Immunogenicity and Safety of the New Inactivated Quadrivalent Influenza Vaccine Vaxigrip Tetra: Preliminary Results in Children ≥6 Months and Older Adults
Abstract
1. Introduction
2. Evaluation of Influenza Vaccine Efficacy: From CHMP Criteria to Post-CHMP Criteria
3. General Information on QIV
4. A Novel Quadrivalent Inactivated Influenza Vaccine: Vaxigrip Tetra
4.1. Clinical Trials Performed on Vaxigrip Tetra
4.1.1. GQM01 Clinical Trial in Adults
4.1.2. GQM04 Clinical Trial in Children, Adolescents, and Adults
4.1.3. GQM02 Clinical Trial in Children (3–8 Years)
4.1.4. GQM09 Clinical Trial in Children and Adolescents
4.1.5. GQM11 Clinical Trial in Younger and Older Adults
4.1.6. GQM07 Clinical Trial in Adults
4.1.7. GQM05 Clinical Trial in Children (6–35 Months)
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Weekly Epidemiological Record. Available online: http://www.who.int/wer/2012/wer8747.pdf?ua=1&ua=1 (accessed on 8 December 2017).
- Kocik, J.; Kołodziej, M.; Joniec, J.; Kwiatek, M.; Bartoszcze, M. Antiviral activity of novel oseltamivir derivatives against some influenza virus strains. Acta Biochim. Pol. 2014, 61, 509–513. [Google Scholar] [PubMed]
- Coughlan, L.; Lambe, T. Measuring Cellular Immunity to Influenza: Methods of Detection, Applications and Challenges. Vaccines 2015, 3, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Molteni, C.G.; Daleno, C.; Valzano, A.; Fossali, E.; Da Dalt, L.; Cecinati, V.; Bruzzese, E.; Giacchino, R.; Giaquinto, C.; et al. Clinical and socioeconomic impact of different types and subtypes of seasonal influenza viruses in children during influenza seasons 2007/2008 and 2008/2009. BMC Infect. Dis. 2011, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Estimates of deaths associated with Seasonal Influenza—United States, 1976–2007. Morb. Mortal. Wkly. Rep. 2010, 59, 1057–1062. [Google Scholar]
- Gianchecchi, E.; Trombetta, C.; Piccirella, S.; Montomoli, E. Evaluating influenza vaccines: Progress and perspectives. Future Virol. 2016, 11, 5. [Google Scholar] [CrossRef]
- Heo, J.Y.; Song, J.Y.; Noh, J.Y.; Choi, M.J.; Yoon, J.G.; Lee, S.N.; Cheong, H.J.; Kim, W.J. Effects of influenza immunization on pneumonia in the elderly. Hum. Vaccines Immunother. 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, A.N. Increasing evidence that influenza is a trigger for cardiovascular disease. J. Infect. Dis. 2012, 206, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Influenza ACIP Vaccine Recommendations. Available online: www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/flu.html (accessed on 8 December 2017).
- Background Paper on Influenza Vaccines and Immunization SAGE Working Group. Available online: http://www.who.int/immunization/sage/meetings/2012/april/1_Background_Paper_Mar26_v13_cleaned.pdf (accessed on 8 December 2017).
- Fiore, A.E.; Uyeki, T.M.; Broder, K.; Finelli, L.; Euler, G.L.; Singleton, J.A.; Iskander, J.K.; Wortley, P.M.; Shay, D.K.; Bresee, J.S.; et al. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm. Rep. 2010, 59, 1–62. [Google Scholar] [PubMed]
- Reperant, L.A.; Grenfell, B.T.; Osterhaus, A.D. Quantifying the risk of pandemic influenza virus evolution by mutation and re-assortment. Vaccine 2015, 33, 6955–6966. [Google Scholar] [CrossRef] [PubMed]
- Nobusawa, E.; Sato, K. Comparison of the mutation rates of human influenza A and B viruses. J. Virol. 2006, 80, 3675–3678. [Google Scholar] [CrossRef] [PubMed]
- Irving, S.A.; Patel, D.C.; Kieke, B.A.; Donahue, J.G.; Vandermause, M.F.; Shay, D.K.; Belongia, E.A. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004–2005 through 2007–2008. Influenza Other Respir. Viruses 2012, 6, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Tafalla, M.; Buijssen, M.; Geets, R.; Vonk Noordegraaf-Schouten, M. A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries. Hum. Vaccines Immunother. 2016, 12, 993–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Influenza (Seasonal). Available online: http://www.who.int/mediacentre/factsheets/fs211/en/ (accessed on 8 December 2017).
- Petrova, V.N.; Russell, C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Barr, I.G.; Russell, C.; Besselaar, T.G.; Cox, N.J.; Daniels, R.S.; Donis, R.; Engelhardt, O.G.; Grohmann, G.; Itamura, S.; Kelso, A. WHO recommendations for the viruses used in the 2013–2014 Northern Hemisphere influenza vaccine: Epidemiology, antigenic and genetic characteristics of influenza A(H1N1) pdm09, A(H3N2) and B influenza viruses collected from October 2012 to January 2013. Vaccine 2014, 32, 4713–4725. [Google Scholar] [CrossRef] [PubMed]
- Klimov, A.I.; Garten, R.; Russell, C.; Barr, I.G.; Besselaar, T.G.; Daniels, R.; Engelhardt, O.G.; Grohmann, G.; Itamura, S.; Kelso, A.; et al. WHO recommendations for the viruses to be used in the 2012 Southern Hemisphere influenza vaccine: Epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from February to September 2011. Vaccine 2012, 30, 6461–6471. [Google Scholar] [CrossRef] [PubMed]
- WHO Writing Group; Ampofo, W.K.; Baylor, N.; Cobey, S.; Cox, N.J.; Daves, S.; Edwards, S.; Ferguson, N.; Grohmann, G.; Hay, A. Improving influenza vaccine virus selection: Report of a WHO informal consultation held at WHO headquarters, Geneva, Switzerland, 14–16 June 2010. Influenza Other Respir. Viruses 2012, 6, 142–152. [Google Scholar]
- Peltola, V.; Ziegler, T.; Ruuskanen, O. Influenza A and B virus infections in children. Clin. Infect. Dis. 2003, 36, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Rota, P.A.; Wallis, T.R.; Harmon, M.W.; Rota, J.S.; Kendal, A.P.; Nerome, K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990, 175, 59–68. [Google Scholar] [CrossRef]
- Chen, J.M.; Guo, Y.J.; Wu, K.Y.; Guo, J.F.; Wang, M.; Dong, J.; Zhang, Y.; Li, Z.; Shu, Y.L. Exploration of the emergence of the Victoria lineage of influenza B virus. Arch. Virol. 2007, 152, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.W.; Xu, X.; Li, Y.; Normand, S.; Ueki, R.T.; Kunimoto, G.Y.; Hall, H.; Klimov, A.; Cox, N.J.; Subbarao, K. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000–2001 and 2001–2002 seasons. Virology 2002, 303, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hannoun, C. The evolving history of influenza viruses and influenza vaccines. Expert Rev. Vaccines 2013, 12, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Influenza activity—United States and worldwide, 2007–08 season. Morb. Mortal. Wkly. Rep. 2008, 57, 692–697. [Google Scholar]
- Caini, S.; Huang, Q.S.; Ciblak, M.A.; Kusznierz, G.; Owen, R.; Wangchuk, S.; Henriques, C.M.; Njouom, R.; Fasce, R.A.; Yu, H.; et al. Epidemiological and virological characteristics of influenza B: Results of the Global Influenza B Study. Influenza Other Respir Viruses 2015, 9, 3–12. [Google Scholar]
- Ambrose, C.S.; Levin, M.J. The rationale for quadrivalent influenza vaccines. Hum. Vaccines. Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010, 28, D45–D53. [Google Scholar] [CrossRef] [PubMed]
- Influenza Virus Characterisation. Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/influenza-virus-characterisation-may-2016.pdf (accessed on 8 December 2017).
- Heikkinen, T.; Ikonen, N.; Ziegler, T. Impact of influenza B lineagelevel mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin. Infect. Dis. 2014, 59, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.; Chan, M.C.; Cheung, J.L.; Lee, N.; Leung, T.F.; Yeung, AC.; Wong, M.C.; Ngai, K.L.; Nelson, E.A.; Hui, D.S. Influenza B lineage circulation and hospitalization rates in a subtropical city, Hong Kong, 2000–2010. Clin. Infect. Dis. 2013, 56, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Coelingh, K.; Ambrose, C.S.; Woo, J.C.; Wu, X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010, 28, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.C.; Beyer, W.E.; Palache, A.M.; Rimmelzwaan, G.F.; Osterhaus, A.D. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J. Med. Virol. 2000, 61, 94–99. [Google Scholar] [CrossRef]
- Vaccines and Related Biological Products Advisory Committee. Available online: https://www.fda.gov/ohrms/dockets/ac/07/transcripts/2007-4282t2.htm (accessed on 8 December 2017).
- Lee, B.Y.; Bartsch, S.M.; Willig, A.M. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine 2012, 30, 7443–7446. [Google Scholar] [CrossRef] [PubMed]
- De Boer, P.T.; Crépey, P.; Pitman, R.J.; Macabeo, B.; Chit, A.; Postma, M.J. Cost-Effectiveness of Quadrivalent versus Trivalent Influenza Vaccine in the United States. Value Health 2016, 19, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Clippard, J.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Petrie, J.G.; McLean, H.Q.; Belongia, E.A.; et al. Early estimates of seasonal influenza vaccine effectiveness- United States, January 2015. Morb. Mortal. Wkly. Rep. 2015, 64, 10–15. [Google Scholar]
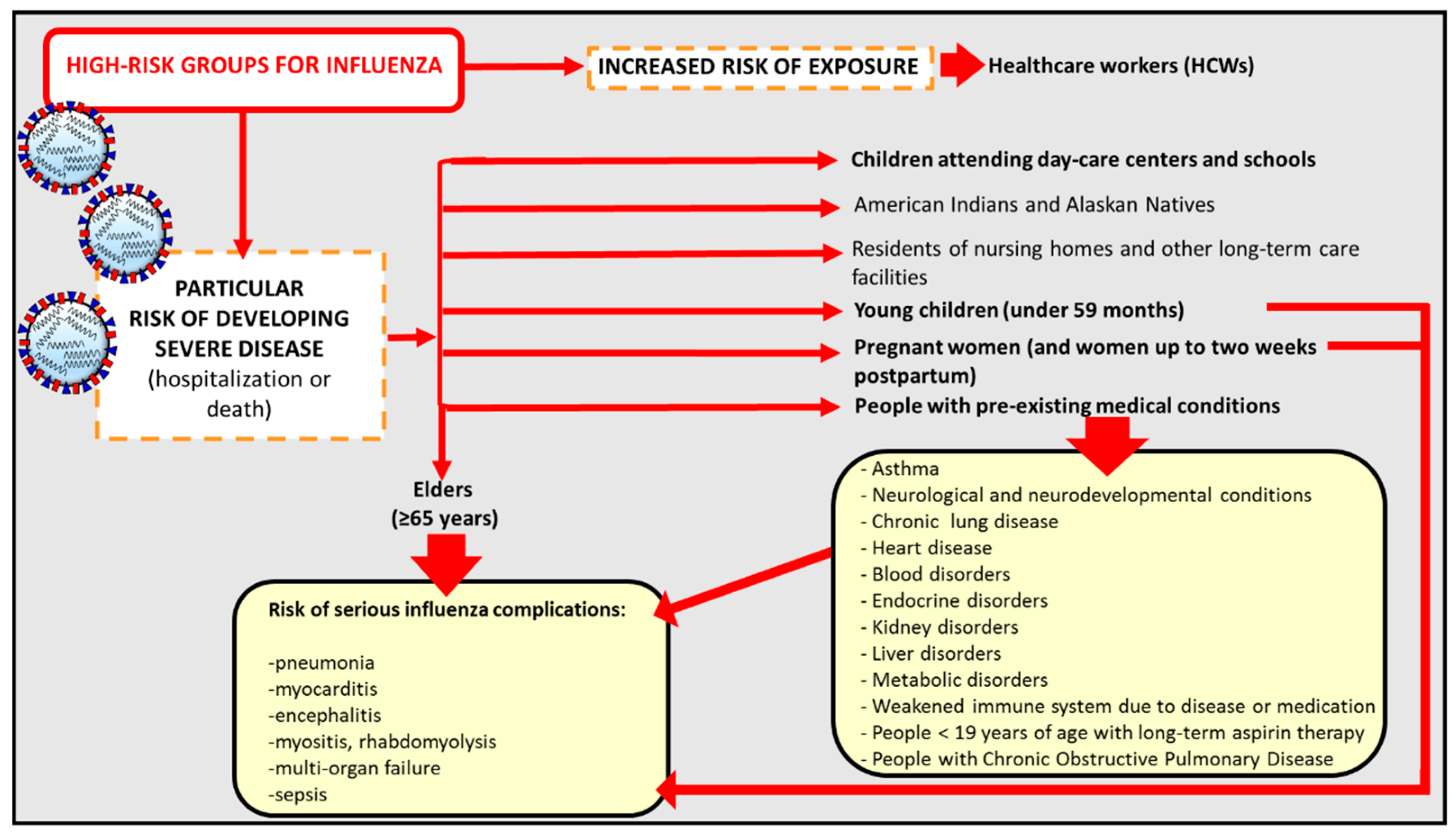
- People at High Risk of Developing Flu–Related Complications. Available online: https://www.cdc.gov/flu/about/disease/high_risk.htm> (accessed on 8 December 2017).
- Wang, B.; Russell, M.L.; Brewer, A.; Newton, J.; Singh, P.; Ward, B.J.; Loeb, M. Single radial haemolysis compared to haemagglutinin inhibition and microneutralization as a correlate of protection against influenza A H3N2 in children and adolescents. Influenza Other Respir. Viruses 2017, 11, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; McCahon, D.; Beare, A. A single radial haemolysis technique for the measurement of influenza antibody. J. Gen. Virol. 1975, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hirst, G.K. Adsorption of influenza hemagglutinins and virus by red blood cells. J. Exp. Med. 1942, 76, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Salk, J.E. A simplified procedure for titrating hemagglutinating capacity of influenza virus and the corresponding antibody. J. Immunol. 1944, 49, 87–98. [Google Scholar]
- Schild, G.; Pereira, M.; Chakraverty, P. Single-radial-haemolysis: A new method for the assay of antibody to influenza haemagglutinin: Applications for diagnosis and seroepidemiologic surveillance of influenza. Bull. World Health Organ. 1975, 52, 43–50. [Google Scholar] [PubMed]
- Trombetta, C.M.; Montomoli, E. Influenza immunology evaluation and correlates of protection: A focus on vaccines. Expert Rev. Vaccines 2016, 15, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Perini, D.; Vitale, L.; Cox, R.J.; Stanzani, V.; Piccirella, S.; Montomoli, E. Validation of Single Radial Haemolysis assay: A reliable method to measure antibodies against influenza viruses. J. Immunol. Methods 2015, 422, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.; Ye, Z.; Xie, H.; Vahl, S.; Dawson, E.; Rowlen, K. Automated interpretation of influenza hemagglutination inhibition (HI) assays: Is plate tilting necessary? PLoS ONE 2017, 12, e0179939. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Gaines-Das, R.E.; Taylor, J.; Chakraverty, P. Comparison of influenza serological techniques by international collaborative study. Vaccine 1994, 12, 167–174. [Google Scholar] [CrossRef]
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Products CFPM. Note for Guidance on Harmonisation of Requirements for Influenza Vaccines; European Agency for the Evaluation of Medicinal Products: London, UK, 1997. [Google Scholar]
- Cox, R.J. Correlates of protection to influenza virus, where do we go from here? Hum. Vaccines Immunother. 2013, 9, 405–408. [Google Scholar] [CrossRef]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Saso, A.; Kampmann, B. Vaccine responses in newborns. Semin. Immunopathol. 2017, 39, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.B.; Rajagopal, S.; Zhu, Y.; Neuzil, K.M.; Fairchok, M.P.; Englund, J.A. Trivalent inactivated influenza vaccine (TIV) immunogenicity in children 6 through 23 months of age: Do children of all ages respond equally? Vaccine 2010, 28, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Levandowski, R.A.; Regnery, H.L.; Staton, E.; Burgess, B.G.; Williams, M.S.; Groothuis, J.R. Antibody responses to influenza B viruses in immunologically unprimed children. Pediatrics 1991, 88, 1031–1036. [Google Scholar] [PubMed]
- Siegrist, C.A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Goronzy, J.J.; Grubeck-Loebenstein, B.; Lambert, P.H.; Mrkvan, T.; Stoddard, J.J.; Doherty, T.M. Fighting against a protean enemy: Immunosenescence, vaccines, and healthy aging. NPJ Aging Mech. Dis. 2017, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Sullivan, M.; Narvaez, C.F.; Holmes, T.H.; Furman, D.; Zheng, N.Y.; Nishtala, M.; Wrammert, J.; Smith, K.; James, J.A.; et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J. Clin. Investig. 2011, 121, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Rivetti, D.; Rivetti, A.; Rudin, M.; Di Pietrantonj, C.; Demicheli, V. Efficacy and effectiveness of influenza vaccines in elderly people: A systematic review. Lancet 2005, 366, 1165–1174. [Google Scholar] [CrossRef]
- Beyer, W.E.; McElhaney, J.; Smith, D.J.; Monto, A.S.; Nguyen-Van-Tam, J.S.; Osterhaus, A.D. Cochrane rearranged: Support for policies to vaccinate elderly people against influenza. Vaccine 2013, 31, 6030–6033. [Google Scholar] [CrossRef] [PubMed]
- Mysliwska, J.; Trzonkowski, P.; Szmit, E.; Brydak, L.B.; Machala, M.; Mysliwski, A. Immunomodulating effect of influenza vaccination in the elderly differing in health status. Exp. Gerontol. 2004, 39, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wallace, D.L.; de Lara, C.M.; Ghattas, H.; Asquith, B.; Worth, A.; Griffin, G.E.; Taylor, G.P.; Tough, D.F.; Beverley, P.C.; et al. In vivo kinetics of human natural killer cells: The effects of ageing and acute and chronic viral infection. Immunology 2007, 121, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Fulbright, J.W.; Crowson, C.S.; Poland, G.A.; O’Fallon, W.M.; Weyand, C.M. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 2001, 75, 12182–12187. [Google Scholar] [CrossRef] [PubMed]
- Saurwein-Teissl, M.; Lung, T.L.; Marx, F.; Gschösser, C.; Asch, E.; Blasko, I.; Parson, W.; Böck, G.; Schönitzer, D.; Trannoy, E.; et al. Lack of antibody production following immunization in old age: Association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 2002, 168, 5893–5899. [Google Scholar] [CrossRef] [PubMed]
- Allman, D.; Miller, J.P. B cell development and receptor diversity during aging. Curr. Opin. Immunol. 2005, 17, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Haynes, L.; Eaton, S.M. The effect of age on the cognate function of CD4+ T cells. The frequencies of CD8+ CD28− T cells can be used to predict influenza vaccine response in elderly people. Immunol. Rev. 2005, 205, 220–228. [Google Scholar]
- Frasca, D.; Riley, R.L.; Blomberg, B.B. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin. Immunol. 2005, 17, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Wijnans, L.; Voordouw, B. A review of the changes to the licensing of influenza vaccines in Europe. Influenza Other Respir. Viruses 2016, 10, 2–8. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Xie, D.; Hager, W.D.; Barry, M.B.; Wang, Y.; Kleppinger, A.; Ewen, C.; Kane, K.P.; Bleackley, R.C. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 2006, 176, 6333–6339. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Davis, M.M. New approaches to understanding the immune response to vaccination and infection. Vaccine 2015, 33, 5271–5281. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.X.; McElhaney, J.E.; Walston, J.D.; Xie, D.; Fedarko, N.S.; Kuchel, G.A. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Czerkinsky, C.C.; Nilsson, L.A.; Nygren, H.; Ouchterlony, O.; Tarkowski, A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 1983, 65, 109–121. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Pankow-Culot, H.; Bautista, M.; Feng, Y.; Claeys, C.; Peeters, M.; Innis, B.L.; Jain, V. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3–17 years. J. Infect. Dis. 2013, 207, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Pyhala, R.; Kleemola, M.; Kumpulainen, V.; Vartiainen, E.; Lappi, S.; Ponka, A.; Cantell, K. Immune response to inactivated influenza virus vaccine: Antibody reactivity with epidemic influenza B viruses of two highly distinct evolutionary lineages. Vaccine 1992, 10, 631–636. [Google Scholar] [CrossRef]
- Baldo, V.; Baldovin, T.; Floreani, A.; Fragapane, E.; Trivello, R.; Family Medicine, G. Response of influenza vaccines against heterovariant influenza virus strains in adults with chronic diseases. J. Clin. Immunol. 2007, 27, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Camilloni, B.; Neri, M.; Lepri, E.; Iorio, A.M. Cross-reactive antibodies in middle-aged and elderly volunteers after MF59-adjuvanted subunit trivalent influenza vaccine against B viruses of the B/Victoria or B/Yamagata lineages. Vaccine 2009, 27, 4099–4103. [Google Scholar] [CrossRef] [PubMed]
- Gresset-Bourgeois, V.; Leventhal, P.S.; Pepin, S.; Hollingsworth, R.; Kazek-Duret, M.P.; De Bruijn, I.; Samson, S.I. Quadrivalent inactivated influenza vaccine (VaxigripTetra™). Expert Rev. Vaccines 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vaxigrip Tetra. Available online: http://mri.cts-mrp.eu/Human/Product/Details/47992 (accessed on 9 December 2017).
- Vaxigrip Tetra Quadrivalent Influenza Vaccine (Split Virion, Inactivated). Available online: https://mri.ctsmrp.eu/Human/Downloads/DE_H_1949_001_PAR.pdf (accessed on 9 December 2017).
- Lu, C.Y.; Ferracin, C.; Chiu, C.H.; Lavis, N.; Huang, C.H.; Huang, L.M. Immunogenicity and safety of a quadrivalent influenza vaccine in children and adolescents in Taiwan: A phase III open-label trial. Trials Vaccinol. 2016, 5, 48–52. [Google Scholar] [CrossRef][Green Version]
- Pepin, S.; Szymanski, H.; Rochín Kobashi, I.A.; Villagomez Martinez, S.; González Zamora, J.F.; Brzostek, J.; Huang, L.M.; Chiu, C.H.; Chen, P.Y.; Ahonen, A.; et al. Safety and immunogenicity of an intramuscular quadrivalent influenza vaccine in children 3 to 8 y of age: A phase III randomized controlled study. Hum. Vaccines Immunother. 2016, 12, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Sesay, S.; Brzostek, J.; Meyer, I.; Donazzolo, Y.; Leroux-Roels, G.; Rouzier, R.; Astruc, B.; Szymanski, H.; Toursarkissian, N.; Vandermeulen, C.; et al. Safety, immunogenicity, and lot-to-lot consistency of a split-virion quadrivalent influenza vaccine in younger and older adults: A phase III randomized, double-blind clinical trial. Hum. Vaccines Immunother. 2017, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Noh, J.Y.; Lee, J.; Choi, J.Y.; Lee, J.S.; Kim, M.S.; Kim, H.S.; Bang, J.; Lavis, N.; Kim, W.J. Immunogenicity and safety of a split-virion quadrivalent influenza vaccine in adults 18–60 years of age in the Republic of Korea. Hum. Vaccines Immunother. 2017, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pépin, S.; Donazzolo, Y.; Jambrecina, A.; Salamand, C.; Saville, M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine 2013, 31, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Cadorna-Carlos, J.B.; Nolan, T.; Borja-Tabora, C.F.; Santos, J.; Montalban, M.C.; de Looze, F.J.; Eizenberg, P.; Hall, S.; Dupuy, M.; Hutagalung, Y.; et al. Safety, immunogenicity, and lot-to-lot consistency of a quadrivalent inactivated influenza vaccine in children, adolescents, and adults: A randomized, controlled, phase III trial. Vaccine 2015, 33, 2485–2492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pepin, S.; Dupuy, M.; Borja-Tabora, C.; Montellano, M.; Bravo, L.; Cadorna-Carlos, J.; Santos, J.; De Castro, J.-A.; Rivera-Medina, D.M.; Cutland, C.; et al. Efficacy, immunogenicity and safety of a quadrivalent inactivated influenza vaccine in children from 6 to 35 months. In Proceedings of the Sixth ESWI Influenza Conference, Riga, Latvia, 10–13 September 2017. [Google Scholar]
- Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf (accessed on 8 December 2017).
- Trombetta, C.M.; Gianchecchi, E.; Montomoli, E. Influenza vaccines: Evaluation of the safety profile. Hum. Vaccines Immunother. 2018, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Zhou, X.; Talbot, H.K.; Soethout, E.; Bleackley, R.C.; Granville, D.J.; Pawelec, G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012, 30, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trial Results: Immunogenicity and Lot-to-Lot Consistency Study of a Quadrivalent Influenza Vaccine in Adult and Elderly Subjects. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-000785-21/results (accessed on 8 December 2017).
- Pasteur, S.; (Rome, Italy). Personal communication, 2018.
- Guideline on Influenza Vaccines. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211324.pdf (accessed on 9 December 2017).
- Moa, A.M.; Chughtai, A.A.; Muscatello, D.J.; Turner, R.M.; MacIntyre, C.R. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: A systematic review and meta analysis of randomised controlled trials. Vaccine 2016, 34, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Risk Assessment for Seasonal Influenza, EU/EEA, 2017–2018. Available online: https://ecdc.europa.eu/sites/portal/files/documents/RRA%20seasonal%20influenza%20EU%20EEA%202017-2018.pdf (accessed on 9 December 2017).
- Uhart, M.; Bricout, H.; Clay, E.; Largeron, N. Public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines compared to trivalent influenza vaccines in Europe. Hum. Vaccines Immunother. 2016, 12, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials for Quadrivalent Vaccine Pregnant Women. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=quadrivalent+vaccine+pregnant+women (accessed on 9 December 2017).
| Ema Criteria for Influenza Vaccine Licensing | |
|---|---|
| Seroconv. Rate or Sig. Incr. % | >30.00 ELD–>40.00 ADU |
| Mean Gmt Increase | >2 ELD–>2.5 ADU |
| Seroprotection Rate % | >60.00 ELD–>70.00 ADU |
| Phase III Clinical Trials on Vaxigrip Tetra | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial Registry No. | Study Design | Type of Study | Objectives | Age | Participants | Countries | Influenza Season | Results | Ref. |
| GQM02 EudraCT No. 2011-005374-33 | Randomized, double-blind, active-controlled, multi-center study | Pivotal investigations testing the QIV licensed | Primary Objective: -Non-inferior immunogenicity of QIV to each TIV for the shared strains Secondary Objective -Humoral immune response induced by QIV -Superior immunogenicity of QIV to each TIV for the alternate B strain-Safety | 3–8 y | 1242 participants immunized with: -QIV (n = 887 *), -TIV-1 with B/Vic (n = 181 *), -an investigational TIV-2 with B/Yam (n = 174 *) * 65 subjects in the QIV group and 31 in the TIV groups were at-risk condition | Finland, Poland, Mexico and Taiwan | 2013/2014 NH | Comparable immunogenicity of QIV vs. TIVs for the shared strains, and superiority for the additional B strain. Safety of QIV. Comparable immune responses induced by QIV in at-risk subjects with underlying comorbidity respect to the overall population after vaccination with QIV | [82] |
| GQM09 WHO Universal Trial No. U1111-1127-7693 | Open-label, uncontrolled | Pivotal investigations testing the QIV licensed | Primary Objective: -Immunogenicity -Safety | 9–17 y | 100 subjects immunized with: QIV | Taiwan | 2013–2014 NH | Immunogenicity and safety demonstrated in children/adolescents | [81] |
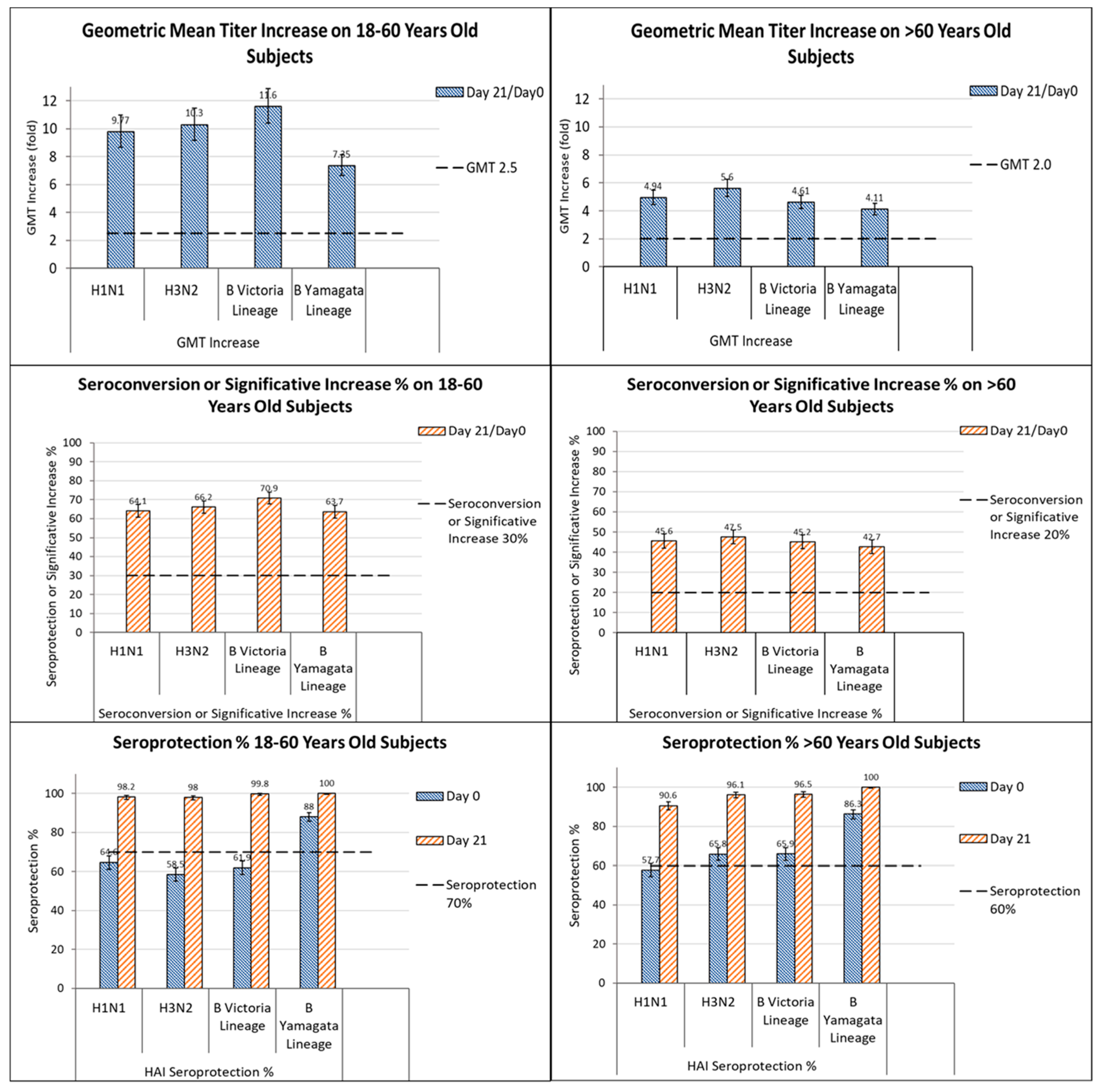
| GQM11 EudraCT No. 2014-000785-21 | Randomized, multicenter, double-blinded for the QIV and TIV-2 groups, single-blinded for the TIV-1 group | Pivotal investigations testing the QIV licensed | Primary Objective: -Similar immunogenicity of 3 QIV lots -Non-inferiority of QIV respect to TIV for the shared strains Secondary Objective -Superior immunogenicity of QIV respect to TIV for the additional strain in each age group -Safety | ≥18 y | 2225 subjects (1114 adults (18–60 y) and 1111 subjects (>60 y) randomized 2:2:2:1:1 to receive: -a single dose of one of three lots of QIV, -the licensed TIV with B/Yam, -an investigational TIV with B/Vic | Belgium, France, Germany, Poland | 2014–2015 NH | Lot-to-lot consistency demonstrated. Non-inferiority demonstrated between QIV and TIV. Higher immunogenicity of QIV against alternate B strains. Similar safety profile of QIV as the licensed TIV Comparable response in at-risk subjects | [83] |
| GQM07 NCT02550197 | Observer-blind, randomized, controlled, multicenter | Evaluation of the QIV licensed | Primary Objective: -Immunogenicity -Safety | 18–60 y | 300 subjects immunized with: -QIV (n = 200) -TIV (n = 100) | Republic of Korea | 2015–2016 NH | Immunogenicity and safety demonstrated in adults | [84] |
| GQM04 NCT01481454 | Randomized, active-controlled, multicenter, double-blind for QIV lots, open-label for QIV vs. TIV | Supportive testing QIV batches produced with the TIV process | Primary Objective: -Safety of QIV and TIV Secondary Objective -Immunogenicity of QIV accordingly with EMA criteria -Immunogenic equivalence of three lots of QIV -Immunogenicity in children/adolescents | 9–60 y | 2090 subjects: -QIV (n = 1977) (1648 adults and 329 children/adolescents) -TIV (n = 111) (56 adults and 55 children/adolescents) | Australia, Philippines | 2011/2012 NH and 2012 SH | Lot-to-lot consistency demonstrated. EMA criteria met in 9–60 years old. Comparable response and safety profiles of QIV to TIV | [86] |
| GQM01 EudraCT: 2011-001976-21 | Randomized, active-controlled, multicenter | Supportive testing QIV batches produced with the TIV process | Primary Objective: -Non-inferiority of QIV respect to a licensed TIV and an investigational TIV Secondary Objective -Safety -Superior ab response to each B strain in the QIV group respect to the corresponding B strain in TIV group lacking this strain | ≥18 y | 1565 participants: -QIV (n = 1116 subjects), -licensed TIV with B/Vic (n = 226), -the investigational TIV with B/Yam (n = 223) | France, Germany | 2011–2012 NH | Safety of QIV and superior immunogenicity of QIV respect TIV for the unmatched strains and non-inferiority for the matched strains | [85] |
| GQM05 EudraCT: 2013-001231-51 | Randomized, double-blind (except TIV groups open-label), placebo-controlled, multicenter | Pediatric investigation | Primary Objective: -Efficacy to prevent at least one of the following endpoints: • laboratory-confirmed influenza illness due to any influenza circulating strain (A or B types) • laboratory-confirmed influenza illness caused by influenza strains similar to those contained in the vaccine Secondary Objective -Non-inferiority of QIV vs. TIV as well as superior immunogenicity for B strains -Safety | 6–35 m | 5805 participants: -QIV (n = 2721 subjects), -placebo (n = 2715), -licensed TIV with B/Vic or B/Yam (n = 369) | Europe, Asia, Latin America, South Africa | 2014–2015 NH TIVYAM or TIVVIC | Preliminary results: Immunogenicity of QIV Prevention of influenza caused by strains similar to the vaccine strains and any circulating strains Protection against severe influenza illness and reduced the risk for AOM and ALRI associated with influenza Reduction of other relevant clinical outcomes for children, including medical visits and antibiotic use Safety | [87] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montomoli, E.; Torelli, A.; Manini, I.; Gianchecchi, E. Immunogenicity and Safety of the New Inactivated Quadrivalent Influenza Vaccine Vaxigrip Tetra: Preliminary Results in Children ≥6 Months and Older Adults. Vaccines 2018, 6, 14. https://doi.org/10.3390/vaccines6010014
Montomoli E, Torelli A, Manini I, Gianchecchi E. Immunogenicity and Safety of the New Inactivated Quadrivalent Influenza Vaccine Vaxigrip Tetra: Preliminary Results in Children ≥6 Months and Older Adults. Vaccines. 2018; 6(1):14. https://doi.org/10.3390/vaccines6010014
Chicago/Turabian StyleMontomoli, Emanuele, Alessandro Torelli, Ilaria Manini, and Elena Gianchecchi. 2018. "Immunogenicity and Safety of the New Inactivated Quadrivalent Influenza Vaccine Vaxigrip Tetra: Preliminary Results in Children ≥6 Months and Older Adults" Vaccines 6, no. 1: 14. https://doi.org/10.3390/vaccines6010014
APA StyleMontomoli, E., Torelli, A., Manini, I., & Gianchecchi, E. (2018). Immunogenicity and Safety of the New Inactivated Quadrivalent Influenza Vaccine Vaxigrip Tetra: Preliminary Results in Children ≥6 Months and Older Adults. Vaccines, 6(1), 14. https://doi.org/10.3390/vaccines6010014






