Cross-Neutralization of Distant Coronaviruses Strongly Correlates with Spike S2-Specific Antibodies from Immunocompetent and Immunocompromised Vaccinated SARS-CoV-2-Infected Patients
Abstract
1. Introduction
2. Methods
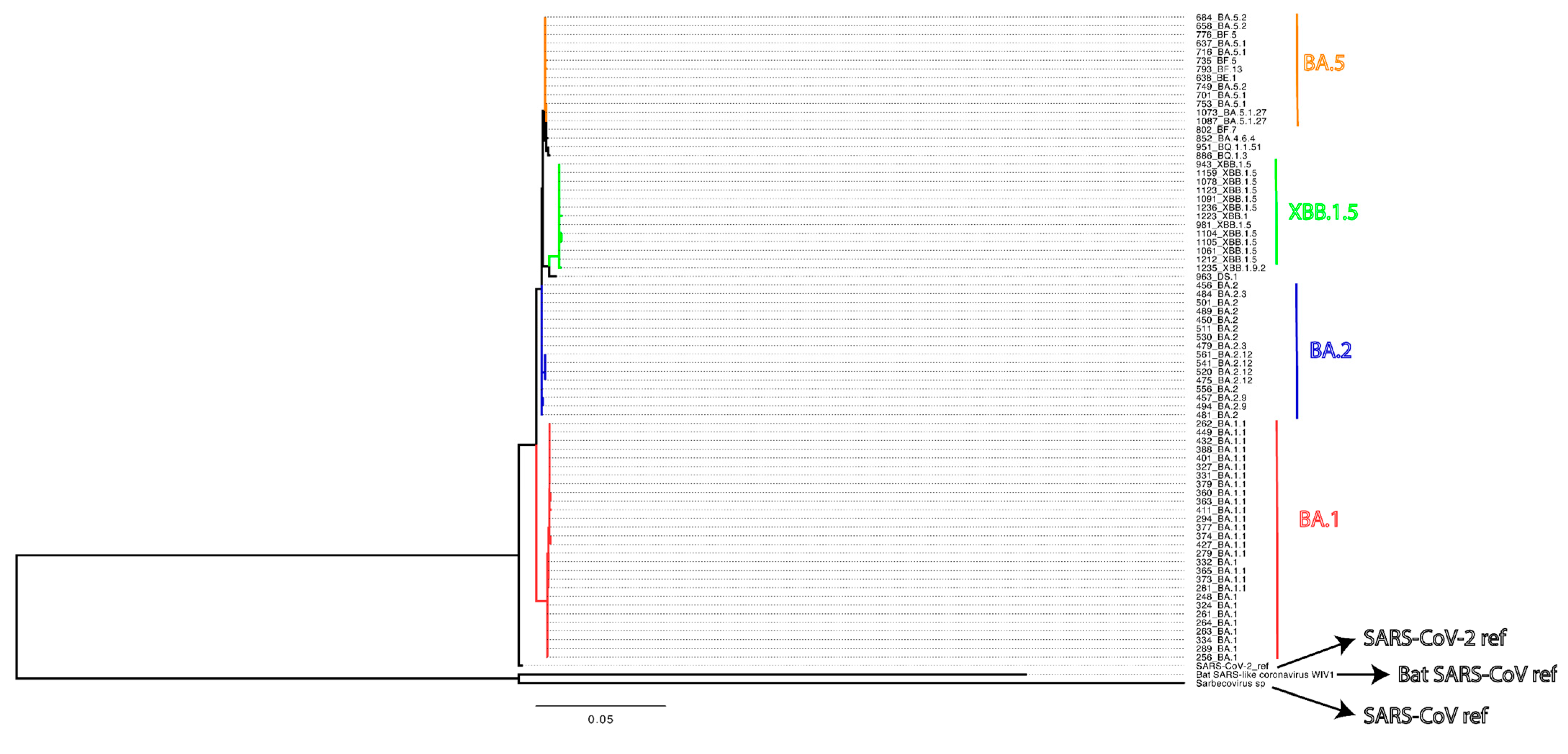
2.1. Study Enrollment and Sample Collection
2.2. Ethics Declaration
2.3. Cell Lines
2.4. SARS-CoV-2-Specific Antibody Measurement by ELISA
2.4.1. ELISA Antigens
2.4.2. S2 Antigen Modification for Solubility
2.4.3. ELISA Procedure
2.5. SARS-CoV-2 Pseudovirus Production
2.5.1. SARS-CoV-2 Pseudovirus Titration
2.5.2. SARS-CoV-2 Pseudovirus Neutralization Assay
2.6. Statistical Analysis
3. Results
3.1. Validity of Modified Soluble S2 as ELISA Antigens
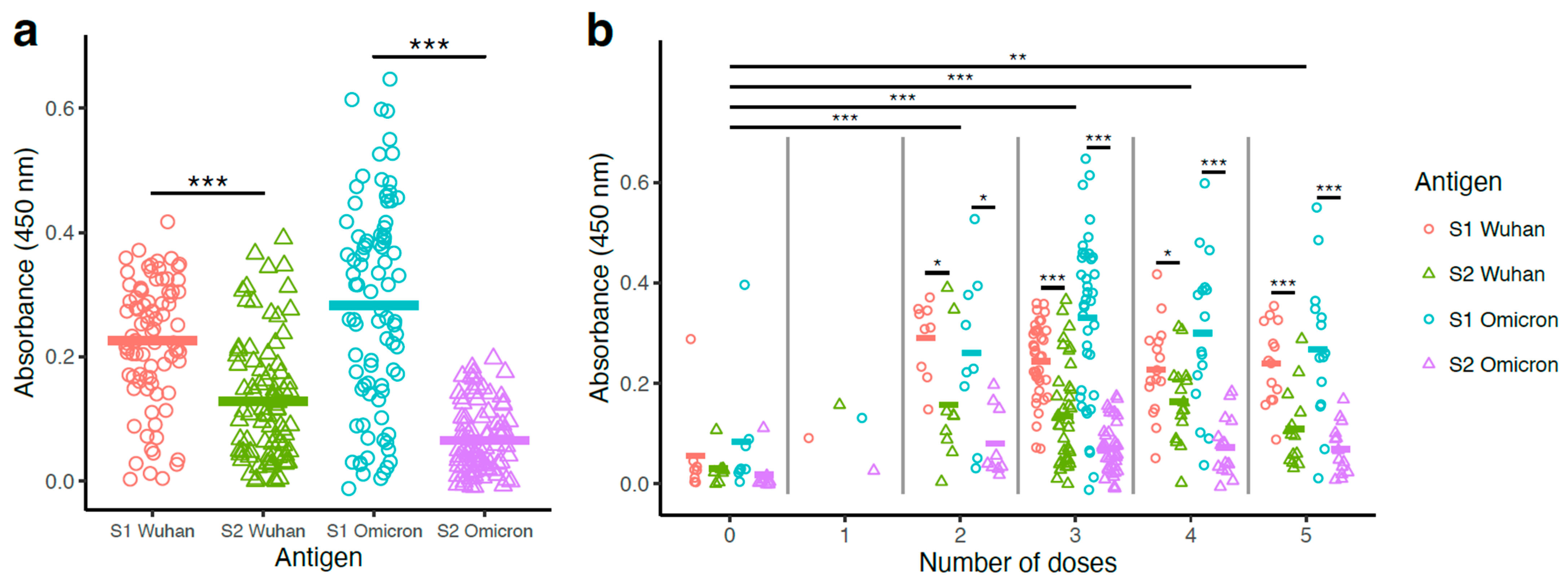
3.2. S1-Specific Antibody Levels Surpassed S2-Specific Antibody Levels
3.3. Binding Antibody Titers Were Higher with Vaccinated Groups Compared to the Non-Vaccinated Group, with No Significant Differences Among the Booster Groups (2–5 Doses)
3.4. Pseudovirus Neutralization Was Higher with Wuhan and Omicron Compared to SARS-CoV and WIV1-CoV
3.5. Vaccination Significantly Improved Pseudovirus Neutralization Potency
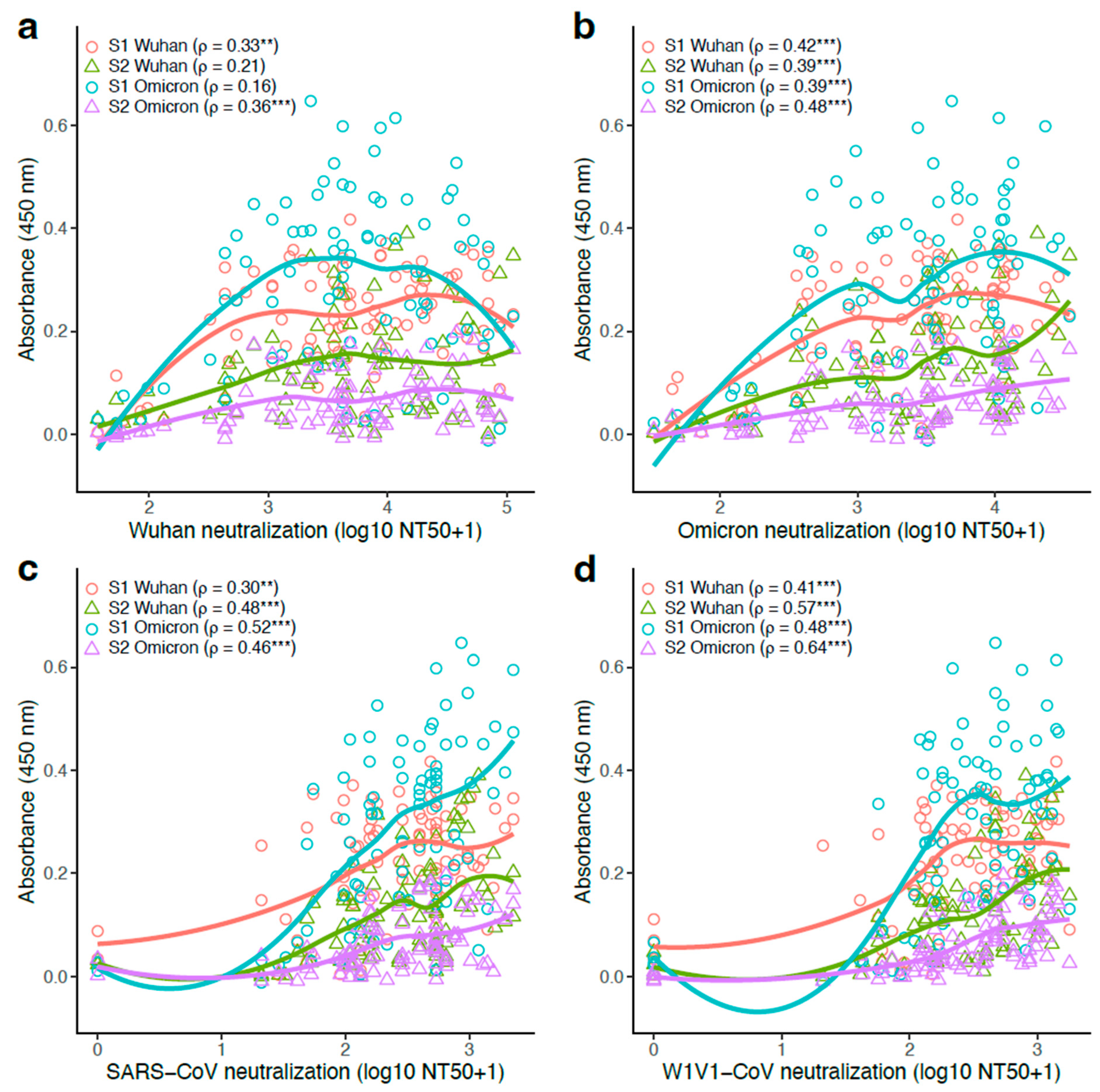
3.6. S1- and S2-Specific Antibody Titers Generally Correlated Positively with Pseudovirus Neutralization Potency
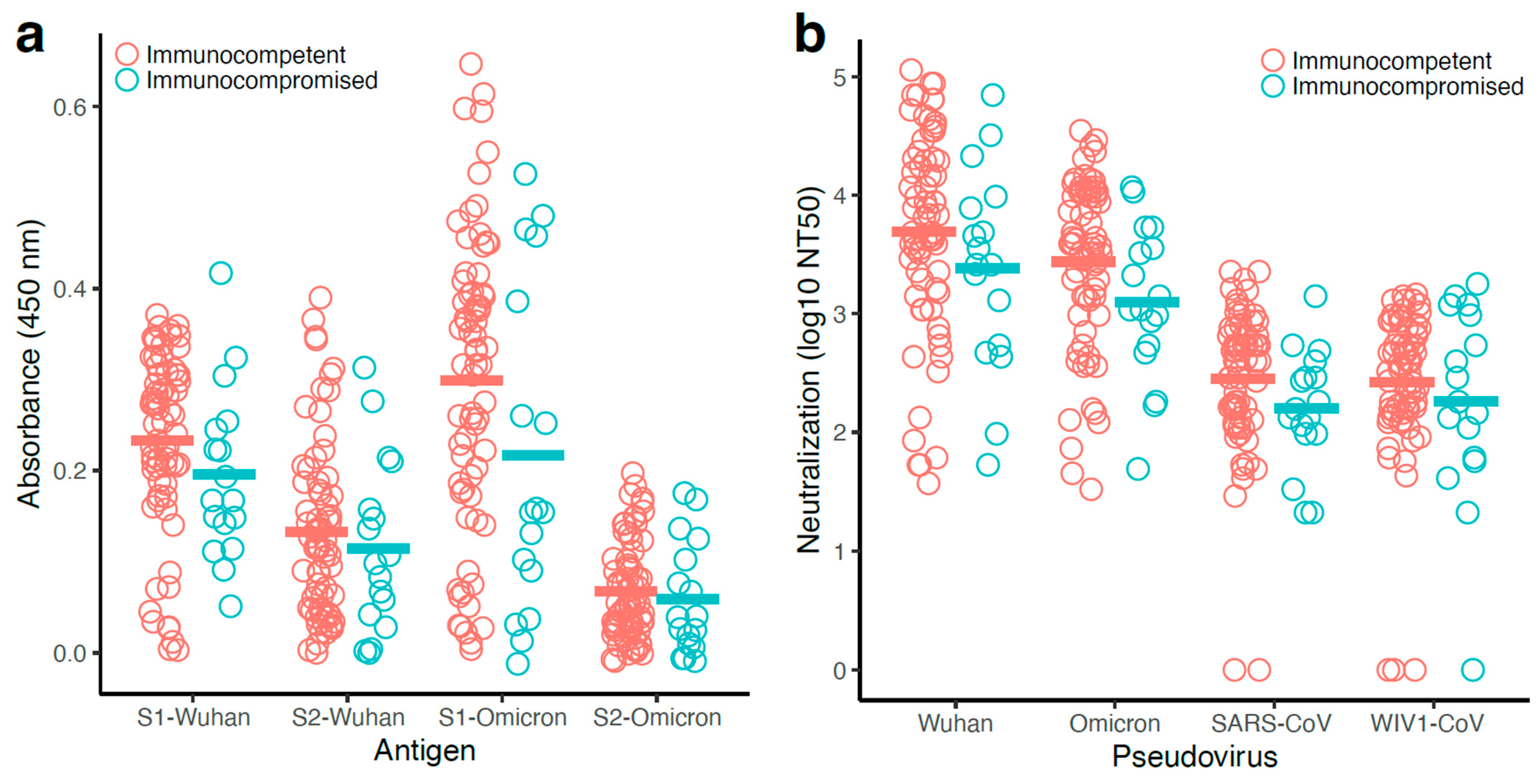
3.7. Antibody Responses Observed with the Immunocompetent Participants Were Generally Higher than That of the Immunocompromised Participants
3.8. Antibody Levels and Pseudovirus Neutralization Were Correlated More Strongly in Immunocompetent Participants
3.9. Increased Number of Booster Doses Resulted in the Maintenance of High Antibody Levels After 2nd Booster
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Stawicki, S.P.; Jeanmonod, R.; Miller, A.C.; Paladino, L.; Gaieski, D.F.; Yaffee, A.Q.; De Wulf, A.; Grover, J.; Papadimos, T.J.; Bloem, C.; et al. The 2019-2020 Novel Coronavirus (Severe Acute Respiratory Syndrome Coronavirus 2) Pandemic: A Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group Consensus Paper. J. Glob. Infect. Dis. 2020, 12, 47–93. [Google Scholar] [CrossRef]
- Joshi, G.; Borah, P.; Thakur, S.; Sharma, P.; Mayank; Poduri, R. Exploring the COVID-19 vaccine candidates against SARS-CoV-2 and its variants: Where do we stand and where do we go? Hum. Vaccin. Immunother. 2021, 17, 4714–4740. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Silk, B.J.; Scobie, H.M.; Duck, W.M.; Palmer, T.; Ahmad, F.B.; Binder, A.M.; Cisewski, J.A.; Kroop, S.; Soetebier, K.; Park, M.; et al. COVID-19 Surveillance After Expiration of the Public Health Emergency Declaration—United States, May 11, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, N.; Ghasemiyeh, P.; Moradishooli, F.; Mohammadi-Samani, S. Update on the effectiveness of COVID-19 vaccines on different variants of SARS-CoV-2. Int. Immunopharmacol. 2023, 117, 109968. [Google Scholar] [CrossRef]
- Basoulis, D.; Mastrogianni, E.; Karamanakos, G.; Gkoufa, A.; Georgakopoulou, V.E.; Makrodimitri, S.; Gamaletsou, M.N.; Markogiannakis, A.; Sipsas, N.V. Efficacy of Tixagevimab/Cilgavimab as Pre-Exposure Prophylaxis against Infection from SARS-CoV-2 and Severe COVID-19 among Heavily Immunocompromised Patients: A Single-Center, Prospective, Real-World Study. Viruses 2024, 16, 1345. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; Dube, S.; Lu, Y.; Yates, M.; Arnetorp, S.; Barnes, E.; Bell, S.; Carty, L.; Evans, K.; Graham, S.; et al. Corrigendum to ‘Impact of COVID-19 on immunocompromised populations during the Omicron era: Insights from the observational population-based INFORM study’ [The Lancet Regional Health—Europe 35 (2023) 100747]. Lancet Reg. Health Eur. 2024, 44, 101008. [Google Scholar] [CrossRef]
- Quek, A.M.L.; Wang, S.; Teng, O.; Shunmuganathan, B.; Er, B.G.C.; Mahmud, N.F.B.; Ng, I.X.Q.; Gupta, R.; Tan, I.S.L.; Tan, N.Y.; et al. Hybrid immunity augments cross-variant protection against COVID-19 among immunocompromised individuals. J. Infect. 2024, 89, 106238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Choudhary, M.C.; Regan, J.; Boucau, J.; Nathan, A.; Speidel, T.; Liew, M.Y.; Edelstein, G.E.; Kawano, Y.; Uddin, R.; et al. SARS-CoV-2 viral clearance and evolution varies by type and severity of immunodeficiency. Sci. Transl. Med. 2024, 16, eadk1599. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2523. [Google Scholar] [CrossRef]
- Chang, M.R.; Ke, H.; Coherd, C.D.; Wang, Y.; Mashima, K.; Kastrunes, G.M.; Huang, C.Y.; Marasco, W.A. Analysis of a SARS-CoV-2 convalescent cohort identified a common strategy for escape of vaccine-induced anti-RBD antibodies by Beta and Omicron variants. EBioMedicine 2022, 80, 104025. [Google Scholar] [CrossRef]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef] [PubMed]
- Zaccaria, M.; Genovese, L.; Dawson, W.; Cristiglio, V.; Nakajima, T.; Johnson, W.; Farzan, M.; Momeni, B. Probing the mutational landscape of the SARS-CoV-2 spike protein via quantum mechanical modeling of crystallographic structures. PNAS Nexus 2022, 1, pgac180. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.R.; Rennick, L.J.; Nambulli, S.; Robinson-McCarthy, L.R.; Bain, W.G.; Haidar, G.; Duprex, W.P. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science 2021, 371, 1139–1142. [Google Scholar] [CrossRef]
- Edelstein, G.E.; Boucau, J.; Uddin, R.; Marino, C.; Liew, M.Y.; Barry, M.; Choudhary, M.C.; Gilbert, R.F.; Reynolds, Z.; Li, Y.; et al. SARS-CoV-2 Virologic Rebound With Nirmatrelvir-Ritonavir Therapy: An Observational Study. Ann. Intern. Med. 2023, 176, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Boucau, J.; Marino, C.; Regan, J.; Uddin, R.; Choudhary, M.C.; Flynn, J.P.; Chen, G.; Stuckwisch, A.M.; Mathews, J.; Liew, M.Y.; et al. Duration of Shedding of Culturable Virus in SARS-CoV-2 Omicron (BA.1) Infection. N. Engl. J. Med. 2022, 387, 275–277. [Google Scholar] [CrossRef]
- Haidar, G.; Agha, M.; Bilderback, A.; Lukanski, A.; Linstrum, K.; Troyan, R.; Rothenberger, S.; McMahon, D.K.; Crandall, M.D.; Sobolewksi, M.D.; et al. Prospective Evaluation of Coronavirus Disease 2019 (COVID-19) Vaccine Responses Across a Broad Spectrum of Immunocompromising Conditions: The COVID-19 Vaccination in the Immunocompromised Study (COVICS). Clin. Infect. Dis. 2022, 75, e630–e644. [Google Scholar] [CrossRef]
- Maneikis, K.; Sablauskas, K.; Ringeleviciute, U.; Vaitekenaite, V.; Cekauskiene, R.; Kryzauskaite, L.; Naumovas, D.; Banys, V.; Peceliunas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Vecchio, J.; Regan, J.; Jiang, Y.; Li, R.; Romain, H.; Yousuf, F.; Adel, T.; Hall, K.; DaCosta, J.M.; Yu, X.; et al. Viral and immunologic evaluation of smokers with severe COVID-19. Sci. Rep. 2023, 13, 17898. [Google Scholar] [CrossRef]
- Mou, H.; Quinlan, B.D.; Peng, H.; Liu, G.; Guo, Y.; Peng, S.; Zhang, L.; Davis-Gardner, M.E.; Gardner, M.R.; Crynen, G.; et al. Mutations derived from horseshoe bat ACE2 orthologs enhance ACE2-Fc neutralization of SARS-CoV-2. PLoS Pathog. 2021, 17, e1009501. [Google Scholar] [CrossRef]
- Roy, V.; Fischinger, S.; Atyeo, C.; Slein, M.; Loos, C.; Balazs, A.; Luedemann, C.; Astudillo, M.G.; Yang, D.; Wesemann, D.R.; et al. SARS-CoV-2-specific ELISA development. J. Immunol. Methods 2020, 484–485, 112832. [Google Scholar] [CrossRef] [PubMed]
- Siebring-van Olst, E.; Vermeulen, C.; de Menezes, R.X.; Howell, M.; Smit, E.F.; van Beusechem, V.W. Affordable luciferase reporter assay for cell-based high-throughput screening. J. Biomol. Screen. 2013, 18, 453–461. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef]
- Burki, T. WHO ends the COVID-19 public health emergency. Lancet Respir. Med. 2023, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Olukitibi, T.A.; Ao, Z.; Warner, B.; Unat, R.; Kobasa, D.; Yao, X. Significance of Conserved Regions in Coronavirus Spike Protein for Developing a Novel Vaccine against SARS-CoV-2 Infection. Vaccines 2023, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zai, X.; Zhang, Z.; Xu, J.; Chen, W. Challenges and developments in universal vaccine design against SARS-CoV-2 variants. NPJ Vaccines 2022, 7, 167. [Google Scholar] [CrossRef]
- Shah, P.; Canziani, G.A.; Carter, E.P.; Chaiken, I. The Case for S2: The Potential Benefits of the S2 Subunit of the SARS-CoV-2 Spike Protein as an Immunogen in Fighting the COVID-19 Pandemic. Front. Immunol. 2021, 12, 637651. [Google Scholar] [CrossRef]
- Pang, W.; Lu, Y.; Zhao, Y.B.; Shen, F.; Fan, C.F.; Wang, Q.; He, W.Q.; He, X.Y.; Li, Z.K.; Chen, T.T.; et al. A variant-proof SARS-CoV-2 vaccine targeting HR1 domain in S2 subunit of spike protein. Cell Res. 2022, 32, 1068–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yuan, M.; Song, G.; Beutler, N.; Shaabani, N.; Huang, D.; He, W.T.; Zhu, X.; Callaghan, S.; Yong, P.; et al. A human antibody reveals a conserved site on beta-coronavirus spike proteins and confers protection against SARS-CoV-2 infection. Sci. Transl. Med. 2022, 14, eabi9215. [Google Scholar] [CrossRef] [PubMed]
- Dacon, C.; Tucker, C.; Peng, L.; Lee, C.D.; Lin, T.H.; Yuan, M.; Cong, Y.; Wang, L.; Purser, L.; Williams, J.K.; et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Y.; Hu, Y.; Chang, F.; Wu, Q.; Yang, J.; Chen, J.; Teng, S.; Zhang, J.; He, R.; et al. Monoclonal antibodies constructed from COVID-19 convalescent memory B cells exhibit potent binding activity to MERS-CoV spike S2 subunit and other human coronaviruses. Front. Immunol. 2022, 13, 1056272. [Google Scholar] [CrossRef]
- Li, C.J.; Chang, S.C. SARS-CoV-2 spike S2-specific neutralizing antibodies. Emerg. Microbes Infect. 2023, 12, 2220582. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 2023, 23, 189–199. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Liu, Z.; Xing, L.; Zhu, Y.; Xu, W.; Xia, S.; Lu, L.; Jiang, S. An engineered recombinant protein containing three structural domains in SARS-CoV-2 S2 protein has potential to act as a pan-human coronavirus entry inhibitor or vaccine antigen. Emerg. Microbes Infect. 2023, 12, 2244084. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Patel, R.S.; Loeffler, K.; Yasuhara, A.; Van De Velde, L.-A.; Yang, J.E.; Chervin, J.; Troxell, C.; Huang, M.; Zheng, N.; et al. Multivalent S2 subunit vaccines provide broad protection against Clade 1 sarbecoviruses in female mice. Nat. Commun. 2025, 16, 462. [Google Scholar] [CrossRef]
- Andrews, S.F.; Cominsky, L.Y.; Shimberg, G.D.; Gillespie, R.A.; Gorman, J.; Raab, J.E.; Brand, J.; Creanga, A.; Gajjala, S.R.; Narpala, S.; et al. An influenza H1 hemagglutinin stem-only immunogen elicits a broadly cross-reactive B cell response in humans. Sci. Transl. Med. 2023, 15, eade4976. [Google Scholar] [CrossRef]
- Haggenburg, S.; Hofsink, Q.; Lissenberg-Witte, B.I.; Broers, A.E.C.; van Doesum, J.A.; van Binnendijk, R.S.; den Hartog, G.; Bhoekhan, M.S.; Haverkate, N.J.E.; Burger, J.A.; et al. Antibody Response in Immunocompromised Patients With Hematologic Cancers Who Received a 3-Dose mRNA-1273 Vaccination Schedule for COVID-19. JAMA Oncol. 2022, 8, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Morgans, H.A.; Bradley, T.; Flebbe-Rehwaldt, L.; Selvarangan, R.; Bagherian, A.; Barnes, A.P.; Bass, J.; Cooper, A.M.; Fischer, R.; Kleiboeker, S.; et al. Humoral and cellular response to the COVID-19 vaccine in immunocompromised children. Pediatr. Res. 2023, 94, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Truong, T.H.; Narendran, A. Evaluation of COVID-19 vaccine response in patients with cancer: An interim analysis. Eur. J. Cancer 2021, 159, 259–274. [Google Scholar] [CrossRef] [PubMed]

| Total | 0 Doses | 1 Dose | 2 Doses | 3 Doses | 4 Doses | 5 Doses | |
| Patients (n = 87) | |||||||
| Female (n = 60), % | 69 | 6.7 | 0 | 8.0 | 34 | 10 | 10 |
| Male (n = 27), % | 31 | 3.4 | 1.1 | 2.3 | 10 | 8.0 | 5.7 |
| Manufacturer of the most recent dose, % | |||||||
| Johnson & Johnson (n = 1), % | 1.1 | 0 | 1.1 | 0 | 0 | 0 | 0 |
| Pfizer (n = 40), % | 46 | 0 | 0 | 6.9 | 24 | 6.9 | 8.0 |
| Moderna (n = 38), % | 44 | 0 | 0 | 3.4 | 21 | 11 | 8.0 |
| None (n = 8), % | 9.2 | NA | NA | NA | NA | NA | NA |
| Race % | |||||||
| White (n = 69), % | 79 | 6.9 | 1.1 | 6.9 | 36 | 13.8 | 13.8 |
| Asian (n = 2), % | 2.3 | 0 | 0 | 0 | 2.3 | 0 | 0 |
| Black or African American (n = 6), % | 6.9 | 0 | 0 | 1.1 | 2.3 | 2.3 | 1.1 |
| Others and unknown (n = 10), % | 11 | 2.3 | 0 | 2.3 | 3.4 | 2.3 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.V.; Leeman, B.M.; Botros, P.J.; Folta, J.; Shahid, D.; Rocque, A.I.; Joyal, A.S.; Vecchio, J.A.; Passell, E.; Tien, D.; et al. Cross-Neutralization of Distant Coronaviruses Strongly Correlates with Spike S2-Specific Antibodies from Immunocompetent and Immunocompromised Vaccinated SARS-CoV-2-Infected Patients. Vaccines 2025, 13, 949. https://doi.org/10.3390/vaccines13090949
Patel SV, Leeman BM, Botros PJ, Folta J, Shahid D, Rocque AI, Joyal AS, Vecchio JA, Passell E, Tien D, et al. Cross-Neutralization of Distant Coronaviruses Strongly Correlates with Spike S2-Specific Antibodies from Immunocompetent and Immunocompromised Vaccinated SARS-CoV-2-Infected Patients. Vaccines. 2025; 13(9):949. https://doi.org/10.3390/vaccines13090949
Chicago/Turabian StylePatel, Sara V., Brooke M. Leeman, Patricia J. Botros, Joanna Folta, Dhiman Shahid, Anya I. Rocque, Andrew S. Joyal, Joseph A. Vecchio, Eliza Passell, Dessie Tien, and et al. 2025. "Cross-Neutralization of Distant Coronaviruses Strongly Correlates with Spike S2-Specific Antibodies from Immunocompetent and Immunocompromised Vaccinated SARS-CoV-2-Infected Patients" Vaccines 13, no. 9: 949. https://doi.org/10.3390/vaccines13090949
APA StylePatel, S. V., Leeman, B. M., Botros, P. J., Folta, J., Shahid, D., Rocque, A. I., Joyal, A. S., Vecchio, J. A., Passell, E., Tien, D., Reynolds, Z., Su, K., Vyas, T. D., Vyas, J. M., Abar, E., Barry, M., Alexandrescu, A., Wallace, Z., DaCosta, J. M., ... Fofana, I. B. (2025). Cross-Neutralization of Distant Coronaviruses Strongly Correlates with Spike S2-Specific Antibodies from Immunocompetent and Immunocompromised Vaccinated SARS-CoV-2-Infected Patients. Vaccines, 13(9), 949. https://doi.org/10.3390/vaccines13090949







