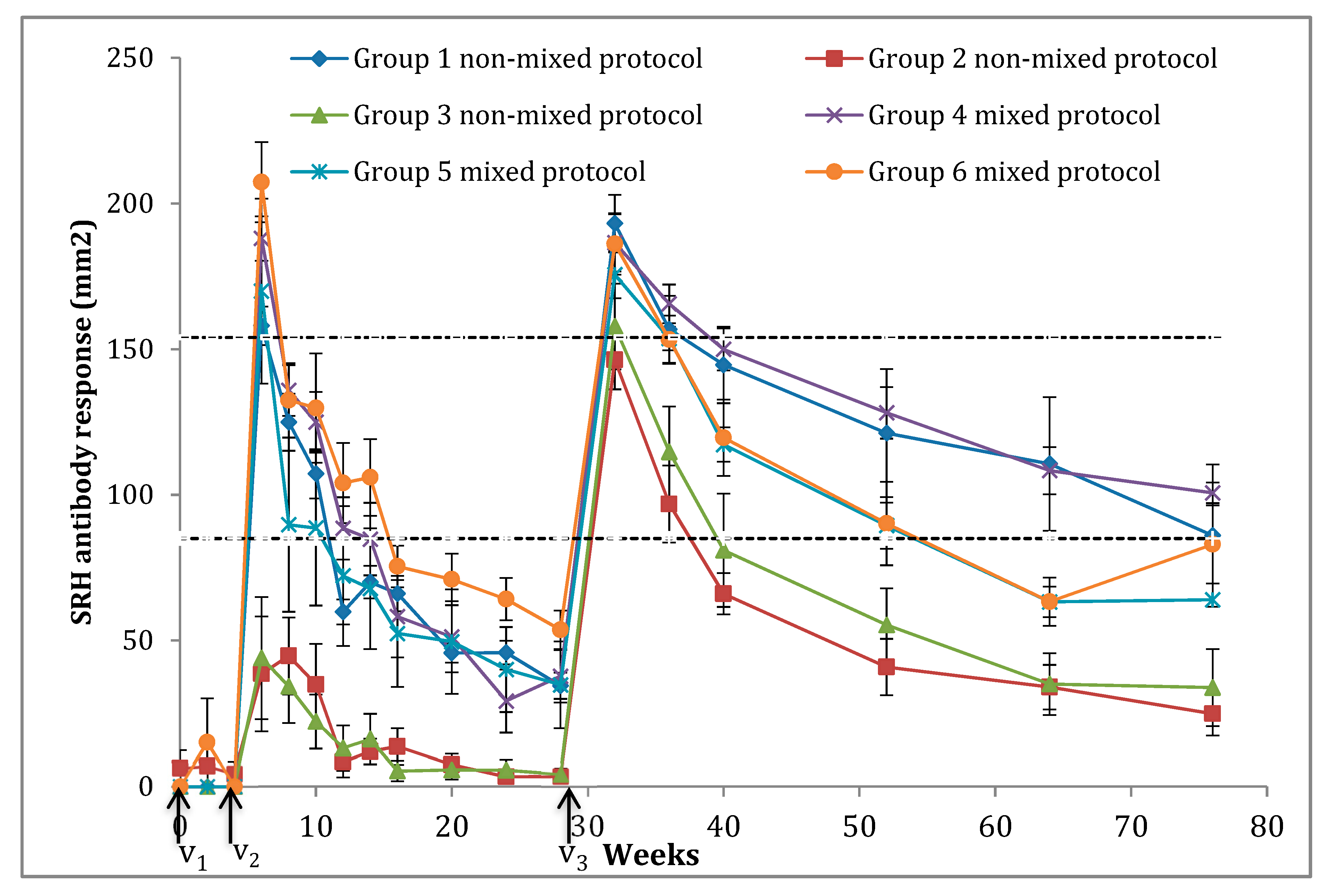
| Weeks | Vaccination | Sampling | Groups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | |||||||||
| Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | |||
| 0 | V1 | S1 | 0 | 0 | 6.2 | 6.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | S2 | 0 | 0 | 6.9 | 6.9 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 15 | |
| 4 | V2 | S3 | 0 | 0 | 4.2 | 4.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | S4 | 158 | 6.6 | 38.5 | 19.7 | 44 | 21 | 188 | 7.6 | 169.9 | 31.7 | 207.3 | 13.7 | |
| 8 | S5 | 124.9 | 9.8 | 44.7 | 13.2 | 34.2 | 12.6 | 135.9 | 8.7 | 89.7 | 29.9 | 132.5 | 12.8 | |
| 10 | S6 | 107.2 | 8.4 | 34.9 | 13.9 | 22.2 | 9.3 | 124.9 | 10.4 | 88.6 | 26.6 | 129.8 | 18.8 | |
| 12 | S7 | 59.8 | 4.3 | 8.3 | 5.2 | 13.1 | 7.7 | 88.5 | 10.7 | 72.1 | 24 | 104 | 13.8 | |
| 14 | S8 | 70 | 5.6 | 11.9 | 4.3 | 16.1 | 8.7 | 84.8 | 12.4 | 67.8 | 20.7 | 106 | 13.1 | |
| 16 | S9 | 66.1 | 6.1 | 13.6 | 6.2 | 5.3 | 3.5 | 58.2 | 14 | 52.4 | 18.4 | 75.4 | 7.1 | |
| 20 | S10 | 45.7 | 3.3 | 7.4 | 3.8 | 5.6 | 3.3 | 51.2 | 12.2 | 49.6 | 17.9 | 71 | 8.8 | |
| 24 | S11 | 45.8 | 4.1 | 3.3 | 2.4 | 5.5 | 3.7 | 29.2 | 10.8 | 40.0 | 14.6 | 64.2 | 7.3 | |
| 28 | V3 | S12 | 34.4 | 4.5 | 3.4 | 2.3 | 4.2 | 2.1 | 37.7 | 9 | 34.8 | 14.9 | 53.6 | 6.6 |
| 32 | S13 | 193.1 | 9.8 | 146.3 | 10.1 | 157.8 | 14.7 | 186.5 | 9.8 | 175.5 | 8.1 | 186.1 | 10.5 | |
| 36 | S14 | 156.8 | 11.5 | 96.8 | 13.3 | 114.7 | 15.6 | 165.5 | 6.7 | 153.7 | 4 | 153.3 | 8.3 | |
| 40 | S15 | 144.6 | 13.2 | 66 | 7.1 | 81 | 19.4 | 150 | 7.2 | 117.2 | 5.9 | 119.6 | 13.1 | |
| 52 | S16 | 121.2 | 22.0 | 40.9 | 9.7 | 55.3 | 12.6 | 128.2 | 8.9 | 89.4 | 7.9 | 90.1 | 14.3 | |
| 64 | S17 | 110.6 | 22.9 | 34 | 7.6 | 35 | 10.6 | 108.3 | 8.1 | 63.2 | 5.3 | 63.3 | 8.3 | |
| 76 | S18 | 86 | 24.4 | 24.9 | 7.5 | 33.8 | 13.2 | 100.7 | 3.5 | 64.0 | 83 | 13.4 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilai, M.; Piro, M.; El Harrak, M.; Fougerolle, S.; Dehhaoui, M.; Dikrallah, A.; Legrand, L.; Paillot, R.; Fassi Fihri, O. Impact of Mixed Equine Influenza Vaccination on Correlate of Protection in Horses. Vaccines 2018, 6, 71. https://doi.org/10.3390/vaccines6040071
Dilai M, Piro M, El Harrak M, Fougerolle S, Dehhaoui M, Dikrallah A, Legrand L, Paillot R, Fassi Fihri O. Impact of Mixed Equine Influenza Vaccination on Correlate of Protection in Horses. Vaccines. 2018; 6(4):71. https://doi.org/10.3390/vaccines6040071
Chicago/Turabian StyleDilai, Mohamed, Mohammed Piro, Mehdi El Harrak, Stéphanie Fougerolle, Mohammed Dehhaoui, Asmaa Dikrallah, Loïc Legrand, Romain Paillot, and Ouafaa Fassi Fihri. 2018. "Impact of Mixed Equine Influenza Vaccination on Correlate of Protection in Horses" Vaccines 6, no. 4: 71. https://doi.org/10.3390/vaccines6040071
APA StyleDilai, M., Piro, M., El Harrak, M., Fougerolle, S., Dehhaoui, M., Dikrallah, A., Legrand, L., Paillot, R., & Fassi Fihri, O. (2018). Impact of Mixed Equine Influenza Vaccination on Correlate of Protection in Horses. Vaccines, 6(4), 71. https://doi.org/10.3390/vaccines6040071






