Adjuvant Probiotics and the Intestinal Microbiome: Enhancing Vaccines and Immunotherapy Outcomes
Abstract
1. Introduction
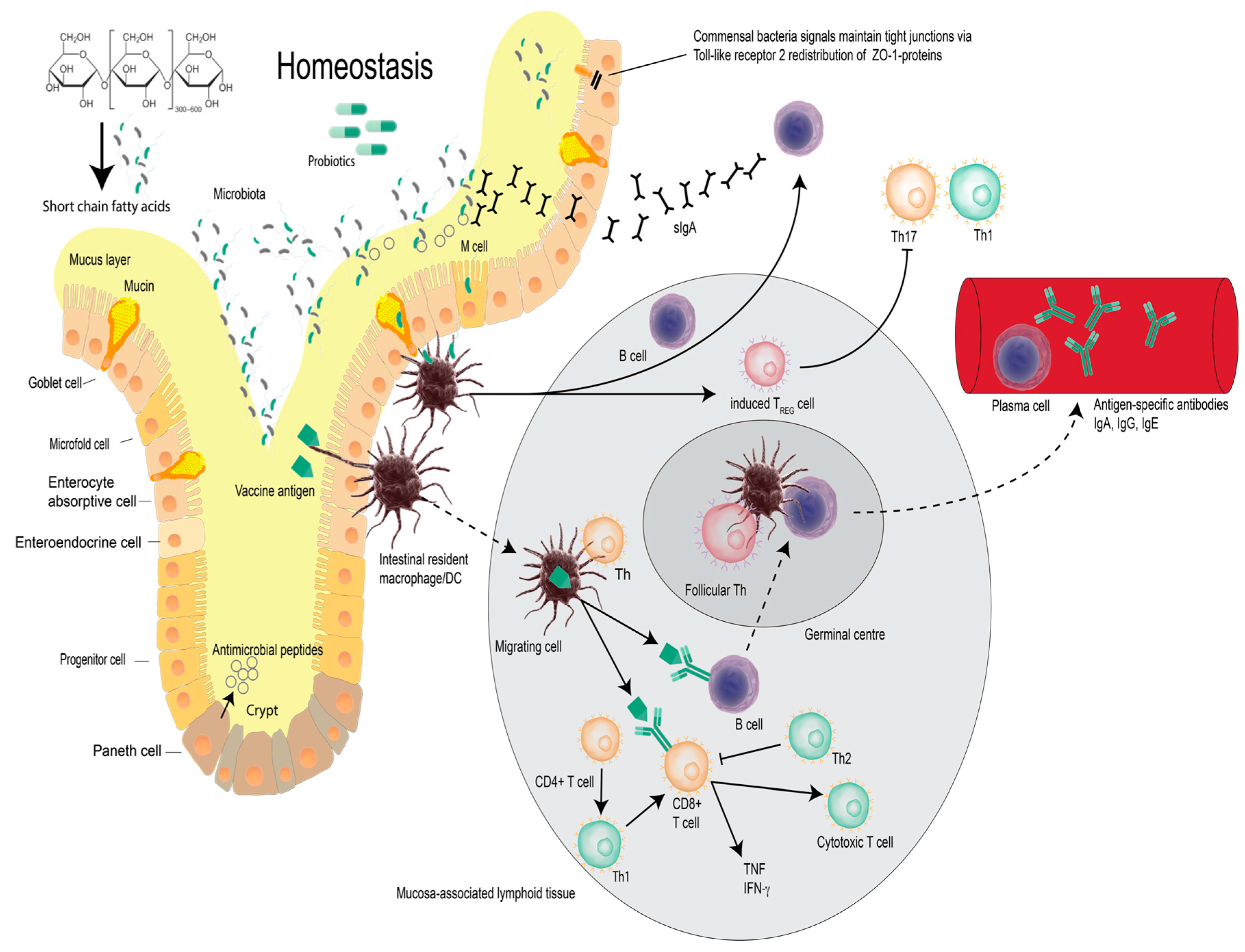
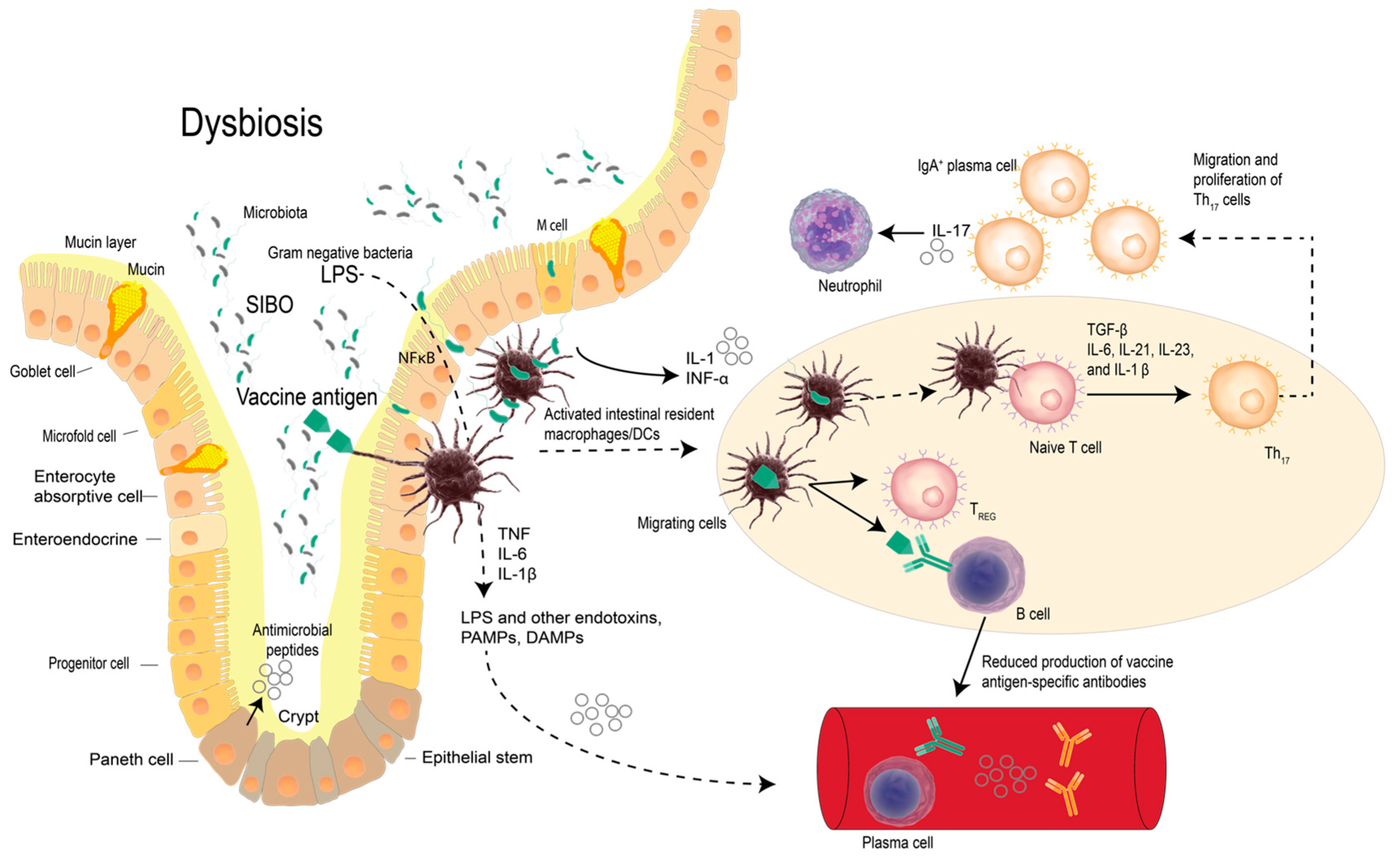
2. Vaccines, Probiotic Bacteria and Modulation of Immunity
3. Probiotics and Vaccines
3.1. Probiotics and Vaccines in Infants
3.2. Probiotics and Vaccines in Adults
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- Arbeter, A.M.; Starr, S.E.; Plotkin, S.A. Varicella vaccine studies in healthy children and adults. Pediatrics 1986, 78, 748–756. [Google Scholar] [PubMed]
- Kuter, B.J.; Weibel, R.E.; Guess, H.A.; Matthews, H.; Morton, D.H.; Neff, B.J.; Provost, P.J.; Watson, B.A.; Starr, S.E.; Plotkin, S.A. Oka/Merck varicella vaccine in healthy children: Final report of a 2-year efficacy study and 7-year follow-up studies. Vaccine 1991, 9, 643–647. [Google Scholar] [CrossRef]
- Youngster, I.; Kozer, E.; Lazarovitch, Z.; Broide, E.; Goldman, M. Probiotics and the immunological response to infant vaccinations: A prospective, placebo controlled pilot study. Arch. Dis. Child. 2011, 96, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.R.; Nemerow, G.R. The role of antibody and complement in the control of viral infections. J. Investig. Dermatol. 1984, 83, 121s–127s. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, R.; Gregori, S.; Roncarolo, M.G. Cd4+ regulatory T cells: Mechanisms of induction and effector function. Autoimmun. Rev. 2005, 4, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Igietseme, J.U.; Eko, F.O.; He, Q.; Black, C.M. Antibody regulation of tcell immunity: Implications for vaccine strategies against intracellular pathogens. Expert Rev. Vaccines 2004, 3, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Ross, P.; Stanton, C. Beneficial microbes: The pharmacy in the gut. Bioengineered 2016, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Hall, S.; Linnane, A.W. Live probiotic cultures and the gastrointestinal tract: Symbiotic preservation of tolerance whilst attenuating pathogenicity. Front. Cell. Infect. Microbiol. 2014, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Palacios, T.; Hall, S.; Coulson, S. Gastrointestinal tract commensal bacteria and probiotics: Influence on end-organ physiology. Prog. Drug Res. 2015, 70, 1–33. [Google Scholar] [PubMed]
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef. Microbes 2015, 6, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; Akbar, A.N. Convergence of innate and adaptive immunity during human aging. Front. Immunol. 2016, 7, 445. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Friedrich, U.; Vogelsang, H.; Jahreis, G. Lactobacillus acidophilus 74–2 and bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur. J. Clin. Nutr. 2008, 62, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Rutherfurd, K.J.; Prasad, J.; Gopal, P.K. Enhancement of natural and acquired immunity by lactobacillus rhamnosus (HN001), lactobacillus acidophilus (HN017) and bifidobacterium lactis (hn019). Br. J. Nutr. 2000, 83, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.-A. 2-Vaccine Immunology A2—Plotkin, Stanley A. In Vaccines, 6th ed.; Orenstein, W.A., Offit, P.A., Eds.; W.B. Saunders: London, UK, 2013; pp. 14–32. [Google Scholar]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Deng, Z.; Hou, Y.; Zhang, G. Regulation of the intestinal barrier function by host defense peptides. Front. Vet. Sci. 2015, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Best Practices for Injections and Related Procedures Toolkit; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Administraiton of Vaccines in Australia. 2016. Available online: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-home~handbook10part2~handbook10-2-2 (accessed on 1 January 2017).
- Mohr, E.; Siegrist, C.A. Vaccination in early life: Standing up to the challenges. Curr. Opin. Immunol. 2016, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.A. Neonatal and early life vaccinology. Vaccine 2001, 19, 3331–3346. [Google Scholar] [CrossRef]
- Einhorn, M.S.; Weinberg, G.A.; Anderson, E.L.; Granoff, P.D.; Granoff, D.M. Immunogenicity in infants of haemophilus influenzae type B polysaccharide in a conjugate vaccine with neisseria meningitidis outer-membrane protein. Lancet 1986, 2, 299–302. [Google Scholar] [CrossRef]
- Murasko, D.M.; Bernstein, E.D.; Gardner, E.M.; Gross, P.; Munk, G.; Dran, S.; Abrutyn, E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp. Gerontol. 2002, 37, 427–439. [Google Scholar] [CrossRef]
- Hainz, U.; Jenewein, B.; Asch, E.; Pfeiffer, K.P.; Berger, P.; Grubeck-Loebenstein, B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 2005, 23, 3232–3235. [Google Scholar] [CrossRef] [PubMed]
- Artz, A.S.; Ershler, W.B.; Longo, D.L. Pneumococcal vaccination and revaccination of older adults. Clin. Microbiol. Rev. 2003, 16, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; De Groot, R.; Hermans, P.W. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Bubnov, R.V.; Spivak, M.Y.; Lazarenko, L.M.; Bomba, A.; Boyko, N.V. Probiotics and immunity: Provisional role for personalized diets and disease prevention. EPMA J. 2015, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.K.; Iwasaki, A. Control of antiviral immunity by pattern recognition and the microbiome. Immunol. Rev. 2012, 245, 209–226. [Google Scholar] [CrossRef] [PubMed]
- GeurtsvanKessel, C.H.; Willart, M.A.; van Rijt, L.S.; Muskens, F.; Kool, M.; Baas, C.; Thielemans, K.; Bennett, C.; Clausen, B.E.; Hoogsteden, H.C.; et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J. Exp. Med. 2008, 205, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Stoeker, L.; Nordone, S.; Gunderson, S.; Zhang, L.; Kajikawa, A.; LaVoy, A.; Miller, M.; Klaenhammer, T.R.; Dean, G.A. Assessment of lactobacillus gasseri as a candidate oral vaccine vector. Clin. Vaccine Immunol. 2011, 18, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Cheng, P.C.; Liao, J.W.; Pan, T.M. Effect of the administration of lactobacillus paracasei subsp. Paracasei NTU 101 on peyer‘s patch-mediated mucosal immunity. Int. Immunopharmacol. 2010, 10, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.F.; Masterson, J.C.; Furuta, G.T. Eosinophils, probiotics, and the microbiome. J. Leukoc. Biol. 2016, 100, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Hajela, N.; Nair, G.B.; Ganguly, N.K. Are probiotics a feasible intervention for prevention of diarrhoea in the developing world? Gut Pathog. 2010, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Sjogren, Y.M.; Persson, J.O.; Nilsson, C.; Sverremark-Ekstrom, E. Early colonization with a group of lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS ONE 2011, 6, e23031. [Google Scholar] [CrossRef] [PubMed]
- Westerholm-Ormio, M.; Vaarala, O.; Tiittanen, M.; Savilahti, E. Infiltration of Foxp3- and Toll-like receptor-4-positive cells in the intestines of children with food allergy. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.; Lahtinen, S.J.; Boyle, R.J. Probiotics and prebiotics: Clinical effects in allergic disease. Curr. Opin. Pediatr. 2010, 22, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Dwivedi, D.; Roy, P.; Kushwah, S.; Singh, V. Probiotics: Multifarious oral vaccine against infectious traumas. FEMS Immunol. Med. Microbiol. 2010, 58, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Hall, S.; Coulson, S. Metabolic Interactions in the Gastrointestinal Tract (GIT): Host, commensal, probiotics, and bacteriophage influences. Microorganisms 2015, 3, 913–932. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.B.; Antunes, L.C.; Finlay, B.B. Should the human microbiome be considered when developing vaccines? PLoS Pathog. 2010, 6, e1001190. [Google Scholar] [CrossRef] [PubMed]
- Valdez, Y.; Brown, E.M.; Finlay, B.B. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014, 35, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Brezar, V.; Godot, V.; Cheng, L.; Su, L.; Levy, Y.; Seddiki, N. T-regulatory cells and vaccination “pay attention and do not neglect them”: Lessons from HIV and cancer vaccine trials. Vaccines 2016, 4, E30. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M. Immunogenicity and efficacy of oral vaccines in developing countries: Lessons from a live cholera vaccine. BMC Biol. 2010, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Korpe, P.S.; Petri, W.A., Jr. Environmental enteropathy: Critical implications of a poorly understood condition. Trends Mol. Med. 2012, 18, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Isolauri, E.; Joensuu, J.; Suomalainen, H.; Luomala, M.; Vesikari, T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by lactobacillus casei GG. Vaccine 1995, 13, 310–312. [Google Scholar] [CrossRef]
- Matsuda, F.; Chowdairy, M.I.; Saha, A.; Asahara, T.; Nomoto, K.; Tarique, A.A.; Ahmed, T.; Nishibuchi, M.; Cravioto, A.; Qadri, F. Evaluation of a probiotics, bifidobacterium breve BBG-01, for enhancement of immunogenicity of an oral inactivated cholera vaccine and safety: A randomized, double-blind, placebo-controlled trial in bangladeshi children under 5 years of age. Vaccine 2011, 29, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Pérez, N.; Iannicelli, J.C.; Girard-Bosch, C.; González, S.; Varea, A.; Disalvo, L.; Apezteguia, M.; Pernas, J.; Vicentin, D.; Cravero, R. Effect of probiotic supplementation on immunoglobulins, isoagglutinins and antibody response in children of low socio-economic status. Eur. J. Nutr. 2010, 49, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Ismail, I.H.; Balloch, A.; Mui, M.; Hoe, E.; Lamb, K.; Tang, M.L. Maternal supplementation with lgg reduces vaccine-specific immune responses in infants at high-risk of developing allergic disease. Front. Immunol. 2013, 4, 381. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Gothefors, L.; Granström, M.; Käyhty, H.; Hammarström, M.L.; Hernell, O. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatr. Allergy Immunol. 2008, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Hale, J.; Wiltschut, J.; Lehmann, H.; Dunstan, J.A.; Prescott, S.L. Evaluation of the effects of probiotic supplementation from the neonatal period on innate immune development in infancy. Clin. Exp. Allergy 2006, 36, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.E.; Ong, D.Q.; Gerez, I.; Zhang, X.; Chollate, P.; Shek, L.P.; Lee, B.W.; Aw, M. Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant hepatitis B vaccination. Vaccine 2010, 28, 2577–2579. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, K.; Nieminen, T.; Poussa, T.; Savilahti, E.; Kuitunen, M. Effect of probiotics on vaccine antibody responses in infancy—A randomized placebo-controlled double-blind trial. Pediatr. Allergy Immunol. 2006, 17, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Olson, S.; Kalina, W.V.; Ruthel, G.; Demmin, G.L.; Warfield, K.L.; Bavari, S.; Klaenhammer, T.R. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 2005, 102, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Mastrangeli, G.; Corinti, S.; Butteroni, C.; Afferni, C.; Bonura, A.; Boirivant, M.; Colombo, P.; Di Felice, G. Effects of live and inactivated VSL#3 probiotic preparations in the modulation of in vitro and in vivo allergen-induced Th2 responses. Int. Arch. Allergy Immunol. 2009, 150, 133–143. [Google Scholar] [PubMed]
- Gibson, R.A.; Barclay, D.; Marshall, H.; Moulin, J.; Maire, J.C.; Makrides, M. Safety of supplementing infant formula with long-chain polyunsaturated fatty acids and bifidobacterium lactis in term infants: A randomised controlled trial. Br. J. Nutr. 2009, 101, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Link-Amster, H.; Rochat, F.; Saudan, K.Y.; Mignot, O.; Aeschlimann, J.M. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol. Med. Microbiol. 1994, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Elina, T.; Heikki, A.; Seppo, S. Modulation of humoral immune response through probiotic intake. FEMS Immunol. Med. Microbiol. 2000, 29, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bunout, D.; Barrera, G.; Hirsch, S.; Gattas, V.; de la Maza, M.P.; Haschke, F.; Steenhout, P.; Klassen, P.; Hager, C.; Avendano, M.; et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living chilean elderly. J. Parenter. Enter. Nutr. 2004, 28, 348–354. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Rautenberg, P.; Laue, C.; Koopmans, M.; Herremans, T.; Schrezenmeir, J. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur. J. Nutr. 2005, 44, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Diaz-Ropero, M.P.; Sierra, S.; Lara-Villoslada, F.; Fonolla, J.; Navas, M.; Rodriguez, J.M.; Xaus, J. Oral intake of lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition 2007, 23, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.A.; Simoneau, G.; Bergmann, J.F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Boge, T.; Remigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; van der Werf, S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.E.; Fiorino, A.M.; Snydman, D.R.; Hibberd, P.L. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: A randomized double-blind placebo-controlled trial. Eur. J. Clin. Nutr. 2011, 65, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Van Puyenbroeck, K.; Hens, N.; Coenen, S.; Michiels, B.; Beunckens, C.; Molenberghs, G.; Van Royen, P.; Verhoeven, V. Efficacy of daily intake of lactobacillus casei shirota on respiratory symptoms and influenza vaccination immune response: A randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am. J. Clin. Nutr. 2012, 95, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains bifidobacterium animalis ssp. Lactis, BB-12(R) and lactobacillus paracasei ssp. Paracasei, l. Casei 431(R) in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Mendez, M.; Perez, M.; Farran, A.; Fuentes, M.C.; Cune, J. Lactobacillus plantarum CECT7315 and CECT7316 stimulate immunoglobulin production after influenza vaccination in elderly. Nutr. Hosp. 2012, 27, 504–509. [Google Scholar] [PubMed]
- Akatsu, H.; Arakawa, K.; Yamamoto, T.; Kanematsu, T.; Matsukawa, N.; Ohara, H.; Maruyama, M. Lactobacillus in jelly enhances the effect of influenza vaccination in elderly individuals. J. Am. Geriatr. Soc. 2013, 61, 1828–1830. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, H.; Iwabuchi, N.; Xiao, J.-Z.; Matsuyama, Z.; Kurihara, R.; Okuda, K.; Yamamoto, T.; Maruyama, M. Clinical effects of probiotic bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. J. Parenter. Enter. Nutr. 2013, 37, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Tarnow, I.; Eskesen, D.; Morberg, C.M.; Michelsen, B.; Bugel, S.; Dragsted, L.O.; Rijkers, G.T.; Calder, P.C. Effect of lactobacillus paracasei subsp. Paracasei, l. Casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. Am. J. Clin. Nutr. 2015, 101, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Abe, R.; Shimono, T.; Iwabuchi, N.; Abe, F.; Xiao, J.Z. The effects of non-viable lactobacillus on immune function in the elderly: A randomised, double-blind, placebo-controlled study. Int. J. Food Sci. Nutr. 2016, 67, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Urdaci, M.C.; Margolles, A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa-bacteria interactions. Microbiology 2010, 156, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L. Lactobacillus acidophilus restores functionality in uremic macrophages: Plausible or lacking evidence? Dig. Dis. Sci. 2016, 61, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Ivec, M.; Botic, T.; Koren, S.; Jakobsen, M.; Weingartl, H.; Cencic, A. Interactions of macrophages with probiotic bacteria lead to increased antiviral response against vesicular stomatitis virus. Antivir. Res. 2007, 75, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Tuohy, K.M.; Thomas, L.V.; Yaqoob, P. Selective effects of lactobacillus casei shirota on t cell activation, natural killer cell activity and cytokine production. Clin. Exp. Immunol. 2010, 161, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Maidens, C.; Childs, C.; Przemska, A.; Dayel, I.B.; Yaqoob, P. Modulation of vaccine response by concomitant probiotic administration. Br. J. Clin. Pharmacol. 2013, 75, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.T.; Shih, P.C.; Liu, S.J.; Lin, C.Y.; Yeh, T.L. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Miyahara, Y.; Wang, H.Y. Toll-like receptors and immune regulation: Implications for cancer therapy. Oncogene 2008, 27, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Peng, G.; Wang, H.Y. Regulatory t cells and toll-like receptors in tumor immunity. Semin. Immunol. 2006, 18, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Vetizou, M.; Waldschmitt, N.; Kroemer, G.; Chamaillard, M.; Boneca, I.G.; Zitvogel, L. Fine-tuning cancer immunotherapy: Optimizing the gut microbiome. Cancer Res. 2016, 76, 4602–4607. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2017. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2017. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferrau, F.; Libra, M. Lactobacillus rhamnosus GG: An overview to explore the rationale of its use in cancer. Front. Pharmacol. 2017, 8, 603. [Google Scholar] [CrossRef] [PubMed]
| Summary of Probiotic Adjuvant Effects to Vaccines | |||
|---|---|---|---|
| Probiotic(s) | Method | Vaccine (Strain) | Biological Effect |
| L. casei GG (LGG) | 5 × 1010 CFU b.i.d. at vaccination and for 1-week following | Oral rotavirus vaccine | Increase in rotavirus-specific IgM antibody secreting cells
Significant rotavirus IgA and IgM seroconversion [49] Improved immunogenicity |
| Streptococcus thermophilus (control) vs. S. thermophilus and L. casei strain CRL431, L. acidophilus strain CRL730, oligofructose and inulin (test product) | Daily CFU doses:
95 × 108 for 16-week for control 95 × 108, 95 × 106 and 95 × 106 for respective test product strains | DTP-Hib/23-valent anti-pneumococcal vaccine | No difference between groups in antibody levels neither before nor after vaccination [51] Less hygienic environment reported Immunogenicity not improved |
| L. rhamnosus GG | 1.8 × 1010 CFU q.d. from 36-week gestation until birth
* maternal administration | DTaP, Hib, PCV7 vaccines | Decreased TT response in infants, decrease PCV response for some. Nil change in Hib/Treg [52] Immunogenicity not improved |
| L. paracasei ssp. paracasei strain F19 | 1 × 108 CFU q.d. for 39-week | DTaP, polio and Hib vaccines | Probiotic enhanced anti-diptheria antibody titres in infants breastfed for less than six months [53] Early life effect (first 6-months) Improved immunogenicity |
| L. acidophilus LAVRIA1 | 3 × 109 CFU q.d. for 26-week | Parenteral tetanus vaccine | Lower IL-10 responses to tetanus antigen in probiotic group [54] Early life effect (first 6-months) Improved immunogenicity |
| B. breve BBG-01 | 4 × 109 CFU b.i.d. for 17-week | Oral cholera vaccine | Significantly lower responders and higher serum-LPS specific IgA in probiotic group and no difference in the vibriocidal antibodies [50] Similar immunogenicity responses, possible effect on intestinal microbiome |
| L. acidophilus ATCC4356 B. bifidum DSMZ20081 B. longum ATCC157078 B. infantis ATCC15697 | 3 × 109 CFU q.d. for 20-week | MMRV vaccine | No difference in vaccine specific IgG antibody titres. Higher proportion reached protective IgG antibody titres in 3 month post-vaccination period in probiotic group [4] Improved immunogenicity |
| B. longum BL999 L. rhamnosus LPR | Total CFU q.d. 2.8 × 108 for 26-week | Hep B vaccine at 1-month and DTPa/HepB vaccine at 6-months | Group treated with probiotics showed a trend towards increased antiHbsAg in infants given probiotic for six months [55] Early life effect (first 6-months) Improved immunogenicity |
| L. rhamnosus GG ATCC53103 L. rhamnosus LG705 B. breve Bbi99 P. freundenreichii ssp. shermanii JS | 5 × 109 CFU
5 × 109 CFU 2 × 108 CFU 2 × 109 CFU 1 capsule b.i.d. to mothers for last gestation month and 1 capsule q.d. + 0.8 g galacto-oligosaccharides newborns for first 24-week | DTwP vaccine/Hib conjugate | Higher frequency of Hib-specific IgG antibody response and a trend for higher Hib-specific IgG GMT [56] Improved immunogenicity |
| Summary of Probiotic Adjuvant Effects to Vaccines | |||
|---|---|---|---|
| Probiotic(s) | Method | Vaccine (Strain) | Biological Effect |
| S. thermophilus, mesophilic streptococci, bifidobacteria Bb12, commercial mixed culture and L. acidophilus La1 | 1 × 107–108 CFU/g of both La1 and Bifidobacteria throughout study period (3-week) | Salmonella typhi Ty21a | Greater increase in vaccine-specific serum IgA antibody titre in probiotic vs. control group [60] Improved immunogenicity |
| L. rhamnosus GG OR L. lactis OR Placebo | 4 × 1010 CFU q.d. for 1-week
OR 3.4 × 1010 CFU q.d. for 1-week OR Ethyl cellulose for 1-week | Attenuated Salmonella typhi Ty21a oral vaccine | Greater increase in specific IgA in LGG group.
L. lactis group had significantly higher expression of CR3 receptor [61] Improved immunogenicity |
| L. paracasei (NCC 2461) | 1 × 109 CFU + 6 g fructo-oligosaccharide daily for 52-week | Parental trivalent influenza vaccine | NK activity increased and less infections reported by supplemented group. Increased innate immunity and protection against infections in supplemented elderly [62] Improved immunogenicity (innate immunity) |
| L. rhamnosus GG OR L. acidophilus CRL431 OR Placebo | 1010 CFU/serving q.d. for 5-week OR
100 g/day acidified milk without probiotics | Live attenuated poliomyelitis vaccine | Probiotic group reported increased poliovirus neutralizing antibody titers and poliovirus-specific serum IgA and IgG in probiotic group [63] Improved immunogenicity |
| L. fermentum CECT5716 | 1 × 1010 CFU containing capsule q.d. for 4-week | Inactivated trivalent influenza vaccine | Probiotic increased vaccine-specific IgA antibodies post-vaccination. Incidence of influenza-like illnesses for 5 months post-vaccination lower in the probiotic group [64] Improved immunogenicity |
| B. lactis (Bi-07 or B1-04) OR L. acidophilus (La-14 or NCFM) ORL. plantarum Lp-115 OR L. paracasei Lpc-37 OR L. salivarius Ls-33 | 1 × 1010 CFU/capsule b.i.d. for 3-week | Oral cholera vaccine | Significant changes in serum Ig concentrations in 6 out of 7 probiotic strains compared to control [65] Improved immunogenicity |
| L. paracasei subsp. paracasei DN-114 001 | 1010 CFU/bottle b.i.d.
pilot study: 7-week confirmatory study:13-week | Parental trivalent influenza vaccine | Influenza-specific antibody titres increased in probiotic group post-vaccination with significantly greater seroconversion rate for B strain in confirmatory study [66] Improved immunogenicity |
| L. rhamnosus GG | 1 × 1010 CFU + 295 mg Inulin b.i.d. for 4-week | Live-attentuated nasal influenza (LAIV) | Protection against H1N1 and B strain vaccine similar for placebo and probiotic group. H3N2 strain showed increased protective titer for LGG group [67] Improved immunogenicity |
| L. casei Shirota | 1.3 × 1010 CFU q.d. for 176 days | Trivalent influenza vaccine | No statistically or clinically significant of
LcS on protection against respiratory symptoms or improvement in seroprotection rates [68] No improved immunogenicity |
| B. animalis ssp. lactis BB-12 OR L. paracasei ssp. paracasei L. casei 431 | 1 × 109 CFU q.d. for 6-week | Parental trivalent influenza vaccine | Significantly greater increase in vaccine-specific IgG antibody titre and mean-fold increases for vaccine-specific secretory IgA antibody in probiotic group [69] Improved immunogenicity |
| Lactobacillus plantarum CECT7315/7316 | Group A: 5 × 109 CFU q.d. for 12-week
Group B: 5 × 108 CFU q.d. for 12-week | Trivalent influenza vaccine | Consumption of probiotics for 3-mo following vaccination increased influenza-specific IgA and IgH antibody levels. Increasing trend in IgM antibodies also observed [70] Improved immunogenicity |
| Heat–killed L. casei | 1 × 1012 CFU/daily dose for 12-week | Trivalent influenza vaccine | IgG, IgM, IgA did not change significantly in either group. HI titers against all 3 antigens significantly higher in probiotic group than baseline whereas only HI titers against A/H3N2 higher in placebo. Seroconversion rate against influenza antigens not statistically significant [71] No improved immunogenicity |
| B. longum BB536 | 5 × 1010 CFU/2 g sachet b.i.d. for 12-week, with 4-week additional follow-up | Trivalent influenza vaccine | Increase in IgA in probiotic group compared to placebo at wk 16. No significant effect on HI titers in probiotic group [72] Beneficial shift in intestinal microbiome Improved immunogenicity |
| L. casei 431® | 109 CFU q.d. 42 days | Trivalent influenza vaccine | Immune responses of probiotic group showed no effect but reported significantly shorter respiratory symptom durations (no differences for symptom incidence or severity) [73] No improved immunogenicity |
| L. paracasei MCC1849 | 109 CFU q.d. for 6-week | Trivalent influenza vaccine | No significant differences in immune parameters between the groups. In oldest of the old subgroup (≥85 y.o) antibody responses to A/H1N1 and B antigens improved only in probiotic group. No significant effects of non-viable L. paracasei MCC1849 observed [74] Partial improved immunogenicity |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitetta, L.; Saltzman, E.T.; Thomsen, M.; Nikov, T.; Hall, S. Adjuvant Probiotics and the Intestinal Microbiome: Enhancing Vaccines and Immunotherapy Outcomes. Vaccines 2017, 5, 50. https://doi.org/10.3390/vaccines5040050
Vitetta L, Saltzman ET, Thomsen M, Nikov T, Hall S. Adjuvant Probiotics and the Intestinal Microbiome: Enhancing Vaccines and Immunotherapy Outcomes. Vaccines. 2017; 5(4):50. https://doi.org/10.3390/vaccines5040050
Chicago/Turabian StyleVitetta, Luis, Emma Tali Saltzman, Michael Thomsen, Tessa Nikov, and Sean Hall. 2017. "Adjuvant Probiotics and the Intestinal Microbiome: Enhancing Vaccines and Immunotherapy Outcomes" Vaccines 5, no. 4: 50. https://doi.org/10.3390/vaccines5040050
APA StyleVitetta, L., Saltzman, E. T., Thomsen, M., Nikov, T., & Hall, S. (2017). Adjuvant Probiotics and the Intestinal Microbiome: Enhancing Vaccines and Immunotherapy Outcomes. Vaccines, 5(4), 50. https://doi.org/10.3390/vaccines5040050






