Engineering Universal Cancer Immunity: Non-Tumor-Specific mRNA Vaccines Trigger Epitope Spreading in Cold Tumors
Abstract
1. Introduction: The Evolution of mRNA-Based Cancer Immunotherapy
1.1. Current Landscape, Limitations, and Comparisons
1.2. Paradigm Shift: Non-Tumor-Specific Immune Activation
2. Mechanistic Foundation: Innate Immunity and Epitope Spreading
2.1. Tumor Immune Phenotypes and Microenvironment Reprogramming
2.2. Detailed Molecular Cascade of Epitope Spreading
2.3. Key Cellular Players in Epitope-Spreading Initiation
3. Alternative Immune Activation Pathways Beyond Type I Interferons
3.1. Inflammasome-Mediated Immunity and Pathway Cross-Talk
3.2. Metabolic Reprogramming Integration
| Metabolic Pathway | Target Enzyme/Protein | mRNA Encoding Strategy | Immune Effects | Validation Status | References |
|---|---|---|---|---|---|
| Glycolysis | HK2, PFKFB3 | Constitutively active forms | Enhanced T-cell effector function | Preclinical proof of concept | [37] |
| Oxidative phosphorylation | PGC-1α, TFAM | Mitochondrial biogenesis factors | Memory T-cell formation | Mouse models validated | [38] |
| Fatty acid oxidation | CPT1A (mutant) | Malonyl-CoA resistant | Sustained T-cell responses | In vitro validation | [39] |
| One-carbon metabolism | MTHFD2, SHMT2 | Folate cycle enzymes | T-cell proliferation | Early development | [40] |
| Amino acid metabolism | CAT-1, ASCT2 | Nutrient transporters | Overcome TME depletion | Preclinical testing | [36] |
| NAD+ metabolism | NAMPT, NMNAT | NAD+ synthesis | Prevent exhaustion | Clinical biomarker | [41] |
3.3. Tissue-Resident Memory Programming
4. Platform Design and Antigen Selection
4.1. Addressing Pre-Existing Immunity Challenges
- Modified Pathogen Antigens Rather than using native pathogen sequences, engineered variants can evade pre-existing immunity while retaining immunogenicity. For the SARS-CoV-2 spike protein, specific modifications include structural modifications such as removal of dominant neutralizing epitopes, retention of conserved T cell epitopes, introduction of stabilizing mutations, and codon optimization [48].
- Consensus and Chimeric Sequences Consensus antigens incorporating epitopes from multiple pathogen strains reduce the likelihood of complete neutralization by any individual’s pre-existing immunity [49].
- Synthetic Immunogens: Completely synthetic antigens designed through computational approaches eliminate concerns about pre-existing immunity [50].
4.2. Advanced Antigen Selection Criteria
| Antigen Category | Specific Example | HLA Coverage (%) | Safety Profile | PRR Activation | Manufacturing Score | Clinical Status | References |
|---|---|---|---|---|---|---|---|
| Modified viral proteins | SARS-CoV-2 Spike (modified) | 85–90 | Proven in billions | TLR7/8, RIG-I | High | Phase II trials | [48,54] |
| Consensus viral proteins | Influenza HA consensus | 80–85 | Decades of use | TLR7/8, RIG-I | High | Phase I completed | [55] |
| Modified bacterial antigens | Flagellin (modified) | 75–80 | Clinical trials | TLR5, NLRC4 | Medium | Phase I ongoing | [24] |
| Bacterial heat shock proteins | HSP70 (low homology) | 70–75 | Preclinical safety | TLR2/4 | Medium | Preclinical | [56] |
| Synthetic multi-epitope | Computationally designed | > 90 | In development | Multiple | High | Design phase | [50,57] |
| Pathogen-associated proteins | Modified OmpA | 75–85 | Preclinical | TLR2/4 | Medium | Research | [58] |
4.3. mRNA Design and Optimization
5. Delivery System Optimization: Beyond Lipid Nanoparticles
5.1. Addressing LNP Limitations: Liver Tropism and Alternative Systems
- Selective Organ-Targeting Lipids Next-generation ionizable lipids have been engineered with tissue-specific targeting capabilities [64].
- Transient Stealth Coating Strategies Two-armed polyethylene glycol (PEG) anchoring to the liver sinusoidal wall represents an innovative approach to redirect nanomedicine distribution [65].
- Alternative Administration Routes: Dosing and administration strategies can minimize liver first-pass effects [66].
5.2. Promising Polymer-Based Alternative Delivery Systems
5.3. RNase Resistance Strategies
| Delivery System | Advantages | Limitations | RNase Protection | Liver Avoidance | Manufacturing | Clinical Status | References |
|---|---|---|---|---|---|---|---|
| Traditional LNPs | Proven efficacy, FDA approved | Liver tropism, inflammation | High (>95%) | Low | Established | Clinical use | [74] |
| Targeted LNPs | Organ-specific delivery | Complex synthesis, cost | High (>95%) | Moderate–High | Development | Phase I | [64] |
| PEI Systems | Enhanced escape, versatile | Potential toxicity concern | Moderate (70–85%) | High | Scalable | Phase I | [67] |
| PBAE Polymers | Biodegradable, tunable | Limited clinical data | Moderate (70–85%) | High | Emerging | Preclinical | [68] |
| Chitosan Systems | Natural, immunostimulatory | Variable quality, consistency | Low (50–70%) | High | Established | Preclinical | [69] |
| Hybrid Systems | Combined advantages | Complexity, characterization | High (85–95%) | Moderate | Research | Research | [75] |
6. Translational Considerations and Clinical Development
6.1. Interspecies Scaling and Human Dose Prediction
6.2. Considerations for Slow-Growing Human Tumors
7. Preclinical Validation Results
7.1. Immunogenicity and Safety Profile
7.2. Evidence of Epitope Spreading
7.3. Synergy with Checkpoint Inhibitors
7.4. Safety and Biodistribution Analysis
8. Comparison with Current Approaches
8.1. Personalized Neoantigen Vaccines
8.2. Shared Antigen Vaccines
8.3. Cytokine-Encoding mRNA Vaccines
9. Clinical Translation Strategy
9.1. Regulatory Pathway and Guidelines
9.2. Phase I Clinical Trial Design
9.3. Biomarker Strategy and Correlative Studies
10. Manufacturing and Global Access Considerations
10.1. Scalable Manufacturing Platform
10.2. Cost-Effectiveness and Health Economics
10.3. Stability and Storage Solutions
11. Challenges and Future Directions
11.1. Key Challenges and Mitigation Strategies
11.2. Future Research Priorities
11.3. Long-Term Vision and Goals
12. Conclusions
12.1. Scientific Foundation and Mechanistic Innovation
12.2. Technological Solutions and Clinical Translation
12.3. Global Health Impact and Access Solutions
12.4. Limitations and Future Development Needs
12.5. Future Research Priorities and Innovation Opportunities
12.6. Concluding Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Mendez-Gomez, H.R.; DeVries, A.; Castillo, P.; von Roemeling, C.; Qdaisat, S.; Stover, B.D.; Xie, C.; Weidert, F.; Zhao, C.; Moor, R.; et al. RNA aggregates harness the danger response for potent cancer immunotherapy. Cell 2024, 187, 2521–2535. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, K.; Lazzaro, S.; Lutz, J.; Rauch, S.; Heidenreich, R. mRNA cancer vaccines. Recent Results Cancer Res. 2016, 209, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Qdaisat, S.; Wummer, B.; Stover, B.D.; Zhang, D.; McGuiness, J.; Weidert, F.; Chardon-Robles, J.; Grippin, A.; DeVries, A.; Zhao, C.; et al. Sensitization of tumours to immunotherapy by boosting early type-I interferon responses enables epitope spreading. Nat. Biomed. Eng. 2025. [Google Scholar] [CrossRef] [PubMed]
- UF Health. Surprising Finding Could Pave Way for Universal Cancer Vaccine; UF Health News: Gainesville, FL, USA, 2025. [Google Scholar]
- Lanese, N. ‘Universal’ Cancer Vaccine Heading to Human Trials Could be Useful for ‘All Forms of Cancer’; Live Science: New York City, NY, USA, 2025. [Google Scholar]
- Bassani-Sternberg, M.; Bräunlein, E.; Klar, R.; Engleitner, T.; Sinitcyn, P.; Audehm, S.; Straub, M.; Weber, J.; Slotta-Huspenina, J.; Specht, K.; et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 2016, 7, 13404. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. Npj Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Fritsch, E.F.; Burkhardt, U.E.; Hacohen, N.; Wu, C.J. Personal Neoantigen Cancer Vaccines: A Road Not Fully Paved. Cancer Immunol. Res. 2020, 8, 1465–1469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. 2015, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Vanderlugt, C.L.; Miller, S.D. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef]
- Andrews, L.P.; Yano, H.; Vignali, D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat. Immunol. 2020, 20, 1425–1434. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Lugrin, J.; Martinon, F. Detection of cytosolic nucleic acids in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2024, 24, 487–502. [Google Scholar] [CrossRef]
- Yamasaki, S.; Ishikawa, E.; Sakuma, M.; Hara, H.; Ogata, K.; Saito, T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 2008, 9, 1179–1188. [Google Scholar] [CrossRef]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Q.; Hu, Y.; He, B.; Cao, T.; Tang, Y.; Zhou, X.P.; Lan, X.P.; Liu, S.Q. Advances in the interaction of glycolytic reprogramming with lactylation. Biomed. Pharmacother. 2023, 177, 116982. [Google Scholar] [CrossRef]
- Bourquin, C.; Anz, D.; Zwiorek, K.; Lanz, A.-L.; Fuchs, S.; Weigel, S.; Wurzenberger, C.; von der Borch, P.; Golic, M.; Moder, S.; et al. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J. Immunol. 2008, 181, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.B.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Speake, C.; Whalen, E.; Chaussabel, D.; Castro, M.G. Copy number loss of the interferon gene cluster in melanomas is linked to reduced T cell infiltrate and poor patient prognosis. PLoS ONE 2014, 9, e109760. [Google Scholar] [CrossRef] [PubMed]
- Zhivaki, D.; Borriello, F.; Chow, O.A.; Doran, B.; Fleming, I.; Theisen, D.J.; Pallis, P.; Shalek, A.K.; Sokol, C.L.; Zanoni, I.; et al. Inflammasomes within Hyperactive Murine Dendritic Cells Stimulate Long-Lived T Cell-Mediated Anti-tumor Immunity. Cell Rep. 2020, 33, 108381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kellermann, G.; Leulliot, N.; Cherfils-Vicini, J.; Blaud, M.; Brest, P. Activated B-cells enhance epitope spreading to support successful cancer immunotherapy. Front. Immunol. 2024, 15, 1382236. [Google Scholar] [CrossRef]
- Tong, G.; Shen, Y.; Li, H.; Qian, H.; Tan, Z. NLRC4 inflammasome in inflammatory bowel disease and cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 65, 1–12. [Google Scholar] [CrossRef]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020, 76, 100924. [Google Scholar] [CrossRef]
- Karki, R.; Man, S.M.; Kanneganti, T.D. Inflammasomes and Cancer. Cancer Immunol 2017, 5, 94–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Cytokine release syndrome in cancer immunotherapy: Mechanisms and man-agement. Cancer Treat. Rev. 2024, 122, 102652. [Google Scholar] [CrossRef]
- Robinson, K.S.; Toh, G.A.; Rozario, P.; Chua, R.; Bauernfried, S.; Sun, Z.; Firdaus, M.J.; Bayat, S.; Nadkarni, R.; Poh, Z.S.; et al. ZAKα-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science 2022, 377, 328–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, C.M.; Vu, T.T.; Nguyen, M.N.; Tran-Nguyen, T.-S.; Huynh, C.T.; Ha, Q.T.; Nguyen, H.-N.; Tran, L.S. Neoantigen-based mRNA vaccine exhibits superior anti-tumor activity compared to synthetic long peptides in an in vivo lung carcinoma model. Cancer Immunol. Immunother. 2025, 74, 145. [Google Scholar] [CrossRef]
- Biswas, S.K. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity 2015, 43, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino acid metabolism in immune cells: Essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Steinert, E.M.; Vasan, K.; Chandel, N.S. Mitochondrial Metabolism Regulation of T Cell-Mediated Immunity. Annu. Rev. Immunol. 2021, 39, 395–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raud, B.; McGuire, P.J.; Jones, R.G.; Sparwasser, T.; Berod, L. Fatty acid oxidation in CD8+ T cell memory. Immunol. Rev. 2024, 317, 123–139. [Google Scholar] [CrossRef]
- Kurniawan, H.; Kobayashi, T.; Brenner, D. The emerging role of one-carbon metabolism in T cells. Curr. Opin. Biotechnol. 2021, 68, 193–201. [Google Scholar] [CrossRef]
- Oliveres, H.; Cascante, M.; Maurel, J. Metabolic interventions to enhance immunotherapy and targeted therapy efficacy in advanced colorectal cancer. Curr. Opin. Chem. Biol. 2023, 77, 102401. [Google Scholar] [CrossRef]
- Navas, L.E.; Carnero, A. NAD+ metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef]
- Poon, M.M.L.; Caron, D.P.; Wang, Z.; Wells, S.B.; Chen, D.; Meng, W.; A Szabo, P.; Lam, N.; Kubota, M.; Matsumoto, R.; et al. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat. Immunol. 2023, 24, 309–319. [Google Scholar] [CrossRef]
- Williams, J.B.; Kupper, T.S. Resident Memory T Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’Malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2024, 625, 381–388. [Google Scholar] [CrossRef]
- Hirai, T.; Yang, Y.; Zenke, Y.; Li, H.; Chaudhri, V.K.; Diaz, J.S.D.L.C.; Zhou, P.Y.; Nguyen, B.A.-T.; Bartholin, L.; Workman, C.J.; et al. Competition for Active TGFβ Cytokine Allows for Selective Retention of Antigen-Specific Tissue-Resident Memory T Cells in the Epidermal Niche. Immunity 2021, 54, 84–98.e5. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Gebhardt, T.; Mackay, L.K. Tissue-Resident Memory T Cells in Cancer Immunosurveillance. Trends Immunol. 2019, 40, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sharma, P.K.; Goedegebuure, S.P.; Gillanders, W.E. Personalized cancer vaccines: Targeting the cancer mutanome. Vaccine 2017, 35, 1094–1100. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Lin, L.; Ting, S.; Yufei, H.; Wendong, L.; Yubo, F.; Jing, Z. Epitope-based peptide vaccines predicted against novel coronavirus disease caused by SARS-CoV-2. Virus Res. 2019, 288, 198082. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Roose, K.; Ballegeer, M.; Zhong, Z.; Sanders, N.N.; De Koker, S.; Saelens, X.; Van Lint, S. The Opposing Effect of Type I IFN on the T Cell Response by Non-modified mRNA-Lipoplex Vaccines Is Determined by the Route of Administration. Mol. Ther. Nucleic. Acids. 2020, 22, 373–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer. 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2008, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Thess, A.; Grund, S.; Mui, B.L.; Hope, M.J.; Baumhof, P.; Fotin-Mleczek, M.; Schlake, T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015, 23, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. Three decades of messenger RNA vaccine development. Nano Today 2019, 28, 100766. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.C.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Leung, A.K.; Tam, Y.Y.C.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic mixing: A general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B 2012, 119, 8698–8706. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- Young, R.E.; Hofbauer, S.I.; Riley, R.S. Overcoming the challenge of long-term storage of mRNA-lipid nanoparticle vaccines. Mol. Ther. 2022, 30, 1792–1793. [Google Scholar] [CrossRef] [PubMed]
- Stadler, C.R.; Bähr-Mahmud, H.; Celik, L.; Hebich, B.; Roth, A.S.; Roth, R.P.; Karikó, K.; Türeci, Ö.; Sahin, U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med. 2017, 23, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Goubier, A.; Kohrt, H.E. A quantitative analysis of therapeutic cancer vaccines in phase 2 or phase 3 trial. J. Immunother. Cancer 2022, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Tan, P.; Nie, G.; Zhu, M. Biomimetic and bioinspired nano-platforms for cancer vaccine development. Exploration 2024, 4, 20230263. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, J.; Lu, L.; Deng, D.; Huang, J.; Tang, Z.; Shi, X.; Lo, P.; Lovell, J.F.; Zheng, Y.; et al. Tumor cell membrane-based vaccines: A potential boost for cancer immunotherapy. Exploration 2024, 4, 20230171. [Google Scholar] [CrossRef]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef]
- Sebastian, M.; Papachristofilou, A.; Weiss, C.; Früh, M.; Cathomas, R.; Hilbe, W.; Wehler, T.; Rippin, G.; Koch, S.D.; Scheel, B.; et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer 2014, 14, 748. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Magoola, M.; Niazi, S.K. Current Progress and Future Perspectives of RNA-Based Cancer Vaccines: A 2025 Update. Cancers 2024, 17, 1882. [Google Scholar] [CrossRef] [PubMed]
- FDA. Clinical Considerations for Therapeutic Cancer Vaccines; U.S. Food and Drug Administration, Center for Biologics Evaluation and Research: Silver Spring, MD, USA, 2024.
- EMA. Guideline on the Quality, Non-Clinical and Clinical Requirements for mRNA-Based Prophylactic Vaccines Against Infectious Diseases; EMA/CHMP/BWP/814208/2024; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2023, 27, 591–600. [Google Scholar] [CrossRef]
- Sava, J. FDA Clears IND Application for EVM14 Across Cancers; FDA: Silver Spring, MD, USA, 2025.
- Singh, P.; Khatib, M.N.; Roopashree, R.; Kaur, M.; Srivastava, M.; Barwal, A.; Rajput, G.V.S.; Rajput, P.; Syed, R.; Sharma, G.; et al. Advancements and challenges in personalized neoantigen-based cancer vaccines. Oncol. Rev. 2025, 19, 1541326. [Google Scholar] [CrossRef]
- Bodner, K.; Irvine, M.; Kwong, J.; Mishra, S. Disparities in COVID-19 clinical studies from high-income and low-middle-income countries. Int. J. Infect. Dis. 2023, 130, 112–120. [Google Scholar] [CrossRef]
- World Economic Forum. A Historic Leap in Cancer Vaccines—Here’s What You Need to Know; World Economic Forum: Geneva, Switzerland, 2024. [Google Scholar]
- Kon, E.; Elia, U.; Peer, D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 2022, 73, 329–336. [Google Scholar] [CrossRef]
- Abreu, A.d.J.L.d.; Mpande, C.A.M.; Helble, M.; Nicholson, M.W.; Cortés, M.d.L.Á.; Ponsa, M.E.P.; Blumenthal, I.R.; Caccavo, F.; Pippo, T.; Sanjuan, J.R.; et al. Investment Opportunities for mRNA Technology in Low- and Middle-Income Countries: Key Findings and Future Perspectives. Vaccines 2025, 13, 112. [Google Scholar] [CrossRef]
- WHO. Potential Benefits and Limitations of mRNA Technology for Vaccine Research and Development in Low- and Middle-Income Countries; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Parhiz, H.; Atochina-Vasserman, E.N.; Weissman, D. mRNA-based therapeutics: Looking beyond COVID-19 vaccines. Lancet 2024, 403, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Zolot, R.S.; Basu, S.; Million, R.P. Antibody-drug conjugates. Nat. Rev. Drug Discov. 2013, 12, 259–260. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Systems immunology approaches for cancer therapy. Cell 2024, 187, 1843–1861. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Dorkin, J.R.; Vegas, A.J.; Chang, P.H.; Veiseh, O.; Matthews, J.; Fenton, O.S.; Zhang, Y.; Olejnik, K.T.; Yesilyurt, V.; et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 2014, 5, 4277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crager, S.E. Improving global access to new vaccines: Intellectual property, technology transfer, and regulatory pathways. Am. J. Public Health 2014, 104, e85–e91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; Van Der Burg, S.H.; et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565, 240–245. [Google Scholar] [CrossRef]

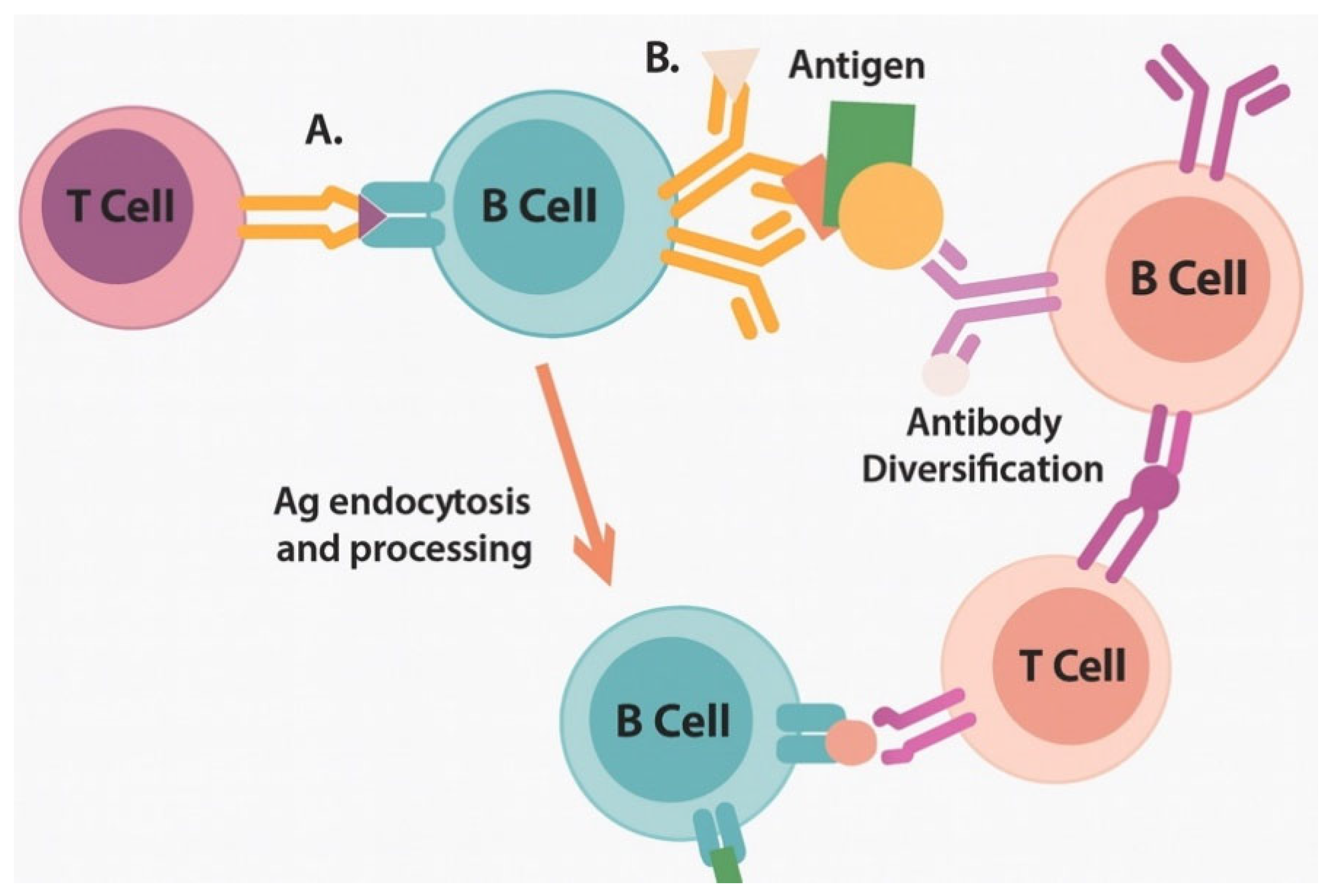
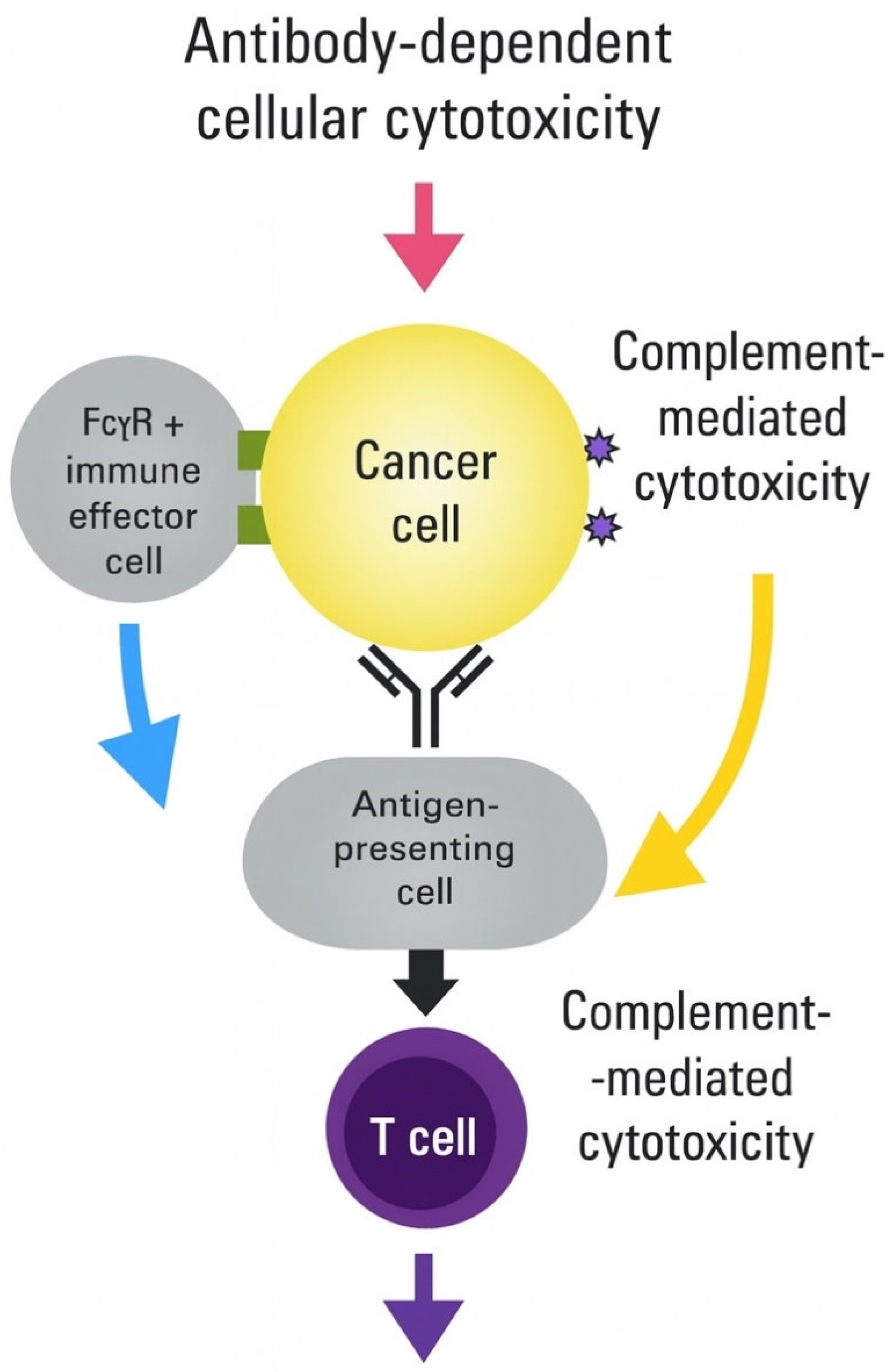
| PRR Family | Specific Receptor | Activating Ligand | Downstream Signaling | Immunological Outcome | References |
|---|---|---|---|---|---|
| Toll-like Receptors | TLR7/8 | Single-stranded mRNA | IRF7 → Type I IFN | DC maturation, T cell priming | [19] |
| Toll-like Receptors | TLR3 | Double-stranded RNA | TRIF → NF-κB | Pro-inflammatory cytokines | [20] |
| RIG-I-like Receptors | RIG-I | 5′-triphosphate mRNA | MAVS → IFN-β | Antiviral response, DC activation | [2] |
| RIG-I-like Receptors | MDA5 | Long dsRNA | MAVS → Type I IFN | Amplified antiviral response | [21] |
| DNA Sensors | cGAS-STING | Mitochondrial DNA | STING → Type I IFN | T-cell cross-priming | [22] |
| DNA Sensors | AIM2 | Cytoplasmic DNA | Inflammasome → IL-1β/IL-18 | Th1/Th17 differentiation | [23] |
| NOD-like Receptors | NLRP3 | Ion flux, ROS | Inflammasome → IL-1β | Inflammatory amplification | [2] |
| NOD-like Receptors | NLRC4 | Flagellin peptides | Inflammasome → IL-1β/IL-18 | Enhanced DC function | [24] |
| Time Point | Cellular Events | Detection Methods (Mouse) | Detection Methods (Human) | Key Markers | References |
|---|---|---|---|---|---|
| 0–6 h | Initial PRR activation | Serum cytokines (ELISA) | Serum cytokines (Luminex) | IFN-α/β, IL-6 | [5] |
| 6–24 h | DC activation | Flow cytometry | Flow cytometry (PBMC) | CD40, CD80, CD86 | [2] |
| 24–48 h | Antigen processing | MHC-peptide elution | Mass spectrometry | Peptide diversity | [8] |
| 48–72 h | T-cell priming | ELISPOT | ELISPOT, tetramer staining | IFN-γ SFU | [21] |
| 3–7 days | B-cell activation | BCR sequencing | BCR deep sequencing | Clonal diversity | [28] |
| 7–14 days | Epitope spreading | Peptide arrays | TCR sequencing | New specificities | [3] |
| 14–28 days | Memory formation | Tetramer staining | Multimer analysis | CD45RO + CCR7+ | [23] |
| 28+ days | Sustained immunity | Rechallenge studies | Clinical response | Survival data | [5] |
| Pathway Interaction | Molecular Mechanism | Net Effect | Optimization Strategy | Clinical Relevance | References |
|---|---|---|---|---|---|
| Type I IFN + NLRP3 | IL-1β → NFκB → IFN genes | Synergistic | Sequential activation | Enhanced efficacy | [30] |
| NLRP3 + cGAS-STING | mtDNA release → STING | Synergistic | Controlled pyroptosis | Amplified immunity | [31] |
| High IL-1β → Type I IFN | STAT1 degradation | Antagonistic | IL-1β blockade | Prevent exhaustion | [32] |
| Chronic inflammasome → DC function | DC pyroptosis | Antagonistic | Pulsed dosing | Maintain the APC pool | [33] |
| Complement + Inflammasome | C5a → enhanced IL-1β | Synergistic | Complement inhibition | Limit inflammation | [34] |
| Strategy | Molecular Target | Implementation | TRM Markers | Functional Outcomes | References |
|---|---|---|---|---|---|
| Route optimization | Local delivery | Intratumoral, orthotopic | CD103 + CD69+ | Local tumor control | [42] |
| Adhesion programming | E-cadherin, CXCR6 | mRNA co-delivery | Tissue retention | Prevents metastasis | [43] |
| Metabolic adaptation | FABP4, FABP5 | Lipid metabolism | Survival in tissue | Long-term protection | [44] |
| Epigenetic priming | TGF-β signaling | Local cytokine encoding | Chromatin remodeling | Stable phenotype | [45] |
| Checkpoint modulation | PD-1 blockade | Combination therapy | Enhanced function | Prevents exhaustion | [46] |
| Parameter | Mouse Models | Human Tumors | Translation Factor | Clinical Adaptation |
|---|---|---|---|---|
| Tumor doubling time | 2–3 days | 50–200 days | 15–50× slower | Extended evaluation periods |
| Immune response onset | 7 days | 14–28 days | 2–4× slower | Delayed biomarker assessment |
| Epitope spreading | 2–3 weeks | 4–8 weeks | 2–3× slower | Extended immune monitoring |
| Treg frequency | 5–10% | 15–30% | 2–3× higher | Combination with Treg depletion |
| Checkpoint expression | Moderate | High | 2–5× higher | Checkpoint inhibitor combinations |
| Treatment duration | 2–4 weeks | 3–6 months | 6–12× longer | Sustained dosing protocols |
| Parameter | Control | Vaccine Alone | Vaccine + Anti-PD-1 | Model System | References |
|---|---|---|---|---|---|
| Tumor growth inhibition (%) | 0 | 60 ± 10 | 85 ± 15 | B16-F10 melanoma | [2,78] |
| Complete response rate (%) | 0 | 30 ± 5 | 70 ± 10 | B16-F10 melanoma | [78] |
| Median survival (days) | 18 ± 2 | 31 ± 4 | 45 ± 6 | B16-F10 melanoma | [79] |
| CD8+ TIL increase (fold) | 1.0 ± 0.2 | 4.2 ± 1.1 | 6.5 ± 1.5 | B16-F10 melanoma | [79] |
| IFN-γ+ TILs (%) | 5 ± 2 | 22 ± 5 | 38 ± 7 | B16-F10 melanoma | [78] |
| Rechallenge protection (%) | 0 | 85 ± 5 | 95 ± 3 | B16-F10 melanoma | [80] |
| Metastasis reduction (%) | 0 | 45 ± 8 | 75 ± 12 | 4T1 breast cancer | [81] |
| Survival extension (%) | 0 | 40 ± 10 | 70 ± 15 | MC38 colon cancer | [82] |
| Safety Parameter | Mouse (C57BL/6) | NHP (Macaca fascicularis) | Clinical Relevance | Monitoring Plan |
|---|---|---|---|---|
| Maximum tolerated dose | >2000 μg/kg | >1000 μg/kg | 100-fold safety margin | Dose escalation study |
| Injection site reactions | Minimal | Mild, reversible | Expected, manageable | Local assessment |
| Constitutional symptoms | None observed | Transient fever | Likely in humans | Symptom monitoring |
| Liver enzyme elevation | None | Mild, reversible | Monitor ALT/AST | Weekly labs |
| Cytokine elevation | Marked (therapeutic) | Moderate | Expected mechanism | Serial cytokine levels |
| Autoimmune reactions | None | None observed | Low risk | ANA, organ-specific Ab |
| Biodistribution concerns | Liver predominant | Similar pattern | Consider dose/schedule | Imaging if needed |
| Feature | Personalized Neoantigen | Shared TAA | Cytokine-Encoding | Universal Non-Specific |
|---|---|---|---|---|
| Time to Treatment | 8–12 weeks | Days-weeks | Days-weeks | Days |
| Cost per Patient | $100,000–200,000 | $10,000–25,000 | $15,000–30,000 | $500–2000 |
| Population Coverage | Limited by HLA/mutations | Moderate | Broad | >90% |
| Manufacturing Complexity | High (personalized) | Moderate | Moderate | Low (standardized) |
| Regulatory Pathway | Complex (individual) | Standard | Standard | Standard |
| Efficacy (ORR) | 15–25% (proven) | 10–15% (proven) | Unknown | 30–40% (projected) |
| Safety Profile | Established | Established | Under evaluation | Favorable (preclinical) |
| Scalability | Poor | Moderate | Moderate | Excellent |
| Global Access | Very limited | Limited | Limited | High potential |
| Resistance Development | High (antigen loss) | Moderate | Unknown | Low (broad targeting) |
| Combination Potential | Moderate | Moderate | Limited | High |
| Study Component | Specification | Rationale | Success Criteria |
|---|---|---|---|
| Design | Modified 3 + 3 dose escalation | Standard oncology phase I | MTD identification |
| Starting dose | 25 μg mRNA | 1/20th of the predicted efficacious dose | No DLTs in first cohort |
| Dose levels | 25, 50, 100, 200 μg | 2-fold escalation steps | Biological activity at ≤200 μg |
| Primary endpoint | Safety and tolerability | Regulatory requirement | <33% DLT rate at MTD |
| Patient population | Advanced solid tumors | Adequate risk/benefit ratio | Diverse tumor representation |
| Sample size | 24–48 patients | Adequate for safety assessment | Enroll within 18 months |
| Treatment schedule | Days 1, 15, 29, then q3monthly | Prime-boost for optimal immunity | ≥80% completion of induction |
| Biomarker plan | Comprehensive immune monitoring | Mechanism confirmation | Evidence of immune activation |
| Timepoint | Sample Type | Assays | Primary Endpoints | Clinical Utility |
|---|---|---|---|---|
| Screening | Blood, archival tissue | HLA typing, TIS, TMB | Patient stratification | Enrollment criteria |
| Baseline | Blood, fresh tissue | Comprehensive immune panel | Predictive markers | Patient selection |
| 6 h | Blood | Cytokines, activation markers | Immediate response | Dose optimization |
| 24 h | Blood | Gene expression, flow cytometry | Early activation | Mechanism confirmation |
| Day 8 | Blood | T-cell responses, ELISPOT | Immune priming | Dose escalation |
| Day 29 | Blood, tissue | Adaptive responses | Vaccine immunogenicity | Efficacy prediction |
| Day 57 | Blood | Epitope spreading | Mechanistic endpoint | Proof of concept |
| Every 2 cycles | Blood | Disease monitoring | Clinical benefit | Response assessment |
| Technology | Development Status | Stability Target | Clinical Timeline | Commercial Viability |
|---|---|---|---|---|
| Current LNP (−70 °C) | Established | 24 months | Available now | Limited global access |
| Lyophilized (2–8 °C) | Development phase | 12–24 months | 2–3 years | Moderate improvement |
| Lyophilized (25 °C) | Research phase | 6–12 months | 3–5 years | Significant improvement |
| Alternative systems | Early research | 12+ months (25 °C) | 5+ years | Game-changing potential |
| Room temperature liquid | Concept stage | 6+ months (25 °C) | 5+ years | Ultimate goal |
| Challenge Category | Impact Level | Probability | Mitigation Strategy | Implementation Timeline | Success Metrics |
|---|---|---|---|---|---|
| Immunological pathway interference | High | Medium | Systems biology modeling | 2–3 years | Optimized dosing protocols |
| CRS/autoimmune toxicity | High | Low-Medium | Enhanced monitoring/prophylaxis | 1–2 years | <5% severe AE rate |
| Manufacturing scale-up | Medium | High | Process automation/standardization | 3–5 years | 10M+ doses annually |
| Regulatory harmonization | Medium | Medium | Global coordination initiatives | 2–4 years | Multi-region approvals |
| Cost optimization | Medium | High | Process innovation | 3–5 years | <$100 per course |
| Individual variability | Medium | High | Personalized approaches | 5–10 years | Predictive algorithms |
| Timeframe | Clinical Milestones | Technical Achievements | Regulatory Goals | Access Targets |
|---|---|---|---|---|
| 2–3 years | Phase I completion, biomarker validation | Improved formulations, delivery optimization | FDA/EMA guidance | Pilot manufacturing |
| 3–5 years | Phase II efficacy data | Room temperature stability | Regulatory submission | Regional production |
| 5–7 years | First approval | AI-optimized design | Market authorization | Global distribution |
| 7–10 years | Multiple indications | Advanced delivery systems | Combination approvals | Cost optimization |
| 10–15 years | Prevention applications | Predictive medicine | Global harmonization | Universal access |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magoola, M.; Niazi, S.K. Engineering Universal Cancer Immunity: Non-Tumor-Specific mRNA Vaccines Trigger Epitope Spreading in Cold Tumors. Vaccines 2025, 13, 970. https://doi.org/10.3390/vaccines13090970
Magoola M, Niazi SK. Engineering Universal Cancer Immunity: Non-Tumor-Specific mRNA Vaccines Trigger Epitope Spreading in Cold Tumors. Vaccines. 2025; 13(9):970. https://doi.org/10.3390/vaccines13090970
Chicago/Turabian StyleMagoola, Matthias, and Sarfaraz K. Niazi. 2025. "Engineering Universal Cancer Immunity: Non-Tumor-Specific mRNA Vaccines Trigger Epitope Spreading in Cold Tumors" Vaccines 13, no. 9: 970. https://doi.org/10.3390/vaccines13090970
APA StyleMagoola, M., & Niazi, S. K. (2025). Engineering Universal Cancer Immunity: Non-Tumor-Specific mRNA Vaccines Trigger Epitope Spreading in Cold Tumors. Vaccines, 13(9), 970. https://doi.org/10.3390/vaccines13090970






