Prophylactic Sublingual Immunization with Mycobacterium tuberculosis Subunit Vaccine Incorporating the Natural Killer T Cell Agonist Alpha-Galactosylceramide Enhances Protective Immunity to Limit Pulmonary and Extra-Pulmonary Bacterial Burden in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Reagents
2.3. Immunization
2.4. IFN-γ ELISpot Assay
2.5. Intracellular Cytokine Production Assay
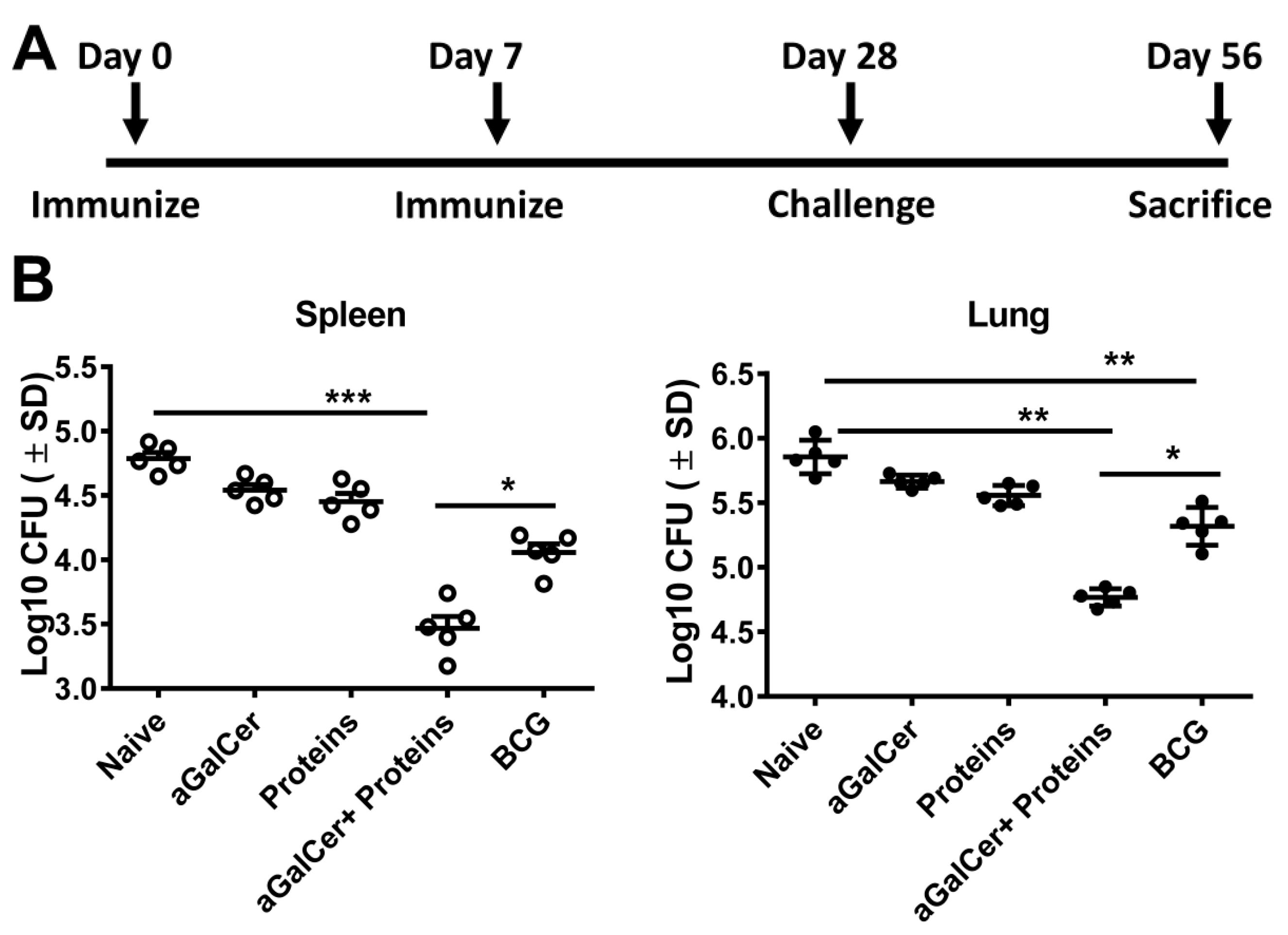
2.6. Mycobacterium Tuberculosis Challenge
2.7. Statistical Analysis
3. Results
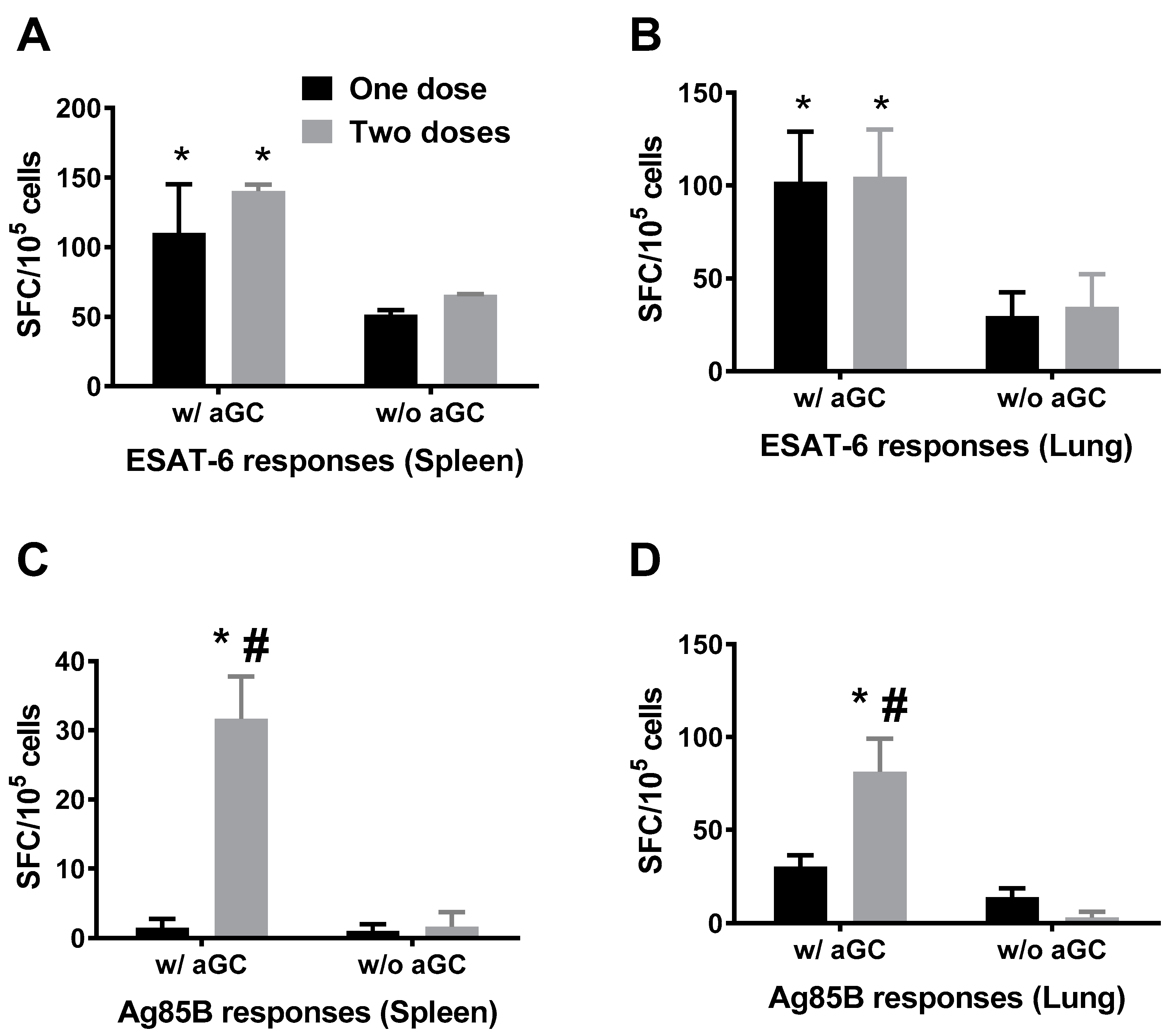
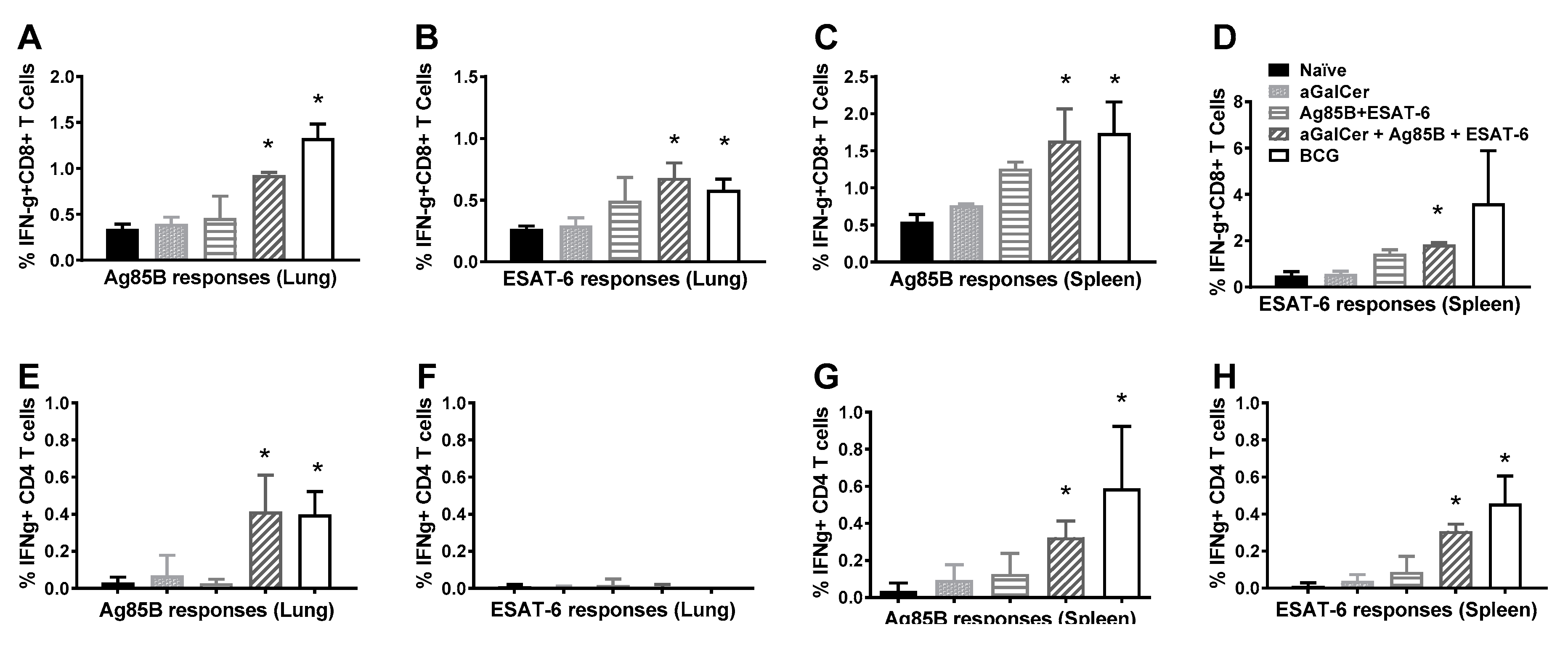
3.1. Immunogenicity of Sublingual Mtb Subunit Vaccination
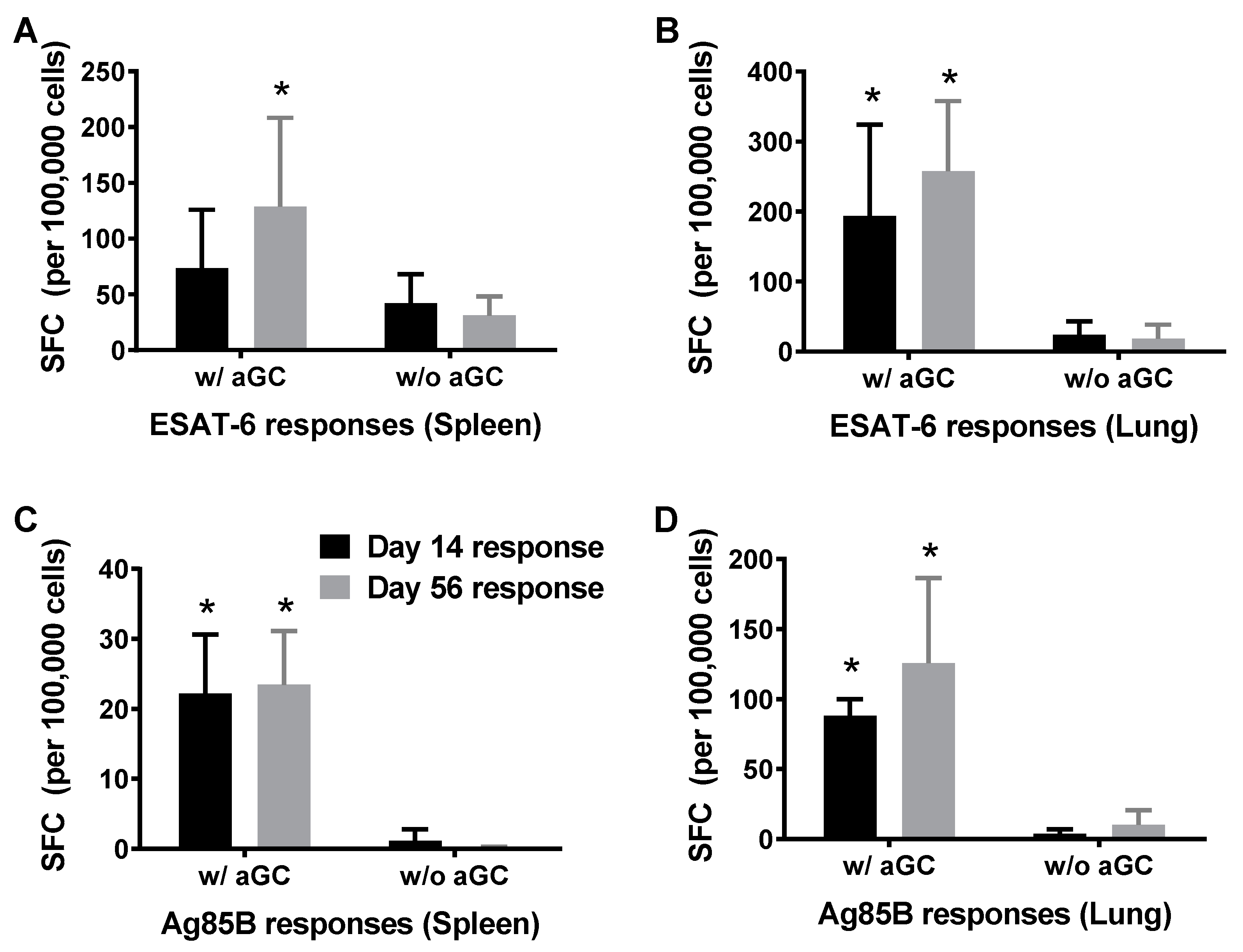
3.2. Persistence of Immunity from Sublingual Vaccination with Mtb Proteins and α-GalCer Adjuvant
3.3. Sublingual Vaccination with Mtb Proteins and α-GalCer Adjuvant Affords Protection against Lung Mtb Challenge
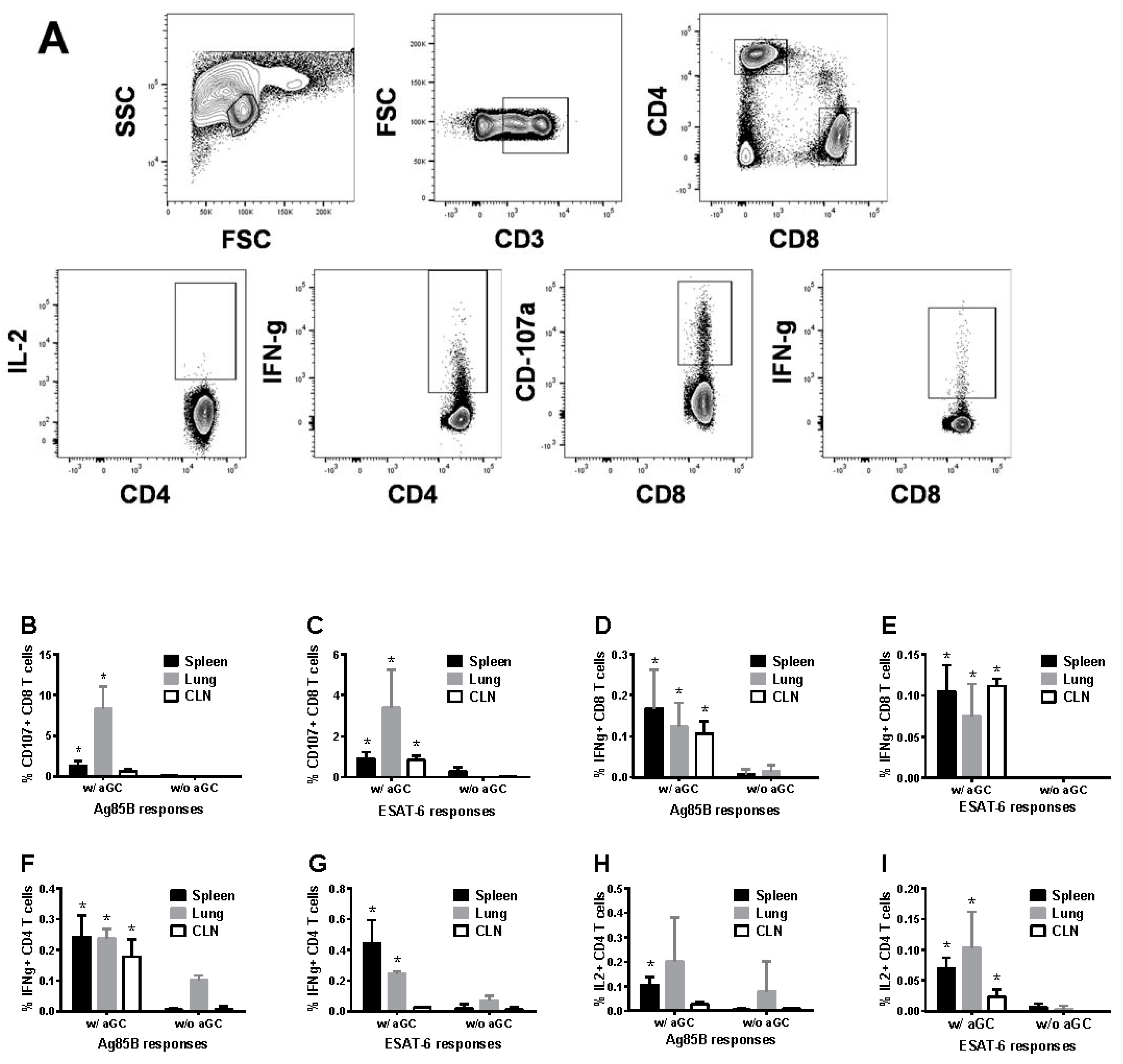
3.4. Sublingual Vaccination Induces T Cell Responses which Correlate with Protection against Lung Mtb Challenge
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2017. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 1 October 2017).
- Adigun, R.; Bhimji, S.S. Tuberculosis. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2017. [Google Scholar]
- Kay, A.; Barry, P.M.; Annambhotla, P.; Greene, C.; Cilnis, M.; Chin-Hong, P.; Arger, N.; McNitt, L.; Neidlinger, N.; Shah, N.; et al. Solid organ transplant-transmitted tuberculosis linked to a community outbreak—California, 2015. Am. J. Transplant. 2017, 17, 2733–2736. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, E.; Greco, A.; Estrada, S.; Cisneros, M.; Colombo, C.; Beltrame, S.; Boncompain, C.; Genero, S. Risk factors associated with tuberculosis mortality in adults in six provinces of argentina. Medicina 2017, 77, 267–273. [Google Scholar] [PubMed]
- Zumla, A.; George, A.; Sharma, V.; Herbert, R.H.N.; Oxley, A.; Oliver, M. The who 2014 global tuberculosis report—Further to go. Lancet Glob. Health 2015, 3, e10–e12. [Google Scholar] [CrossRef]
- Kwan, C.K.; Ernst, J.D. Hiv and tuberculosis: A deadly human syndemic. Clin. Microbiol. Rev. 2011, 24, 351–376. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.M.; Fronteira, I.; Couto, I.; Machado, D.; Viveiros, M.; Abecasis, A.B.; Dias, S. HIV and tuberculosis co-infection among migrants in europe: A systematic review on the prevalence, incidence and mortality. PLoS ONE 2017, 12, e0185526. [Google Scholar] [CrossRef] [PubMed]
- Brewer, T.F. Preventing tuberculosis with bacillus calmette-guerin vaccine: A meta-analysis of the literature. Clin. Infect. Dis. 2000, 31, S64–S67. [Google Scholar] [CrossRef] [PubMed]
- Zenteno-Cuevas, R. Successes and failures in human tuberculosis vaccine development. Expert Opin. Biol. Ther. 2017, 17, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Hesseling, A.C.; Rabie, H.; Marais, B.J.; Manders, M.; Lips, M.; Schaaf, H.S.; Gie, R.P.; Cotton, M.F.; van Helden, P.D.; Warren, R.M.; et al. Bacille Calmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin. Infect. Dis. 2006, 42, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.W.; Williams, A.; Okkels, L.M.; Hatch, G.; Andersen, P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85b and esat-6 in the aerosol guinea pig model. Infect. Immun. 2004, 72, 6148–6150. [Google Scholar] [CrossRef] [PubMed]
- Weinrich Olsen, A.; van Pinxteren, L.A.; Meng Okkels, L.; Birk Rasmussen, P.; Andersen, P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect. Immun. 2001, 69, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Bean, A.G.; Roach, D.R.; Briscoe, H.; France, M.P.; Korner, H.; Sedgwick, J.D.; Britton, W.J. Structural deficiencies in granuloma formation in tnf gene-targeted mice underlie the heightened susceptibility to aerosol mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immun. 1999, 162, 3504–3511. [Google Scholar] [PubMed]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Czerkinsky, C.; Holmgren, J. Mucosal delivery routes for optimal immunization: Targeting immunity to the right tissues. Curr. Top. Microbiol. Immunol. 2012, 354, 1–18. [Google Scholar] [PubMed]
- Patel, H.; Yewale, C.; Rathi, M.N.; Misra, A. Mucosal immunization: A review of strategies and challenges. Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 273–303. [Google Scholar] [CrossRef]
- Woodrow, K.A.; Bennett, K.M.; Lo, D.D. Mucosal vaccine design and delivery. Annu. Rev. Biomed. Eng. 2012, 14, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Gowda, D.V.; Madhunapantula, S.V.; Shinde, C.G.; Iyer, M. Mucosal vaccines: A paradigm shift in the development of mucosal adjuvants and delivery vehicles. APMIS 2015, 123, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Yang, G.; Schluns, K.S.; Anthony, S.M.; Sastry, K.J. Sublingual vaccination induces mucosal and systemic adaptive immunity for protection against lung tumor challenge. PLoS ONE 2014, 9, e90001. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Svennerholm, A.M. Vaccines against mucosal infections. Curr. Opin. Immunol. 2012, 24, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Shimizu, K.; Hemmi, H.; Fukui, M.; Bonito, A.J.; Chen, G.; Franck, R.W.; Tsuji, M.; Steinman, R.M. Glycolipid alpha-c-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc. Natl. Acad. Sci. USA 2006, 103, 11252–11257. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Horiguchi, S.; Tanaka, Y.; Yamamoto, H.; Kunii, N.; Motohashi, S.; Taniguchi, M.; Nakayama, T.; Okamoto, Y. Phase i study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol. Immunother. 2008, 57, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhou, A.; Meng, M.; Wang, L.; Han, Y.; Guo, J.; Zhou, H.; Cong, H.; Zhao, Q.; Zhu, X.Q.; et al. Alpha-galactosylceramide enhances protective immunity induced by DNA vaccine of the sag5d gene of toxoplasma gondii. BMC Infect. Dis. 2014, 14, 3862. [Google Scholar] [CrossRef] [PubMed]
- Courtney, A.N.; Thapa, P.; Singh, S.; Wishahy, A.M.; Zhou, D.; Sastry, J. Intranasal but not intravenous delivery of the adjuvant alpha-galactosylceramide permits repeated stimulation of natural killer T cells in the lung. Eur. J. Immunol. 2011, 41, 3312–3322. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Godfrey, D.I.; Smyth, M.J. Alpha-galactosylceramide: Potential immunomodulatory activity and future application. Curr. Med. Chem. 2004, 11, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, M.; Persson, J.; Thorn, K.; Harandi, A.M. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J. Immunol. 2009, 182, 6435–6443. [Google Scholar] [CrossRef] [PubMed]
- Courtney, A.N.; Nehete, P.N.; Nehete, B.P.; Thapa, P.; Zhou, D.; Sastry, K.J. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of hiv peptide antigens. Vaccine 2009, 27, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Cuburu, N.; Kweon, M.N.; Song, J.H.; Hervouet, C.; Luci, C.; Sun, J.B.; Hofman, P.; Holmgren, J.; Anjuere, F.; Czerkinsky, C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 2007, 25, 8598–8610. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nehete, P.N.; Yang, G.; He, H.; Nehete, B.; Hanley, P.W.; Barry, M.A.; Sastry, K.J. Enhancement of mucosal immunogenicity of viral vectored vaccines by the nkt cell agonist alpha-galactosylceramide as adjuvant. Vaccines 2014, 2, 686–706. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, A.J.; Gruppo, V.; Brooks, J.V.; Orme, I.M. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 2003, 47, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Elias, E.J.; Timm, J.; Botha, T.; Chan, W.T.; Gomez, J.E.; McKinney, J.D. Replication dynamics of mycobacterium tuberculosis in chronically infected mice. Infect. Immun. 2005, 73, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Kitamura, H.; Iwakabe, K.; Yahata, T.; Ohta, A.; Sato, M.; Takeda, K.; Okumura, K.; Van Kaer, L.; Kawano, T.; et al. The interface between innate and acquired immunity: Glycolipid antigen presentation by cd1d-expressing dendritic cells to nkt cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 2000, 12, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Burdin, N.; Brossay, L.; Koezuka, Y.; Smiley, S.T.; Grusby, M.J.; Gui, M.; Taniguchi, M.; Hayakawa, K.; Kronenberg, M. Selective ability of mouse cd1 to present glycolipids: Alpha-galactosylceramide specifically stimulates v alpha 14+ nk T lymphocytes. J. Immunol. 1998, 161, 3271–3281. [Google Scholar] [PubMed]
- Sada-Ovalle, I.; Skold, M.; Tian, T.; Besra, G.S.; Behar, S.M. Alpha-galactosylceramide as a therapeutic agent for pulmonary mycobacterium tuberculosis infection. Am. J. Respir. Crit. Care Med. 2010, 182, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, X.; Barnes, P.F.; Tang, H.; Townsend, J.C.; Samten, B. The Mycobacterium tuberculosis Early Secreted Antigenic Target of 6 kDa Inhibits T Cell Interferon-γ Production through the p38 Mitogen-activated Protein Kinase Pathway. J. Biol. Chem. 2011, 286, 24508–24518. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.-R.; Choi, J.-H.; Shin, S.J.; Park, J.-H. Mycobacterium tuberculosis ESAT6 induces IFN-β gene expression in Macrophages via TLRs-mediated signaling. Cytokine 2017. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.A.; Johndrow, J.E.; Manzanillo, P.; Cox, J.S. The Type I IFN Response to Infection with Mycobacterium tuberculosis Requires ESX-1-Mediated Secretion and Contributes to Pathogenesis. J. Immunol. 2007, 178, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Iwakabe, K.; Yahata, T.; Nishimura, S.; Ohta, A.; Ohmi, Y.; Sato, M.; Takeda, K.; Okumura, K.; Van Kaer, L.; et al. The natural killer T (nkt) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (il)-12 production by dendritic cells and il-12 receptor expression on nkt cells. J. Exp. Med. 1999, 189, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hong, S.; Scherer, D.C.; Serizawa, I.; Burdin, N.; Kronenberg, M.; Koezuka, Y.; Van Kaer, L. Cutting edge: Activation of nk T cells by cd1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 1999, 163, 2373–2377. [Google Scholar] [PubMed]
- Kronenberg, M.; Gapin, L. The unconventional lifestyle of nkt cells. Nat. Rev. Immunol. 2002, 2, 557–568. [Google Scholar] [PubMed]
- Chackerian, A.; Alt, J.; Perera, V.; Behar, S.M. Activation of nkt cells protects mice from tuberculosis. Infect. Immun. 2002, 70, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Kee, S.J.; Kwon, Y.S.; Park, Y.W.; Cho, Y.N.; Lee, S.J.; Kim, T.J.; Lee, S.S.; Jang, H.C.; Shin, M.G.; Shin, J.H.; et al. Dysfunction of natural killer T cells in patients with active mycobacterium tuberculosis infection. Infect. Immun. 2012, 80, 2100–2108. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Motoki, K.; Akimoto, K.; Natori, T.; Sakai, T.; Sawa, E.; Yamaji, K.; Koezuka, Y.; Kobayashi, E.; Fukushima, H. Structure-activity relationship ofAlpha-galactosylceramides against b16-bearing mice. J. Med. Chem. 1995, 38, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Kakimi, K.; Guidotti, L.G.; Koezuka, Y.; Chisari, F.V. Natural killer T cell activation inhibits hepatitis b virus replication in vivo. J. Exp. Med. 2000, 192, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aseguinolaza, G.; Van Kaer, L.; Bergmann, C.C.; Wilson, J.M.; Schmieg, J.; Kronenberg, M.; Nakayama, T.; Taniguchi, M.; Koezuka, Y.; Tsuji, M. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 2002, 195, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Nguyen, H.H.; Cuburu, N.; Horimoto, T.; Ko, S.Y.; Park, S.H.; Czerkinsky, C.; Kweon, M.N. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Paget, C.; Trottein, F. Role of type 1 natural killer T cells in pulmonary immunity. Mucosal Immunol. 2013, 6, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Rothchild, A.C.; Jayaraman, P.; Nunes-Alves, C.; Behar, S.M. iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathog. 2014, 10, e1003805. [Google Scholar] [CrossRef] [PubMed]
- Middendorp, S.; Nieuwenhuis, E.E. Nkt cells in mucosal immunity. Mucosal Immunol. 2009, 2, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G.; Brawand, P.; MacDonald, H.R. Selective bystander proliferation of memory cd4+ and cd8+ T cells upon nk T or T cell activation. J. Immunol. 2000, 165, 4305–4311. [Google Scholar] [CrossRef] [PubMed]
- Orme, I.M. The achilles heel of bcg. Tuberculosis 2010, 90, 329–332. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Singh, S.; Galvan, G.; Jagannath, C.; Sastry, K.J. Prophylactic Sublingual Immunization with Mycobacterium tuberculosis Subunit Vaccine Incorporating the Natural Killer T Cell Agonist Alpha-Galactosylceramide Enhances Protective Immunity to Limit Pulmonary and Extra-Pulmonary Bacterial Burden in Mice. Vaccines 2017, 5, 47. https://doi.org/10.3390/vaccines5040047
Khan A, Singh S, Galvan G, Jagannath C, Sastry KJ. Prophylactic Sublingual Immunization with Mycobacterium tuberculosis Subunit Vaccine Incorporating the Natural Killer T Cell Agonist Alpha-Galactosylceramide Enhances Protective Immunity to Limit Pulmonary and Extra-Pulmonary Bacterial Burden in Mice. Vaccines. 2017; 5(4):47. https://doi.org/10.3390/vaccines5040047
Chicago/Turabian StyleKhan, Arshad, Shailbala Singh, Gloria Galvan, Chinnaswamy Jagannath, and K. Jagannadha Sastry. 2017. "Prophylactic Sublingual Immunization with Mycobacterium tuberculosis Subunit Vaccine Incorporating the Natural Killer T Cell Agonist Alpha-Galactosylceramide Enhances Protective Immunity to Limit Pulmonary and Extra-Pulmonary Bacterial Burden in Mice" Vaccines 5, no. 4: 47. https://doi.org/10.3390/vaccines5040047
APA StyleKhan, A., Singh, S., Galvan, G., Jagannath, C., & Sastry, K. J. (2017). Prophylactic Sublingual Immunization with Mycobacterium tuberculosis Subunit Vaccine Incorporating the Natural Killer T Cell Agonist Alpha-Galactosylceramide Enhances Protective Immunity to Limit Pulmonary and Extra-Pulmonary Bacterial Burden in Mice. Vaccines, 5(4), 47. https://doi.org/10.3390/vaccines5040047





