Anti-IgE Qb-VLP Conjugate Vaccine Self-Adjuvants through Activation of TLR7
Abstract
:1. Introduction
2. Material and Methods
2.1. Vaccine Antigens
2.2. Adjuvants
2.3. In Vitro Stimulation of Human Immune Cells and IFN-α ELISA
2.4. Animals
2.5. Immunization of Mice
2.6. Anti-IgE/Qb Ab ELISA
2.7. Statistical Analysis
3. Results and Discussion
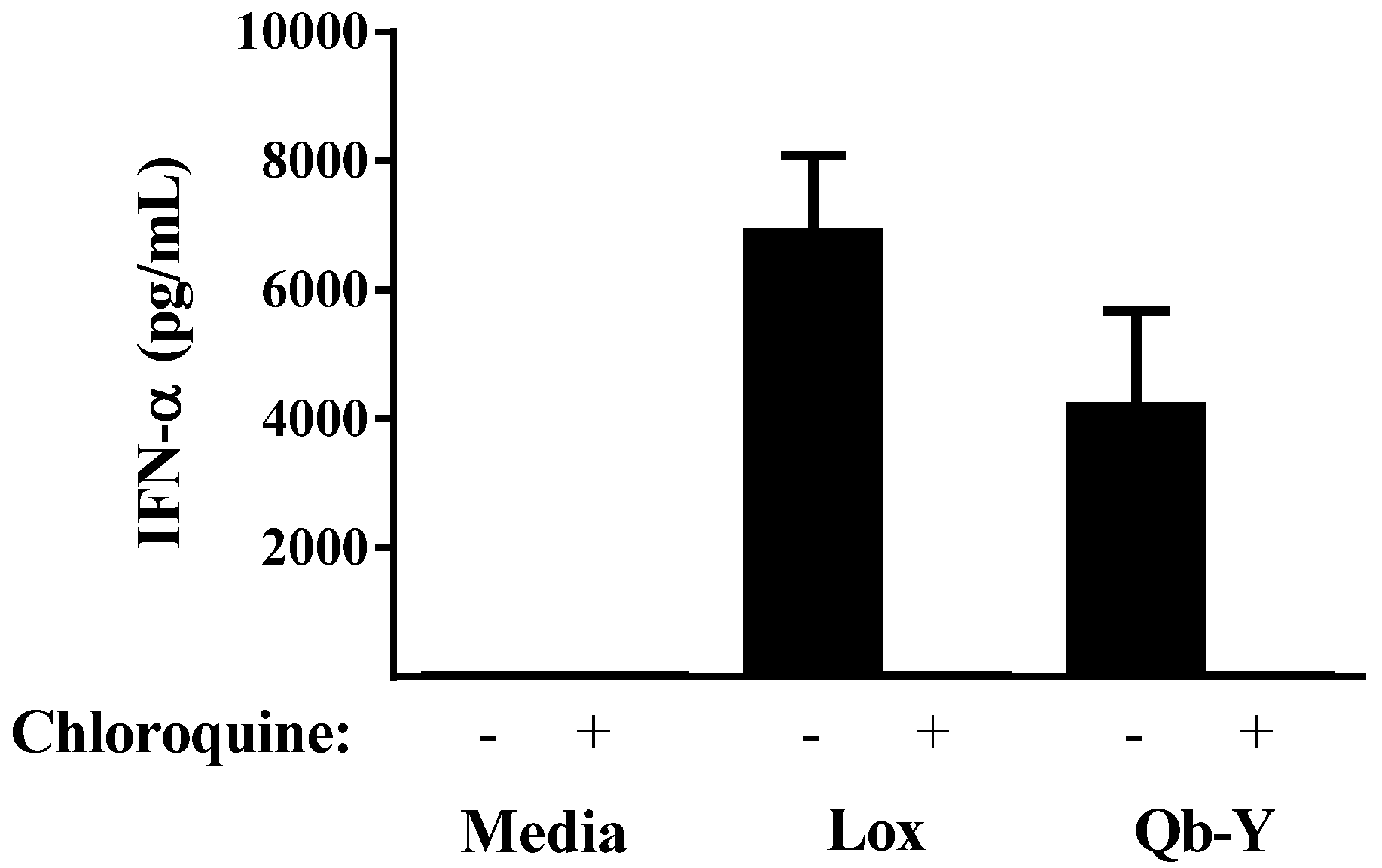
3.1. Qb-VLPs Induce IFN-α Secretion in Human PBMCs
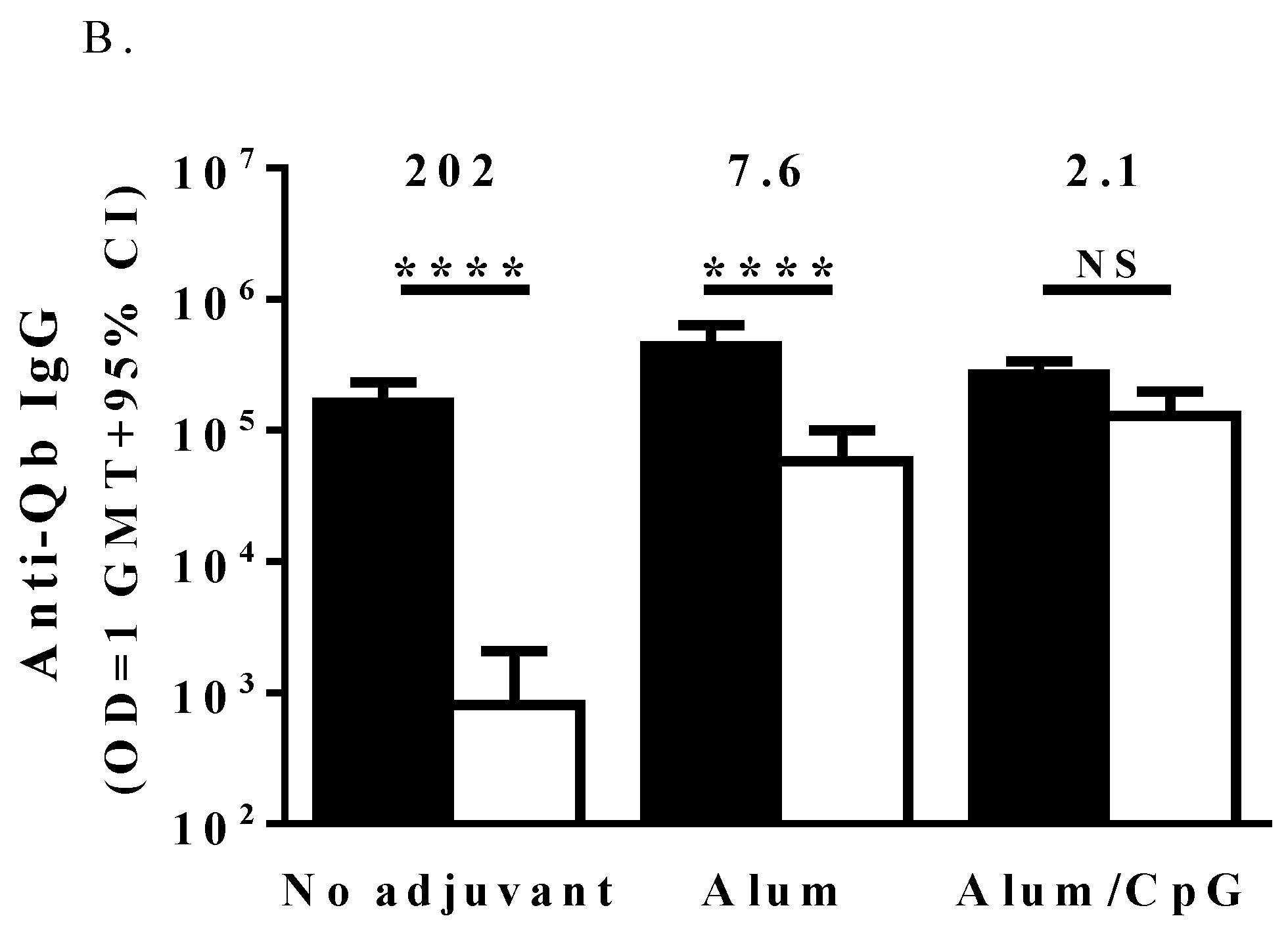
3.2. Immune Responses to the IgE Peptide Qb-VLP Conjugates in WT Mice

3.3. TLR7 Activity Mediates the Induction of Anti-IgE Immune Responses by the IgE Peptide Qb-VLP Conjugates
| Mouse Strain | Vaccine Formulation | IgG1 | IgG2c | IgG2c/IgG1 | |||
|---|---|---|---|---|---|---|---|
| GMT | 95% CI | GMT | 95% CI | GMT | 95% CI | ||
| WT | Unadjuvanted | 130 | 58.9–287.2 | 2528 | 1567.6–4075.4 | 19.43 | 7.75–48.73 |
| Alum | 1010 | 571.7–1785.5 | 3408 | 2124.1–5469.4 | 3.37 | 1.56–7.28 | |
| Alum/CpG | 797 | 351–1809.6 | 7440 | 4599.5–12,034.2 | 9.33 | 4.57–19.09 | |
| TLR7 KO | Unadjuvanted | 144 | 44.6–465.1 | 11 | 9.2–12.6 | 0.07 | 0.02–0.23 |
| Alum | 3185 | 1666.5–6087.9 | 92 | 28.1–304.1 | 0.03 | 0.01–0.08 | |
| Alum/CpG | 2859 | 1689.8–4835.5 | 2183 | 939.4–4635.2 | 0.76 | 0.43–1.37 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bachmann, M.F.; Jennings, G.T. Therapeutic vaccines for chronic diseases: Successes and technical challenges. Philos. Trans. R. Soc. Lond B Biol. Sci. 2011, 366, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Cornuz, J.; Zwahlen, S.; Jungi, W.F.; Osterwalder, J.; Klingler, K.; van Melle, G.; Bangala, Y.; Guessous, I.; Muller, P.; Willers, J.; et al. A vaccine against nicotine for smoking cessation: A randomized controlled trial. PLoS ONE 2008, 3, e2547. [Google Scholar] [CrossRef] [PubMed]
- Tissot, A.C.; Maurer, P.; Nussberger, J.; Sabat, R.; Pfister, T.; Ignatenko, S.; Volk, H.D.; Stocker, H.; Muller, P.; Jennings, G.T.; et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: A double-blind, randomised, placebo-controlled phase IIa study. Lancet 2008, 371, 821–827. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Rohrer, U.H.; Kundig, T.M.; Burki, K.; Hengartner, H.; Zinkernagel, R.M. The influence of antigen organization on B cell responsiveness. Science 1993, 262, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.P.; Ling, C.M.; Overby, L.R. Self-assembly of Q-beta and MS2 phage particles: Possible function of initiation complexes. Science 1969, 166, 1638–1640. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Winter, G.; Vogt, L.; Zurcher, A.; Dorigo, B.; Schimmele, B. Rational design of a stable, freeze-dried virus-like particle-based vaccine formulation. Drug Dev. Ind. Pharm. 2009, 35, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.; Kopf, M.; Bachmann, M.F. Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J. Immunol. 2010, 184, 4615–4619. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Beerli, R.R.; Bauer, M.; Jegerlehner, A.; Dietmeier, K.; Maudrich, M.; Pumpens, P.; Saudan, P.; Bachmann, M.F. Universal vaccine against influenza virus: Linking TLR signaling to anti-viral protection. Eur. J. Immunol. 2012, 42, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Champion, B.R.; Stead, D.R.; Wright, P. IGE CH3 Peptide Vaccine. U.S. Patent 8722053, 8 December 2011. [Google Scholar]
- Zheng, L.; Li, B.; Qian, W.; Zhao, L.; Cao, Z.; Shi, S.; Gao, J.; Zhang, D.; Hou, S.; Dai, J.; et al. Fine epitope mapping of humanized anti-IgE monoclonal antibody omalizumab. Biochem. Biophys. Res. Commun. 2008, 375, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Weeratna, R.; Payette, P.; Jurk, M.; Schetter, C.; Laucht, M.; Wader, T.; Tluk, S.; Liu, M.; Davis, H.L.; et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004, 34, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.D.; Bola, G.; Lee, A.C.; MacLachlan, I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 2006, 13, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Ahmad-Nejad, P.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Gellert, T.; Dietrich, H.; Lipford, G.; Takeda, K.; Akira, S.; et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 2003, 33, 2987–2997. [Google Scholar] [CrossRef] [PubMed]
- Beignon, A.S.; McKenna, K.; Skoberne, M.; Manches, O.; Dasilva, I.; Kavanagh, D.G.; Larsson, M.; Gorelick, R.J.; Lifson, J.D.; Bhardwaj, N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 2005, 115, 3265–3275. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Takeuchi, O.; Kawai, T.; Hoshino, K.; Akira, S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 2001, 166, 5688–5694. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Thorn, J.; Gervais, D.P.; Stead, D.R.; Zhang, N.; Benoit, M.; Cartier, J.; Kim, I.J.; Bhattacharya, K.; Finneman, J.I.; et al. Anti-nicotine vaccines: Comparison of adjuvanted CRM and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int. Immunopharmacol. 2015, 29, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.S.; Buza, J.J.; Potter, A.; Babiuk, L.A.; Mutwiri, G.K. Co-stimulation with TLR7/8 and TLR9 agonists induce down-regulation of innate immune responses in sheep blood mononuclear and B cells. Dev. Comp. Immunol. 2010, 34, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Jegerlehner, A.; Maurer, P.; Bessa, J.; Hinton, H.J.; Kopf, M.; Bachmann, M.F. TLR9 signaling in B cells determines class switch recombination to IgG2a. J. Immunol. 2007, 178, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Brazolot Millan, C.L.; Weeratna, R.; Krieg, A.M.; Siegrist, C.A.; Davis, H.L. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15553–15558. [Google Scholar] [CrossRef] [PubMed]
- Ambuhl, P.M.; Tissot, A.C.; Fulurija, A.; Maurer, P.; Nussberger, J.; Sabat, R.; Nief, V.; Schellekens, C.; Sladko, K.; Roubicek, K.; et al. A vaccine for hypertension based on virus-like particles: Preclinical efficacy and phase I safety and immunogenicity. J. Hypertens. 2007, 25, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Cytos Biotechnology Ltd. Cytos Biotechnology Updates on the Development of the Hypertension Vaccine CYT006-AngQb. Cytos_Press_E_091110. 11-10-2009. Available online: http://cytos.com/uploads/news/id127/Cytos_Press_E_091110.pdf (accessed on 20 January 2016).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akache, B.; Weeratna, R.D.; Deora, A.; Thorn, J.M.; Champion, B.; Merson, J.R.; Davis, H.L.; McCluskie, M.J. Anti-IgE Qb-VLP Conjugate Vaccine Self-Adjuvants through Activation of TLR7. Vaccines 2016, 4, 3. https://doi.org/10.3390/vaccines4010003
Akache B, Weeratna RD, Deora A, Thorn JM, Champion B, Merson JR, Davis HL, McCluskie MJ. Anti-IgE Qb-VLP Conjugate Vaccine Self-Adjuvants through Activation of TLR7. Vaccines. 2016; 4(1):3. https://doi.org/10.3390/vaccines4010003
Chicago/Turabian StyleAkache, Bassel, Risini D. Weeratna, Aparna Deora, Jennifer M. Thorn, Brian Champion, James R. Merson, Heather L. Davis, and Michael J. McCluskie. 2016. "Anti-IgE Qb-VLP Conjugate Vaccine Self-Adjuvants through Activation of TLR7" Vaccines 4, no. 1: 3. https://doi.org/10.3390/vaccines4010003
APA StyleAkache, B., Weeratna, R. D., Deora, A., Thorn, J. M., Champion, B., Merson, J. R., Davis, H. L., & McCluskie, M. J. (2016). Anti-IgE Qb-VLP Conjugate Vaccine Self-Adjuvants through Activation of TLR7. Vaccines, 4(1), 3. https://doi.org/10.3390/vaccines4010003






