Immune Response to MVA-BN Vaccination for Mpox: Current Evidence and Future Directions
Abstract
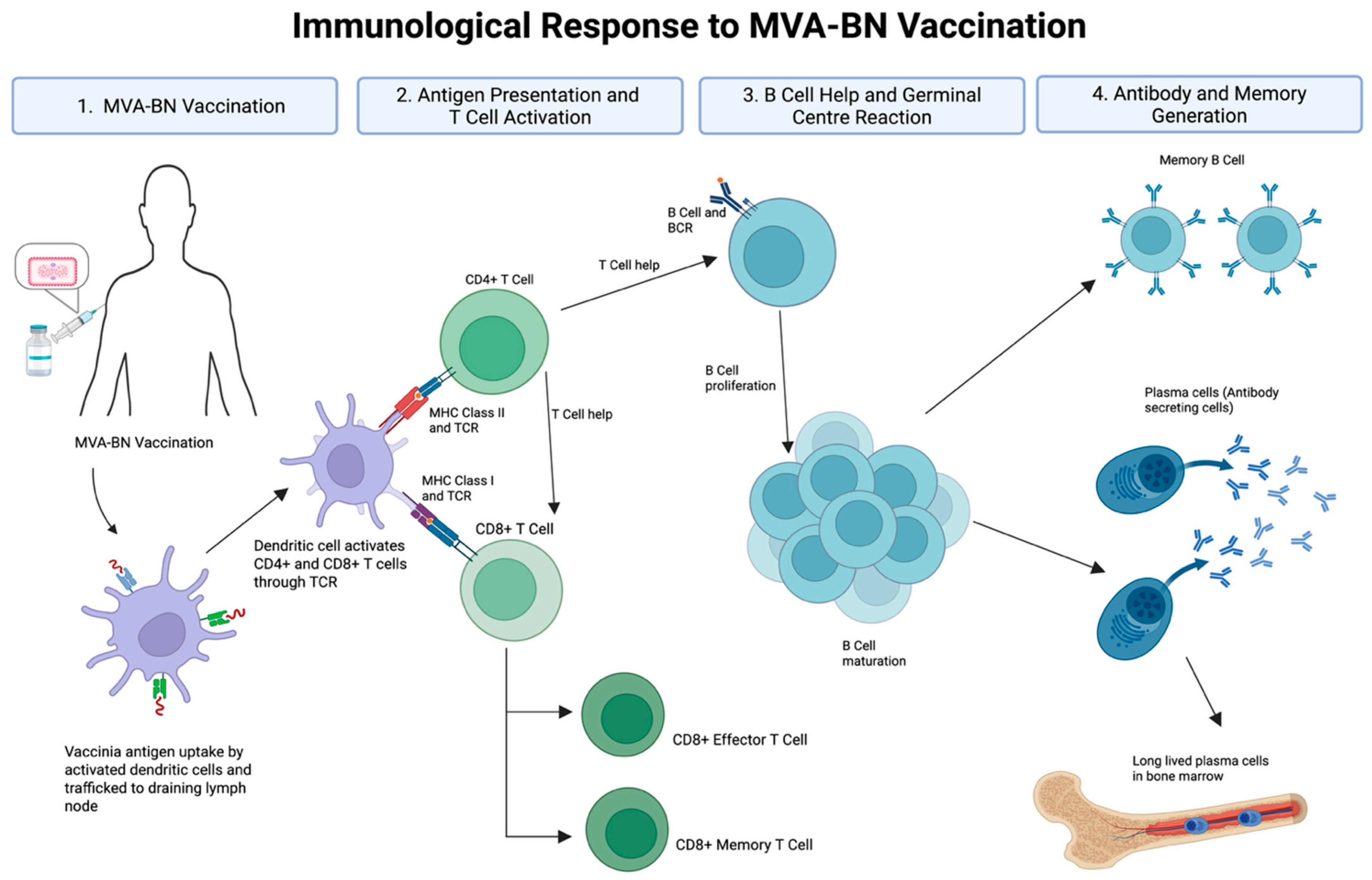
1. Introduction
2. MVA-BN Vaccine
3. Challenges in Serologic Assays for Antibody Evaluation
4. Seropositivity Thresholds for Antibody Responses
5. Kinetics and Magnitude of Binding Antibody Responses
6. Durability of Humoral Immunity
7. Determinants of Antibody Responses
8. Mucosal Antibody Responses and Site-Specific Immunity
9. Neutralising Antibodies
10. Fc-Mediated Effector Functions
11. B Cell Memory
12. T Cell Responses
13. Correlates of Protection
14. Role of Booster Vaccines
15. Future Directions
16. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86 Pt 10, 2661–2672. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Mpox in the United States and Around the World: Current Situation. Available online: https://www.cdc.gov/mpox/situation-summary/index.html#:~:text=The%20ongoing%20global%20outbreak%20of,caused%20by%20the%20subclade%20IIb (accessed on 22 January 2025).
- World Health Organisation. Mpox (Monkeypox) Outbreak 2022—Global. 2024. Available online: https://www.who.int/emergencies/situations/monkeypox-oubreak-2022 (accessed on 17 June 2024).
- World Health Organisation. WHO Director-General Declares Mpox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern (accessed on 15 August 2024).
- Hendrickson, R.C.; Wang, C.; Hatcher, E.L.; Lefkowitz, E.J. Orthopoxvirus genome evolution: The role of gene loss. Viruses 2010, 2, 1933–1967. [Google Scholar] [CrossRef]
- National Library of Medicine. Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 15 August 2024).
- Nigam, P.; Earl, P.L.; Americo, J.L.; Sharma, S.; Wyatt, L.S.; Edghill-Spano, Y.; Chennareddi, L.S.; Silvera, P.; Moss, B.; Robinson, H.L.; et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology 2007, 366, 73–83. [Google Scholar] [CrossRef]
- Greenberg, R.N.; Hay, C.M.; Stapleton, J.T.; Marbury, T.C.; Wagner, E.; Kreitmeir, E.; Roesch, S.; von Krempelhuber, A.; Young, P.; Nichols, R.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN(R)) in 56-80-Year-Old Subjects. PLoS ONE 2016, 11, e0157335. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.T.; Lawrence, S.J.; Wagner, E.; Nopora, K.; Rosch, S.; Young, P.; Schmidt, D.; Kreusel, C.; De Carli, S.; Meyer, T.P.; et al. Immunogenicity and safety of three consecutive production lots of the non replicating smallpox vaccine MVA: A randomised, double blind, placebo controlled phase III trial. PLoS ONE 2018, 13, e0195897. [Google Scholar] [CrossRef] [PubMed]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Jezek, Z.; Marennikova, S.S.; Mutumbo, M.; Nakano, J.H.; Paluku, K.M.; Szczeniowski, M. Human monkeypox: A study of 2510 contacts of 214 patients. J. Infect. Dis. 1986, 154, 551–555. [Google Scholar] [CrossRef]
- Berry, M.T.; Khan, S.R.; Schlub, T.E.; Notaras, A.; Kunasekaran, M.; Grulich, A.E.; MacIntyre, C.R.; Davenport, M.P.; Khoury, D.S. Predicting vaccine effectiveness for mpox. Nat. Commun. 2024, 15, 3856. [Google Scholar] [CrossRef]
- Guagliardo, S.A.J.; Kracalik, I.; Carter, R.J.; Braden, C.; Free, R.; Hamal, M.; Tuttle, A.; McCollum, A.M.; Rao, A.K. Monkeypox Virus Infections After 2 Preexposure Doses of JYNNEOS Vaccine—United States, May 2022–May 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 460–466. [Google Scholar] [CrossRef]
- Hazra, A.; Zucker, J.; Bell, E.; Flores, J.; Gordon, L.; Mitjà, O.; Suñer, C.; Lemaignen, A.; Jamard, S.; Nozza, S.; et al. Mpox in people with past infection or a complete vaccination course: A global case series. Lancet Infect. Dis. 2024, 24, 57–64. [Google Scholar] [CrossRef]
- Byrne, J.; Saini, G.; Garcia-Leon, A.; Alalwan, D.; Doran, P.; Landay, A.; Luong Nguyen, L.B.; O’Broin, C.; Savinelli, S.; O’Halloran, J.A.; et al. Development and validation of a quantitative Orthopoxvirus immunoassay to evaluate and differentiate serological responses to Mpox infection and vaccination. eBioMedicine 2025, 113, 105622. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.-r.Y.; McMahan, K.; Jacob-Dolan, C.; Liu, J.; Borducchi, E.N.; Moss, B.; Barouch, D.H. Decline of Mpox Antibody Responses After Modified Vaccinia Ankara–Bavarian Nordic Vaccination. JAMA 2024, 332, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Carson, W.C.; Ortega, E.; Navarra, T.; Tran, S.; Smith, T.G.; Pukuta, E.; Muyamuna, E.; Kabamba, J.; Nguete, B.U.; et al. Serological responses to the MVA-based JYNNEOS monkeypox vaccine in a cohort of participants from the Democratic Republic of Congo. Vaccine 2022, 40, 7321–7327. [Google Scholar] [CrossRef] [PubMed]
- Drennan, P.G.; Provine, N.M.; Harris, S.A.; Otter, A.; Hollett, K.; Cooper, C.; De Maeyer, R.P.H.; Nassanga, B.; Ateere, A.; Pudjohartono, M.F.; et al. Immunogenicity of MVA-BN vaccine deployed as mpox prophylaxis: A prospective, single-centre, cohort study and analysis of transcriptomic predictors of response. Lancet Microbe 2025, 6, 101045. [Google Scholar] [CrossRef]
- Kottkamp, A.C.; Samanovic, M.I.; Duerr, R.; Oom, A.L.; Belli, H.M.; Zucker, J.R.; Rosen, J.B.; Mulligan, M.J.; NYC OSMI Study Group. Antibody Titers against Mpox Virus after Vaccination. N. Engl. J. Med. 2023, 389, 2299–2301. [Google Scholar] [CrossRef]
- Matusali, G.; Cimini, E.; Mazzotta, V.; Colavita, F.; Maggi, F.; Antinori, A. Mpox Immune response elicited by MVA-BN vaccine over 12 months of follow-up. J. Infect. 2024, 89, 106309. [Google Scholar] [CrossRef]
- Oom, A.L.; Wilson, K.K.; Yonatan, M.; Rettig, S.; Youn, H.A.; Tuen, M.; Shah, Y.; DuMont, A.L.; Belli, H.M.; Zucker, J.R.; et al. The two-dose MVA-BN mpox vaccine induces a nondurable and low avidity MPXV-specific antibody response. J. Virol. 2025, e00253-25. [Google Scholar] [CrossRef]
- Ilchmann, H.; Samy, N.; Reichhardt, D.; Schmidt, D.; Powell, J.D.; Meyer, T.P.H.; Silbernagl, G.; Nichols, R.; Weidenthaler, H.; De Moerlooze, L.; et al. One- and Two-Dose Vaccinations with Modified Vaccinia Ankara-Bavarian Nordic Induce Durable B-Cell Memory Responses Comparable to Replicating Smallpox Vaccines. J. Infect. Dis. 2023, 227, 1203–1213. [Google Scholar] [CrossRef]
- Mazzotta, V.; Lepri, A.C.; Matusali, G.; Cimini, E.; Piselli, P.; Aguglia, C.; Lanini, S.; Colavita, F.; Notari, S.; Oliva, A.; et al. Immunogenicity and reactogenicity of modified vaccinia Ankara pre-exposure vaccination against mpox according to previous smallpox vaccine exposure and HIV infection: Prospective cohort study. eClinicalMedicine 2024, 68, 102420. [Google Scholar] [CrossRef]
- Byrne, J.; Garcia-Leon, A.; Murphy, A.; Saini, G.; Banik, I.; Landay, A.; Nguyen, L.B.L.; Savinelli, S.; O’Broin, C.; Horgan, M.; et al. Antibody Responses are Sustained Two Years Post Mpox Infection but not Following MVA-BN Vaccination. Open Forum Infect. Dis. 2025, ofaf536. [Google Scholar] [CrossRef]
- Overton, E.T.; Lawrence, S.J.; Stapleton, J.T.; Weidenthaler, H.; Schmidt, D.; Koenen, B.; Silbernagl, G.; Nopora, K.; Chaplin, P. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 2020, 38, 2600–2607. [Google Scholar] [CrossRef]
- Greenberg, R.N.; Overton, E.T.; Haas, D.W.; Frank, I.; Goldman, M.; von Krempelhuber, A.; Virgin, G.; Bädeker, N.; Vollmar, J.; Chaplin, P. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J. Infect. Dis. 2013, 207, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, N.; Raccagni, A.R.; Bianchi, M.; Diotallevi, S.; Lolatto, R.; Candela, C.; Uberti Foppa, C.; Gismondo, M.R.; Castagna, A.; Nozza, S.; et al. Mpox neutralising antibodies at 6 months from mpox infection or MVA-BN vaccination: A comparative analysis. Lancet Infect. Dis. 2023, 23, e455–e456. [Google Scholar] [CrossRef] [PubMed]
- Crandell, J.; Monteiro, V.S.; Pischel, L.; Fang, Z.; Conde, L.; Zhong, Y.; Lawres, L.; de Asis, G.M.; Maciel, G.; Zaleski, A.; et al. The impact of orthopoxvirus vaccination and Mpox infection on cross-protective immunity: A multicohort observational study. The Lancet Microbe 2025, 6, 101098. [Google Scholar] [CrossRef]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Gotz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Grüner, E.; Grossegesse, M.; Stern, D.; Ober, V.; Eser, T.M.; Reiling, G.; Stirner, R.; Ibarra, G.; Postel, N.; Conca, R.; et al. Mpox-Specific Immune Responses Elicited by Vaccination or Infection in People with HIV. J. Infect. Dis. 2024, 230, 1110–1119. [Google Scholar] [CrossRef]
- Cohn, H.; Bloom, N.; Cai, G.Y.; Clark, J.J.; Tarke, A.; Bermudez-Gonzalez, M.C.; Altman, D.R.; Lugo, L.A.; Lobo, F.P.; Marquez, S.; et al. Mpox vaccine and infection-driven human immune signatures: An immunological analysis of an observational study. Lancet Infect. Dis. 2023, 23, 1302–1312. [Google Scholar] [CrossRef]
- Sisteré-Oró, M.; Du, J.; Wortmann, D.D.J.; Filippi, M.D.; Cañas-Ruano, E.; Arrieta-Aldea, I.; Marcos-Blanco, A.; Castells, X.; Grau, S.; García-Giralt, N.; et al. Pan-pox-specific T-cell responses in HIV-1-infected individuals after JYNNEOS vaccination. J. Med. Virol. 2024, 96, e29317. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia virus vaccines: Past, present and future. Antiviral Res. 2009, 84, 1–13. [Google Scholar] [CrossRef]
- Cono, J.; Casey, C.G.; Bell, D.M. Smallpox vaccination and adverse reactions: Guidance for clinicians. MMWR Recomm. Rep. 2003, 52, 1–28. [Google Scholar]
- Lane, J.M.; Ruben, F.L.; Neff, J.M.; Millar, J.D. Complications of Smallpox Vaccination, 1968: National surveillance in the United States. N. Engl. J. Med. 1969, 281, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Antoine, G.; Scheiflinger, F.; Dorner, F.; Falkner, F.G. The complete genomic sequence of the modified vaccinia Ankara strain: Comparison with other orthopoxviruses. Virology 1998, 244, 365–396. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, A.; Williamson, A.L.; Weidenthaler, H.; Meyer, T.P.H.; Robertson, J.S.; Excler, J.L.; Condit, R.C.; Evans, E.; Smith, E.R.; Kim, D.; et al. The Brighton Collaboration standardized template for collection of key information for risk/benefit assessment of a Modified Vaccinia Ankara (MVA) vaccine platform. Vaccine 2021, 39, 3067–3080. [Google Scholar] [CrossRef] [PubMed]
- Verheust, C.; Goossens, M.; Pauwels, K.; Breyer, D. Biosafety aspects of modified vaccinia virus Ankara (MVA)-based vectors used for gene therapy or vaccination. Vaccine 2012, 30, 2623–2632. [Google Scholar] [CrossRef]
- Overton, E.T.; Stapleton, J.; Frank, I.; Hassler, S.; Goepfert, P.A.; Barker, D.; Wagner, E.; von Krempelhuber, A.; Virgin, G.; Meyer, T.P.; et al. Safety and Immunogenicity of Modified Vaccinia Ankara-Bavarian Nordic Smallpox Vaccine in Vaccinia-Naive and Experienced Human Immunodeficiency Virus-Infected Individuals: An Open-Label, Controlled Clinical Phase II Trial. Open Forum Infect. Dis. 2015, 2, ofv040. [Google Scholar] [CrossRef]
- von Sonnenburg, F.; Perona, P.; Darsow, U.; Ring, J.; von Krempelhuber, A.; Vollmar, J.; Roesch, S.; Baedeker, N.; Kollaritsch, H.; Chaplin, P. Safety and immunogenicity of modified vaccinia Ankara as a smallpox vaccine in people with atopic dermatitis. Vaccine 2014, 32, 5696–5702. [Google Scholar] [CrossRef]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Ennis, F.; Abate, G.; Hoft, D.F.; Monath, T.P. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax in healthy vaccinia-naive adults. Vaccine 2009, 27, 1637–1644. [Google Scholar] [CrossRef]
- Fine, P.E.; Jezek, Z.; Grab, B.; Dixon, H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988, 17, 643–650. [Google Scholar] [CrossRef]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J.; Hartmann, C.J.; Jackson, D.L.; Kulesh, D.A.; et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004, 428, 182–185. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Espenshade, O.; Bassler, J.; Gong, K.; Lin, S.; Peters, E.; Rhodes, L., Jr.; Spano, Y.E.; et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. USA 2008, 105, 10889–10894. [Google Scholar] [CrossRef]
- Frey, S.E.; Wald, A.; Edupuganti, S.; Jackson, L.A.; Stapleton, J.T.; El Sahly, H.; El-Kamary, S.S.; Edwards, K.; Keyserling, H.; Winokur, P.; et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naive subjects. Vaccine 2015, 33, 5225–5234. [Google Scholar] [CrossRef]
- O’Reilly, S.; Byrne, J.; Feeney, E.R.; Mallon, P.W.G.; Gautier, V. Navigating the Landscape of B Cell Mediated Immunity and Antibody Monitoring in SARS-CoV-2 Vaccine Efficacy: Tools, Strategies and Clinical Trial Insights. Vaccines 2024, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Totmenin, A.V.; Safronov, P.F.; Mikheev, M.V.; Gutorov, V.V.; Ryazankina, O.I.; Petrov, N.A.; Babkin, I.V.; Uvarova, E.A.; Sandakhchiev, L.S.; et al. Analysis of the monkeypox virus genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Shamim, M.; Whitbeck, J.C.; Sfyroera, G.; Lambris, J.D.; Isaacs, S.N. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 2004, 325, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Matho, M.H.; Schlossman, A.; Meng, X.; Benhnia, M.R.; Kaever, T.; Buller, M.; Doronin, K.; Parker, S.; Peters, B.; Crotty, S.; et al. Structural and Functional Characterization of Anti-A33 Antibodies Reveal a Potent Cross-Species Orthopoxviruses Neutralizer. PLoS Pathog. 2015, 11, e1005148. [Google Scholar] [CrossRef]
- Sagdat, K.; Batyrkhan, A.; Kanayeva, D. Exploring monkeypox virus proteins and rapid detection techniques. Front. Cell. Infect. Microbiol. 2024, 14, 1414224. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, K.; Zhou, H. Immunogenic proteins and potential delivery platforms for mpox virus vaccine development: A rapid review. Int. J. Biol. Macromol. 2023, 245, 125515. [Google Scholar] [CrossRef]
- Jones, S.; Hicks, B.; Callaby, H.; Bailey, D.; Gordon, N.C.; Rampling, T.; Houlihan, C.; Jones, R.; Pond, M.; Mehta, R.; et al. Assessment of MpoxPlex, a high-throughput and multiplexed immunoassay: A diagnostic accuracy study. Lancet Microbe 2025, 6, 100987. [Google Scholar] [CrossRef]
- Li, E.; Guo, X.; Hong, D.; Gong, Q.; Xie, W.; Li, T.; Wang, J.; Chuai, X.; Chiu, S. Duration of humoral immunity from smallpox vaccination and its cross-reaction with Mpox virus. Signal Transduct. Target. Ther. 2023, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Chang, Y.X.; Izmailyan, R.; Tang, Y.L.; Chang, W. Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells. J. Virol. 2010, 84, 8422–8432. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.N.; Dowell, A.C.; Jones, S.; Hicks, B.; Rowe, C.; Begum, J.; Wailblinger, D.; Wright, J.; Owens, S.; Pickering, A.; et al. Early evaluation of the safety, reactogenicity, and immune response after a single dose of modified vaccinia Ankara-Bavaria Nordic vaccine against mpox in children: A national outbreak response. Lancet Infect. Dis. 2023, 23, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.C.; Rest, E.C.; Lloyd-Smith, J.O.; Bansal, S. The global landscape of smallpox vaccination history and implications for current and future orthopoxvirus susceptibility: A modelling study. Lancet Infect. Dis. 2023, 23, 454–462. [Google Scholar] [CrossRef]
- Crotty, S.; Felgner, P.; Davies, H.; Glidewell, J.; Villarreal, L.; Ahmed, R. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003, 171, 4969–4973. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.G.C.; Dörner, T.; Hiepe, F. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef]
- Mitja, O.; Alemany, A.; Marks, M.; Lezama Mora, J.I.; Rodriguez-Aldama, J.C.; Torres Silva, M.S.; Corral Herrera, E.A.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in people with advanced HIV infection: A global case series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef]
- Wharton, M.; Strikas, R.A.; Harpaz, R.; Rotz, L.D.; Schwartz, B.; Casey, C.G.; Pearson, M.L.; Anderson, L.J. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm. Rep. 2003, 52, 1–16. [Google Scholar]
- Desai, S.; Landay, A. Early Immune Senescence in HIV Disease. Curr. HIV/AIDS Rep. 2010, 7, 4–10. [Google Scholar] [CrossRef]
- Troy, J.D.; Hill, H.R.; Ewell, M.G.; Frey, S.E. Sex difference in immune response to vaccination: A participant-level meta-analysis of randomized trials of IMVAMUNE smallpox vaccine. Vaccine 2015, 33, 5425–5431. [Google Scholar] [CrossRef] [PubMed]
- Dashraath, P.; Nielsen-Saines, K.; Mattar, C.; Musso, D.; Tambyah, P.; Baud, D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet 2022, 400, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.T. Maternal and Fetal Outcomes Among Pregnant Women with Human Monkeypox Infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Katoto, P.D.; Muttamba, W.; Bahizire, E.; Malembaka, E.B.; Bosa, H.K.; Kazadi, D.M.; Lubambo, G.; Siangoli, F.B.; Bakamutumaho, B.; Wayengera, M.; et al. Shifting transmission patterns of human mpox in South Kivu, DR Congo. Lancet Infect. Dis. 2024, 24, e354–e355. [Google Scholar] [CrossRef]
- Heron, S.E.; Elahi, S. HIV Infection and Compromised Mucosal Immunity: Oral Manifestations and Systemic Inflammation. Front. Immunol. 2017, 8, 241. [Google Scholar] [CrossRef]
- Zhang, A.; Stacey, H.D.; D’Agostino, M.R.; Tugg, Y.; Marzok, A.; Miller, M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat. Rev. Immunol. 2023, 23, 381–396. [Google Scholar] [CrossRef]
- Lederer, K.; Bettini, E.; Parvathaneni, K.; Painter, M.M.; Agarwal, D.; Lundgreen, K.A.; Weirick, M.; Muralidharan, K.; Castano, D.; Goel, R.R.; et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 2022, 185, 1008–1024.e15. [Google Scholar] [CrossRef]
- Chen, J.-L.; Wang, B.; Lu, Y.; Antoun, E.; Bird, O.; Drennan, P.G.; Yin, Z.; Liu, G.; Yao, X.; Pidoux, M.; et al. T cell memory response to MPXV infection exhibits greater effector function and migratory potential compared to MVA-BN vaccination. Nat. Commun. 2025, 16, 4362. [Google Scholar] [CrossRef]
- Calle-Jiménez, O.; Casado-Fernández, G.; Armenteros-Yeguas, I.; Lemus-Aguilar, L.; Baza, B.; Pérez-García, J.A.; Rodríguez-Añover, J.; Mateos, E.; Homen, R.; Orviz-García, E.; et al. Low Specific T-Cell Immunity Against Mpox Elicited in People with HIV-1 and PrEP Users After Subcutaneous Vaccination Compared to Natural Infection. J. Med. Virol. 2025, 97, e70498. [Google Scholar] [CrossRef]
- Grifoni, A.; Zhang, Y.; Tarke, A.; Sidney, J.; Rubiro, P.; Reina-Campos, M.; Filaci, G.; Dan, J.M.; Scheuermann, R.H.; Sette, A. Defining antigen targets to dissect vaccinia virus and monkeypox virus-specific T cell responses in humans. Cell Host Microbe 2022, 30, 1662–1670.e4. [Google Scholar] [CrossRef]
- Byrne, J.; Alalwan, D.; Garcia-Leon, A.; O’Broin, C.; Mallon, P.W.G.; Feeney, E.R. Breakthrough atypical mpox in a vaccinated individual with high antibody titres. Lancet Infect. Dis. 2025, 25, e439. [Google Scholar] [CrossRef]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E.; et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef]
- Panchanathan, V.; Chaudhri, G.; Karupiah, G. Correlates of protective immunity in poxvirus infection: Where does antibody stand? Immunol. Cell Biol. 2008, 86, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Hagan, T.; Rouphael, N.; Wu, S.-Y.; Xie, X.; Kazmin, D.; Wimmers, F.; Gupta, S.; van der Most, R.; Coccia, M.; et al. System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans. Nat. Immunol. 2025, 26, 116–130. [Google Scholar] [CrossRef]
- Du, M.; Niu, B.; Liu, J. The research and development landscape for mpox vaccines. Lancet Infect. Dis. 2025, 25, e198–e199. [Google Scholar] [CrossRef]
- Byrne, J.; Gu, L.; Garcia-Leon, A.; Gaillard, C.M.; Saini, G.; Alalwan, D.; Tomás-Cortázar, J.; Kenny, G.; Donohue, S.; Reynolds, B.; et al. Robust and persistent B-cell responses following SARS-CoV-2 vaccine determine protection from SARS-CoV-2 infection. Front. Immunol. 2024, 15, 1445653. [Google Scholar] [CrossRef]
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e4. [Google Scholar] [CrossRef]
- Mucker, E.M.; Freyn, A.W.; Bixler, S.L.; Cizmeci, D.; Atyeo, C.; Earl, P.L.; Natarajan, H.; Santos, G.; Frey, T.R.; Levin, R.H.; et al. Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates. Cell 2024, 187, 5540–5553.e10. [Google Scholar] [CrossRef]
- Tai, W.; Tian, C.; Shi, H.; Chai, B.; Yu, X.; Zhuang, X.; Dong, P.; Li, M.; Yin, Q.; Feng, S.; et al. An mRNA vaccine against monkeypox virus inhibits infection by co-activation of humoral and cellular immune responses. Nat. Commun. 2025, 16, 2971. [Google Scholar] [CrossRef]
- Brasu, N.; Elia, I.; Russo, V.; Montacchiesi, G.; Stabile, S.A.; De Intinis, C.; Fesi, F.; Gizzi, K.; Macagno, M.; Montone, M.; et al. Memory CD8+ T cell diversity and B cell responses correlate with protection against SARS-CoV-2 following mRNA vaccination. Nat. Immunol. 2022, 23, 1445–1456. [Google Scholar] [CrossRef]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef]
| Study (Ref) | Study Type | Vaccine Regimen | Study Population | Location | Timepoints Analysed | Immunoassay | Key Findings | |
|---|---|---|---|---|---|---|---|---|
| Antibody | Greenberg et al., PloS One, 2016 [9] | Randomised, double-blind, placebo-controlled Phase II trial | 2× MVA vs. 1× MVA after placebo | VACV-experienced adults (n = 120) | USA | Weeks 0, 2, 6; month 6 | ELISA, PRNT | High seroconversion IgG rates and nAb post-dose 2. GMTs were higher in the two-dose group. A single dose elicited a strong memory response. |
| Pittman et al., NEJM, 2019 [11] | Phase III RCT | 2× MVA + 1× ACAM2000 vs. 1× ACAM2000 | Healthy, VACV-naïve adults (n = 440) | USA, South Korea | Weeks 1, 2, 4, 6, 8, 12 | ELISA, PRNT | Similar VACV IgG seroconversion rates (MVA-BN 90.8% vs. ACAM2000 91.8%). MVA was noninferior to ACAM2000 for peak nAb titres (GMT 153.5 vs. 79.3). | |
| Byrne et al., eBioMedicine, 2025 [16] | Observational Cohort Study | 2× MVA | Adults (n = 167), including PWH | Ireland | Up to 1 year | ECL Assay | High VACV IgG seroconversion rates within the first 90 days, significant waning of IgG titres up to 1 year. | |
| Collier et al., JAMA, 2024 [17] | Observational Cohort Study | 1 or 2× MVA | Adults (n = 45) | USA | Weeks 0, 3; months 3, 6, 9, 12 | ELISA, flow cytometry-based live virus neutralisation assay | VACV IgG peaked at 3 weeks and then declined significantly by 12 months. NAb titres were low for 3 months. | |
| Priyamvada et al., Vaccine, 2022 [18] | Observational Cohort Study | 2× MVA | Healthcare workers (n = 999), stratified by prior VACV vaccination | DRC | Weeks 0, 2, 4, 6; months 6, 12, 18, 24 | PRNT, ELISA | Both naïve and previously vaccinated participants mounted robust VACV- and MPXV-nAbs, peaking at week 6. IgG titres waned by 2 years, but seropositivity was retained in most. | |
| Drennan et al., Lancet Microbe, 2025 [19] | Observational Cohort Study | 2× MVA | Adults (n = 34), HIV-negative | United Kingdom | Days 0, 1, 14, 28 (post-dose 1), 28, 90 (post-dose 2) | Luminex | 89% seropositivity by day 90 post-dose 2, though <50% seroconverted by day 28 post-dose 1. | |
| Kottkamp et al., NEJM, 2023 [20] | Observational Cohort Study | 1 or 2× MVA | Adults (n = 145), 24% with HIV, 20% VACV-primed | USA | Up to 3 months post-vaccine | ELISA | IgG titres declined over time (half-life ~108 days). Titres were similar regardless of route (ID vs. SC), dose interval, or HIV status. Prior VACV vaccination conferred greater antibody durability. | |
| Matusali et al., Journal of Infection, 2024 [21] | Observational Cohort Study | 1 or 2× MVA | Adults (n = 50), 42% PWH, 50% VACV-primed | Italy | Months 0, 1, 6, 12 | ELISA, pseudovirus-based neutralisation assay | In primed individuals, 72% remained seropositive at 12 months. MPXV-neutralising antibodies declined over time, detectable in 32% of non-primed individuals at 12 months. No difference by HIV status. | |
| Oom et al., Journal of Virology, 2025 [22] | Observational Cohort Study | 2× MVA | Adults (n = 159), including PWH, 31% prior VACV vaccination | USA | Weeks 0, 3; Months 3, 9 | ELISA, Luminex, live-virus microneutralisation | nABs wane by 6 months in VACV-naïve. Both IgG and nAb were more durable in those with prior VACV vaccination. | |
| Ilchmann et al., JID, 2022 [23] | Phase II Randomised, Placebo-Controlled (With Follow-Up Booster Study) | 1× or 2× MVA, or placebo; single MVA booster at 2 years | Adults (n = 745), adults (n = 152) in booster study | Germany | Weeks 0, 2, 4, 6, 8, 30, and 2 years; post-booster at weeks 1, 2, 4, and month 6 | PRNT, ELISA | Boosting 2 years later led to rapid increases in nAbs. Total antibodies post-booster were highest in the 2× MVA group. | |
| Mazzotta et al., eClinicalMedicine, 2024 [24] | Observational Cohort Study | 1× MVA VACV naïve, 2× MVA VACV experienced | Adults (n = 164), including 46% PWH | Italy | Months 0, 1 | ELISA, PRNT | No difference in IgG between 1 dose vs. 2 doses overall, but 2 doses were more effective at eliciting nAbs in PWH. | |
| Byrne et al., 2025 [OFID, 2025] [25] | Observational Cohort Study | 2× MVA | Adults post-vaccination (n = 122), 25% PWH | Ireland | Up to 2 years | ECL assay | Mean IgG titres declined below the seropositivity threshold by 15.5 months. At 2 years, 32% remained seropositive. PWH had significantly lower odds of sustained seropositivity at 2 years. | |
| Overton et al., Vaccine, 2020 [26] | Randomised Phase II Trial | 2× MVA-BN (standard), 2× double dose, or 2× standard + booster at week 12 | PWH with a history of advanced HIV (n = 87) | USA | Weeks 0, 4, 6, 12, 14, 30, 56 | PRNT, ELISA | Double dose offered no immunogenicity advantage over standard. The booster improved peak and sustained nAbs. | |
| Greenberg et al., JID, 2013 [27] | Phase I/II Trial | 2× MVA-BN in VACV-naïve, 1× in VACV-experienced | Adults (n = 151), including PWH (n = 91) | USA | Days 0, 14, 42; week 8; month 6 | PRNT, ELISA | NaB and IgG responses were comparable across groups. Boosting in VACV-experienced participants showed strong anamnestic responses. GMTs were lower in PWH. | |
| Moschetta et al., Lancet Infect Dis, 2023 [28] | Observational Cohort Study | 2× MVA | Adults (n = 85), including PWH | Italy | Month 6 | PRNT | 12% had no detectable nAb. PWH had higher odds of low titres. | |
| Crandell et al., Lancet Microbe, 2025 [29] | Observational Cohort Study | 2× MVA | Adults (n = 111), including VACV-experienced (n = 36) | USA, Brazil, Portugal | Up to 11 months | ELISA, PRNT | MVA induced strong anti-VACV responses but limited cross-neutralisation against MPXV. MPXV nAbs waned to baseline within 6–11 months. Boosting with MVA in VACV-experienced enhanced breadth and durability. | |
| Zaeck et al., Nat Med, 2023 [30] | Observational Cohort Study | 2× MVA | Adults (n = 105), all VACV-naïve | Netherlands | Days 0, 28, 56 | ELISA, PRNT | 77% developed detectable MPXV nAbs, but titres were low. IgG to MPXV antigens was lower compared to VACV antigens. | |
| Grüner et al., J Infect Dis, 2024 [31] | Observational Cohort Study | 2× MVA | Adults (n = 17), all PWH, 11 VACV-experienced | Germany | Months 0, 3 | ELISA, live-virus microneutralisation assay | Enhanced IgG and nAb responses in VACV-experienced. | |
| B Cell | Oom et al., Journal of Virology, 2025 [22] | Observational Cohort Study | 2× MVA | Adults (n = 159), including PWH, 31% prior VACV vaccination | USA | Weeks 0, 3; months 3, 9 | Memory B cell ELISpot | Memory B cells were detectable in low proportions (less than 40%) at 1 year. |
| Cohn et al., Lancet Infect Dis., 2023 [32] | Observational Cohort Study | 1 or 2× MVA | Adults (n = 10) | USA | 6–60 days post-vaccine | Transcriptomics (single-cell RNAseq) | MVA induced limited B cell activation with negligible gene-level plasmablast and antibody responses. | |
| T Cell | Collier et al., JAMA, 2024 [17] | Observational Cohort Study | 1 or 2× MVA | Adults (n = 45) | USA | Weeks 0, 3; months 3, 6, 9, 12 | Flow cytometry-based ICS against VACV | Low CD4+ and CD8+ T cell responses to VACV at 9 months. |
| Drennan et al., Lancet Microbe, 2025 [19] | Observational Cohort Study | 2× MVA | Adults (n = 34), HIV-negative | United Kingdom | Days 0, 1, 14, 28 (post-dose 1), 28, 90 (post-dose 2) | ELISpot, AIM assay | Peak CD4+ and CD8+ T cell responses were seen by day 14. | |
| Matusali et al., Journal of Infection, 2024 [21] | Observational Cohort Study | 1 or 2× MVA | Adults (n = 50), 42% PWH, 50% VACV-experienced | Italy | Months 0, 1, 6, 12 | IFN-γ ELISpot | T cell responses were robust and durable in both groups for up to one year. | |
| Mazzotta et al., eClinicalMedicine, 2024 [24] | Observational Cohort Study | 1× MVA VACV-naïve, 2× MVA VACV-experienced | Adults (n = 164), including 46% PWH | Italy | Months 0, 1 | IFN-γ ELISpot | 2 doses led to stronger T cell responses. | |
| Crandell et al., Lancet Microbe, 2025 [29] | Observational Cohort Study | 2× MVA | Adults (n = 111), including VACV-experienced (n = 36) | USA, Brazil, Portugal | Up to 11 months | Flow cytometry, OPXV peptide stimulation | T cell cross-reactivity to MPXV was robust and long-lasting but declined with age. | |
| Cohn et al., Lancet Infect Dis., 2023 [32] | Observational Cohort Study | 1 or 2× MVA | Adults (n = 10) | USA | Up to 2 months | AIM/ICS T cell assays | MVA induced robust CD4+ and CD8+ T cell responses. | |
| Grüner et al., J Infect Dis, 2024 [31] | Observational Cohort Study | 2× MVA | Adults (n = 17), all PWH | Germany | Months 0, 3 | AIM assay | CD8+ T cell responses increased after the second MVA dose, but CD4+ responses were modest. | |
| Sisteré-Oró et al., J Med Virol, 2024 [33] | Observational Cohort Study | 1 or 2× MVA | Adults (n = 24), all PWH | Spain | Days 0, 28 | ELISpot, ICS, | Intradermal vaccination induced stronger T cell responses than subcutaneous. CD4+ count correlated with response. |
| Virion Form | MPXV Antigen | VACV Homologue (% Homology) | Known/Proposed Function | Assay Type and Study |
|---|---|---|---|---|
| EEV | B6R | B5R (95.9%) | Envelope glycoprotein required for efficient cell spread, complement control | Anti-MPXV B6R IgG (ELISA) [17,19,29,55] Anti-VACVB5 IgG (ELISA) [29] Anti-MPXV B6R IgG, Anti-VACVB5 IgG (Luminex®) [19] Anti-MPXV B6R IgG, Anti-VACVB5 IgG (Electrochemiluminescence Assay) [16] |
| A35R | A33R (96.1%) | Envelope glycoprotein, needed for the formation of actin-containing microvilli and cell-to-cell spread | Anti-MPXV A35R IgG (Luminex®) [19,22] Anti-VACVA33R IgG (Luminex®) [19] MPXV A35R-specific Memory B Cell (ELISpot) [22] Anti-MPXV A35R IgG (ELISA) [17,29,32,55] Anti-VACVB5 A33R IgG (ELISA) [29] | |
| B2 | A56 (93%) | Type I membrane glycoprotein haemagglutinin | Anti-MPXV B2 IgG (Luminex®) [19] | |
| IMV | A29L | A27L (93.6%) | Surface membrane fusion protein, binds cell surface heparan | Anti-MPXV A29L IgG (ELISA) [17,29,32,55] Anti MPXVA29L, Anti-VACV A27L IgG (Luminex®) [19] Anti-VACV A27L IgG (ELISA) [29] |
| H3L | H3 (92.3%) | Heparan-binding surface membrane protein, attaches to the cell surface by binding to glycosaminoglycan (GAG) | Anti-MPXV H3L IgG (Luminex®) [19,22] Anti-MPXV H3L IgG (ELISA), MPXV H3L-specific Memory B Cell (ELISpot) [22] Anti-H3L IgG (ELISA) [17,20] | |
| M1R | L1 (98.8%) | Surface membrane protein, mediates virus entry into cells independently of GAG | Anti-MPXV M1R IgG (ELISA) [17,29,55] Anti-MPXV M1R IgG (Luminex®) [19] Anti-VACV L1R IgG (ELISA) [29] | |
| A27L | Deleted from MVA-BN genome | Surface membrane protein | Anti-MPXV A27L IgG (Electrochemiluminescence Assay) [16] Anti-MPXV A27L IgG (Luminex®) [19] Anti-MPXV A27L IgG (ELISA) [32] | |
| E8L | D8 (94.7%) | Surface membrane protein, binds cell surface chondroitin sulfate, IMV adsorption to cell surface | Anti-MPXV E8L IgG (Luminex®) [19] Anti-MPXV E8L IgG (ELISA) [29,32] Anti-VACV D8 IgG (ELISA) [29] | |
| A5 | A4 (95%) | Immunodominant virion core protein | Anti-MPXV A5 IgG (Luminex®) [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, J.; Katoto, P.D.M.C.; Kirenga, B.; Sabiiti, W.; Obuku, A.; Gautier, V.; Mallon, P.W.G.; Feeney, E.R. Immune Response to MVA-BN Vaccination for Mpox: Current Evidence and Future Directions. Vaccines 2025, 13, 930. https://doi.org/10.3390/vaccines13090930
Byrne J, Katoto PDMC, Kirenga B, Sabiiti W, Obuku A, Gautier V, Mallon PWG, Feeney ER. Immune Response to MVA-BN Vaccination for Mpox: Current Evidence and Future Directions. Vaccines. 2025; 13(9):930. https://doi.org/10.3390/vaccines13090930
Chicago/Turabian StyleByrne, Joanne, Patrick D. M. C. Katoto, Bruce Kirenga, Wilber Sabiiti, Andrew Obuku, Virginie Gautier, Patrick W. G. Mallon, and Eoin R. Feeney. 2025. "Immune Response to MVA-BN Vaccination for Mpox: Current Evidence and Future Directions" Vaccines 13, no. 9: 930. https://doi.org/10.3390/vaccines13090930
APA StyleByrne, J., Katoto, P. D. M. C., Kirenga, B., Sabiiti, W., Obuku, A., Gautier, V., Mallon, P. W. G., & Feeney, E. R. (2025). Immune Response to MVA-BN Vaccination for Mpox: Current Evidence and Future Directions. Vaccines, 13(9), 930. https://doi.org/10.3390/vaccines13090930







