Correction: Hernández-Pedro et al. Impact of Tyrosine Kinase Inhibitors on the Immune Response to SARS-CoV-2 Vaccination in Patients with Thoracic Malignancies. Vaccines 2023, 11, 1612
| Clinical Characteristics | SARS-COV-2 Antigen (>20) | SARS-COV-2 Antigen (<20) | p-Value | SARS-COV-2 Titles (−) | SARS-COV-2 Titles (+) | p-Value |
|---|---|---|---|---|---|---|
| N = 92 | % (n) | % (n) | % (n) | % (n) | ||
| Sex | ||||||
| Male: 39 | 46.2 (18) | 53.8 (21) | 46.2 (18) | 53.8 (21) | ||
| Female: 53 | 45.3 (24) | 54.7 (29) | 0.934 | 50.9 (27) | 49.1 (26) | 0.650 |
| Age | ||||||
| <59: 41 | 34.1 (14) | 65.9 (27) | 39.0 (16) | 61.0 (25) | ||
| ≥59: 51 | 54.9 (28) | 45.1 (23) | 0.047 | 56.9 (29) | 43.1 (22) | 0.089 |
| Smoking status | ||||||
| No: 67 | 41.8 (28) | 58.2 (39) | 46.3 (31) | 53.7 (36) | ||
| Yes: 25 | 56.0 (14) | 44.0 (11) | 0.224 | 56.0 (14) | 44.0 (11) | 0.406 |
| Woodsmoke exposure | ||||||
| No: 72 | 48.6 (35) | 51.4 (37) | 50.0 (36) | 50.0 (36) | ||
| Yes: 20 | 35.0 (7) | 65.0 (13) | 0.280 | 45.0 (9) | 55.0 (11) | 0.692 |
| Asbestos exposure | ||||||
| No: 82 | 47.6 (39) | 52.4 (43) | 50.0 (41) | 50.0 (41) | ||
| Yes: 10 | 30.0 (3) | 70.0 (7) | 0.336 * | 40.0 (4) | 60.0 (6) | 0.740 * |
| ECOG PS | ||||||
| 0–1: 86 | 47.7 (41) | 52.3 (45) | 51.2 (44) | 48.8 (42) | ||
| >2: 6 | 16.7 (1) | 83.3 (5) | 0.214 * | 16.7 (1) | 83.3 (5) | 0.204 * |
| Clinical stage | ||||||
| I–III: 10 | 60.0 (6) | 40.0 (4) | 60.0 (6) | 40.0 (4) | ||
| IV: 82 | 43.9 (36) | 56.1 (46) | 0.503 * | 47.6 (39) | 52.4 (43) | 0.518 * |
| Histology | ||||||
| Adenocarcinoma: 79 | 44.3 (35) | 55.7 (44) | 48.1 (38) | 51.9 (41) | ||
| Squamous: 4 | 75.0 (3) | 25.0 (1) | 75.0 (3) | 25.0 (1) | ||
| Mesothelioma: 5 | 20.0 (1) | 80.0 (4) | 20.0 (1) | 80.0 (4) | ||
| Others: 4 | 75.0 (3) | 25.0 (1) | 0.245 | 75.0 (3) | 25.0 (1) | 0.276 |
| Histological grade | ||||||
| Low: 15 | 46.7 (7) | 53.3 (8) | 53.3 (8) | 46.7 (7) | ||
| Intermediate: 27 | 51.9 (14) | 48.1 (13) | 51.9 (14) | 48.1 (13) | ||
| High: 23 | 34.8 (8) | 65.2 (15) | 34.8 (8) | 65.2 (15) | ||
| n/a: 14 | 42.9 (6) | 57.1 (8) | 0.679 | 57.1 (8) | 42.9 (6) | 0.492 |
| Metastases | ||||||
| CNS: 23 | 47.8 (11) | 52.2 (12) | 0.809 | 52.2 (12) | 47.8 (11) | 0.718 |
| Liver: 7 | 42.9 (3) | 57.1 (4) | 1.00 * | 42.9 (3) | 57.1 (4) | 1.00 * |
| Lung: 60 | 48.3 (29) | 51.7 (31) | 0.480 | 51.7 (31) | 48.3 (29) | 0.469 |
| Ganglia: 8 | 62.5 (5) | 37.5 (3) | 0.317 * | 62.5 (5) | 37.5 (3) | 0.481 * |
| Bone: 28 | 35.7 (10) | 64.3 (18) | 0.206 | 42.9 (12) | 57.1 (16) | 0.442 |
| Pulmonary effusion: 31 | 51.6 (16) | 48.4 (15) | 0.413 | 58.1 (18) | 41.9 (13) | 0.211 |
| EGFR | ||||||
| Wild type: 54 | 44.4 (24) | 55.6 (30) | 46.3 (25) | 53.7 (29) | ||
| Mutant: 38 | 47.4 (18) | 52.6 (20) | 0.782 | 52.6 (20) | 47.4 (18) | 0.549 |
| EGFR subtype | ||||||
| Exon 19 del: 26 | 42.3 (11) | 57.7 (15) | 0.686 | 46.2 (12) | 53.8 (14) | 0.740 |
| Exon 21 L858R: 12 | 50.0 (6) | 50.0 (6) | 0.746 | 58.3 (7) | 41.7 (5) | 0.547 * |
| Exon 20 T790M: 1 | 100 (1) | 0.0 (0) | 0.457 * | 100 (1) | 0.0 (0) | 0.489 * |
| ALK | ||||||
| Wild type: 68 | 51.5 (35) | 48.5 (33) | 54.4 (37) | 45.6 (31) | ||
| Mutant: 24 | 29.2 (7) | 70.8 (17) | 0.059 | 33.3 (8) | 66.7 (16) | 0.076 |
| TKI treatment | ||||||
| No: 20 | 55.0 (11) | 45.0 (9) | 55.0 (11) | 45.0 (9) | ||
| Yes: 55 | 40.0 (22) | 60.0 (33) | 0.247 | 45.5 (25) | 54.5 (30) | 0.464 |
| QT treatment | ||||||
| No: 61 | 41.0 (25) | 59.0 (36) | 45.9 (28) | 54.1 (33) | ||
| Yes: 31 | 54.8 (17) | 45.2 (14) | 0.207 | 54.8 (17) | 45.2 (14) | 0.418 |
| Vaccine | ||||||
| BNT162b2: 31 | 48.4 (15) | 51.6 (16) | 48.4 (15) | 51.6 (16) | ||
| AZD1222: 37 | 45.9 (17) | 54.1 (20) | 48.6 (18) | 51.4 (19) | ||
| Sputnik: 10 | 50.0 (5) | 50.0 (5) | 50.0 (5) | 50.0 (5) | ||
| Sinovac: 13 | 38.5 (5) | 61.5 (8) | 46.2 (6) | 53.8 (7) | ||
| Cansino: 1 | 0.0 (0) | 100 (1) | 0.864 | 100 (1) | 0 (0) | 0.895 |
| COVID-19 | ||||||
| No: 82 | 47.6 (39) | 52.4 (43) | 51.2 (42) | 48.8 (40) | ||
| Yes: 10 | 30.0 (3) | 70.0 (7) | 0.336 * | 30.0 (3) | 70.0 (7) | 0.317 * |
- 12.
- Tapia-Conyer, R.; Kuri-Morales, P.; González-Urbán, L.; Sarti, E. Evaluation and Reform of Mexican National Epidemiological Surveillance System. Am. J. Public Health 2001, 91, 1758–1760. https://doi.org/10.2105/ajph.91.11.1758.
| Clinical Characteristics | Neutralization Antibodies for SARS-COV-2 Virus (Cut-Off > 20) | Neutralization Antibodies for SARS-COV-2 Virus (Cut-Off < 20) | p-Value | SARS-COV-2 Antibodies Titles (−) | SARS-COV-2 Antibodies Titles (+) | p-Value |
|---|---|---|---|---|---|---|
| N = 92 | % (n) | % (n) | % (n) | % (n) | ||
| Sex | ||||||
| Male: 39 | 46.2 (18) | 53.8 (21) | 46.2 (18) | 53.8 (21) | ||
| Female: 53 | 45.3 (24) | 54.7 (29) | 0.934 | 50.9 (27) | 49.1 (26) | 0.678 |
| Age | ||||||
| <59: 41 | 34.1 (14) | 65.9 (27) | 39.0 (16) | 61.0 (25) | ||
| ≥59: 51 | 54.9 (28) | 45.1 (23) | 0.047 | 56.9 (29) | 43.1 (22) | 0.098 |
| Smoking status | ||||||
| No: 67 | 41.8 (28) | 58.2 (39) | 46.3 (31) | 53.7 (36) | ||
| Yes: 25 | 56.0 (14) | 44.0 (11) | 0.224 | 56 (14) | 44.0 (11) | 0.485 |
| Woodsmoke exposure | ||||||
| No: 72 | 48.6 (35) | 51.4 (37) | 50.0 (36) | 50.0 (36) | ||
| Yes: 20 | 35.0 (7) | 65.0 (13) | 0.280 | 45.0 (9) | 55.0 (11) | 0.802 |
| Asbestos exposure | ||||||
| No: 82 | 47.6 (39) | 52.4 (43) | 50 (41) | 50 (41) | ||
| Yes: 10 | 30.0 (3) | 70.0 (7) | 0.293 | 40 (4) | 60 (6) | 0.740 |
| ECOG PS | ||||||
| 0–1: 86 | 47.7 (41) | 52.3 (45) | 51.2 (44) | 48.8 (42) | ||
| >2: 6 | 16.7 (1) | 83.3 (5) | 0.214 | 16.7 (1) | 83.3 (5) | 0.204 |
| Clinical stage | ||||||
| I–III: 10 | 60.0 (6) | 40.0 (4) | 60.0 (6) | 40.0 (4) | ||
| IV: 82 | 43.9 (36) | 56.1 (46) | 0.503 | 47.6 (39) | 52.4 (43) | 0.518 |
| Histology | ||||||
| Adenocarcinoma: 79 | 44.3 (35) | 55.7 (44) | 48.1 (38) | 51.9 (41) | ||
| Squamous: 4 | 75.0 (3) | 25.0 (1) | 75.0 (3) | 25.0 (1) | ||
| Mesothelioma: 5 | 20.0 (1) | 80.0 (4) | 20.0 (1) | 80.0 (4) | ||
| Others: 4 | 75.0 (3) | 25.0 (1) | 0.245 | 75.0 (3) | 25.0 (1) | 0.276 |
| Histological grade | ||||||
| Low: 15 | 46.7 (7) | 53.3 (8) | 53.3 (8) | 46.7 (7) | ||
| Intermediate: 27 | 51.9 (14) | 48.1 (13) | 51.9 (14) | 48.1 (13) | ||
| High: 23 | 34.8 (8) | 65.2 (15) | 34.8 (8) | 65.2 (15) | ||
| n/a: 14 | 42.9 (6) | 57.1 (8) | 0.679 | 57.1 (8) | 42.9 (6) | 0.492 |
| Metastases * | ||||||
| CNS: 23 | 47.8 (11) | 52.2 (12) | 0.809 | 52.2 (12) | 47.8 (11) | 0.811 |
| Liver: 7 | 42.9 (3) | 57.1 (4) | 0.877 | 42.9 (3) | 57.1 (4) | 1.00 |
| Lung: 60 | 48.3 (29) | 51.7 (31) | 0.480 | 51.7 (31) | 48.3 (29) | 0.517 |
| Ganglia: 8 | 62.5 (5) | 37.5 (3) | 0.317 | 62.5 (5) | 37.5 (3) | 0.481 |
| Bone: 28 | 35.7 (10) | 64.3 (18) | 0.206 | 42.9 (12) | 57.1 (16) | 0.501 |
| Pulmonary effusion: 31 | 51.6 (16) | 48.4 (15) | 0.413 | 58.1 (18) | 41.9 (13) | 0.271 |
| EGFR | ||||||
| Wild type: 54 | 44.4 (24) | 55.6 (30) | 46.3 (25) | 53.7 (29) | ||
| Mutant: 38 | 47.4 (18) | 52.6 (20) | 0.782 | 52.6 (20) | 47.4 (18) | 0.672 |
| EGFR subtype | ||||||
| Exon 19 del: 26 | 42.3 (11) | 57.7 (15) | 0.686 | 46.2 (12) | 53.8 (14) | 0.819 |
| Exon 21 L858R: 12 | 50.0 (6) | 50.0 (6) | 0.746 | 58.3 (7) | 41.7 (5) | 0.547 |
| Exon 20 T790M: 1 | 100 (1) | 0.0 (0) | 0.273 | 100 (1) | 0.0 (0) | 0.489 |
| ALK | ||||||
| Wild type: 68 | 51.5 (35) | 48.5 (33) | 54.4 (37) | 45.6 (31) | ||
| Mutant: 24 | 29.2 (7) | 70.8 (17) | 0.059 | 33.3 (8) | 66.7 (16) | 0.098 |
| TKI treatment | ||||||
| No: 20 | 55.0 (11) | 45.0 (9) | 55.0 (11) | 45.0 (9) | ||
| Yes: 55 | 40.0 (22) | 60.0 (33) | 0.247 | 45.5 (25) | 54.5 (30) | 0.602 |
| QT treatment | ||||||
| No: 61 | 41.0 (25) | 59.0 (36) | 45.9 (28) | 54.1 (33) | ||
| Yes: 31 | 54.8 (17) | 45.2 (14) | 0.207 | 54.8 (17) | 45.2 (14) | 0.509 |
| Vaccine | ||||||
| BNT162b2: 31 | 48.4 (15) | 51.6 (16) | 48.4 (15) | 51.6 (16) | ||
| AZD1222: 36 | 45.9 (17) | 54.1 (20) | 48.6 (18) | 51.4 (19) | ||
| Sputnik: 10 | 50.0 (5) | 50.0 (5) | 50.0 (5) | 50.0 (5) | ||
| Sinovac: 13 | 38.5 (5) | 61.5 (8) | 46.2 (6) | 53.8 (7) | ||
| Cansino: 1 | 0.0 (0) | 100 (1) | 0.864 | 100 (1) | 0 (0) | 0.895 |
| COVID-19 | ||||||
| No: 82 | 47.6 (39) | 52.4 (43) | 51.2 (42) | 48.8 (40) | ||
| Yes: 10 | 30.0 (3) | 70.0 (7) | 0.293 | 30.0 (3) | 70.0 (7) | 0.317 |
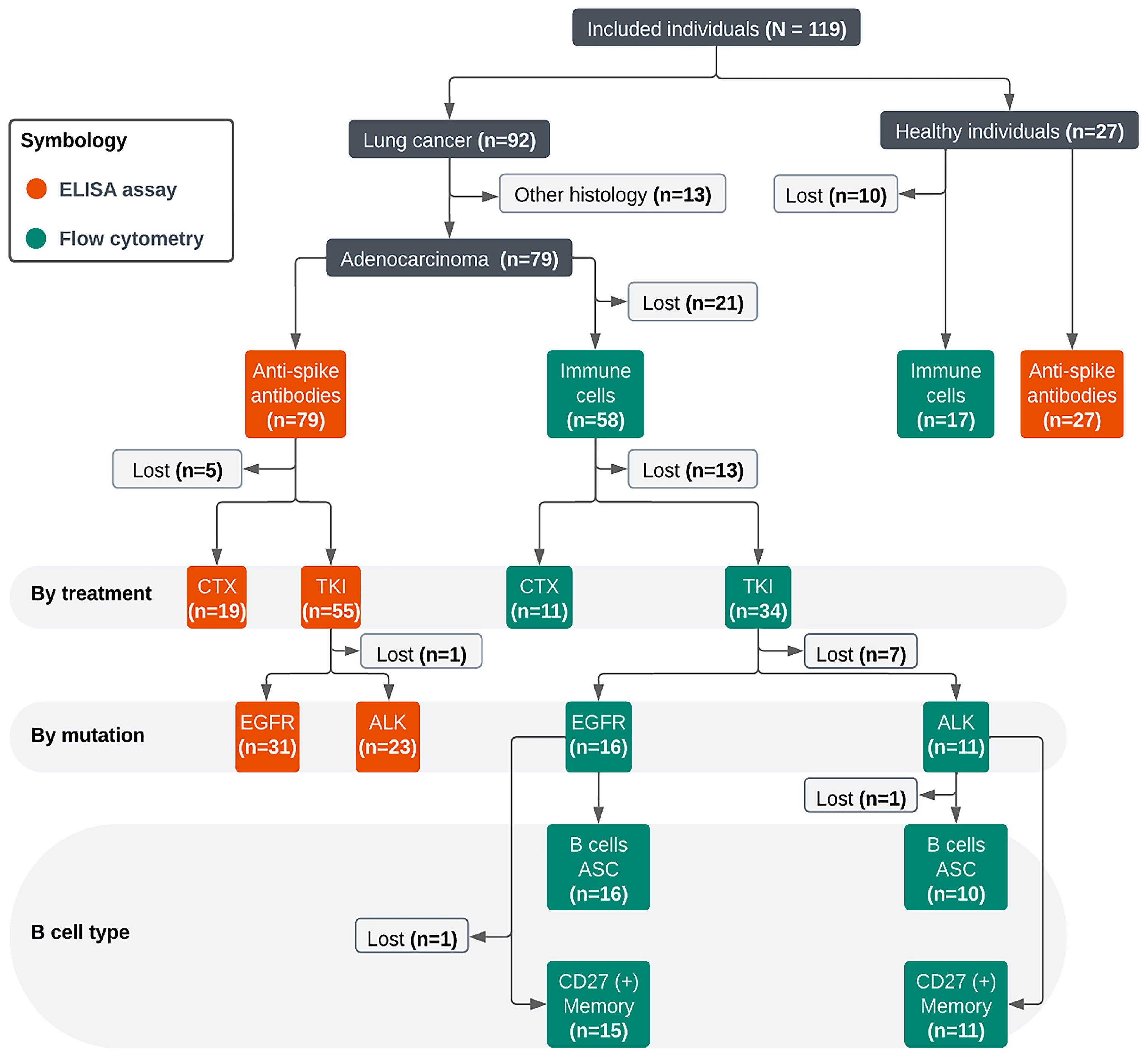
Reference
- Hernández-Pedro, N.; Arroyo-Hernández, M.; Barrios-Bernal, P.; Romero-Nuñez, E.; Sosa-Hernandez, V.A.; Ávila-Ríos, S.; Maravillas-Montero, J.L.; Pérez-Padilla, R.; de Miguel-Perez, D.; Rolfo, C.; et al. Impact of Tyrosine Kinase Inhibitors on the Immune Response to SARS-CoV-2 Vaccination in Patients with Thoracic Malignancies. Vaccines 2023, 11, 1612. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Pedro, N.; Arroyo-Hernández, M.; Barrios-Bernal, P.; Romero-Nuñez, E.; Sosa-Hernandez, V.A.; Ávila-Ríos, S.; Maravillas-Montero, J.L.; Pérez-Padilla, R.; de Miguel-Perez, D.; Rolfo, C.; et al. Correction: Hernández-Pedro et al. Impact of Tyrosine Kinase Inhibitors on the Immune Response to SARS-CoV-2 Vaccination in Patients with Thoracic Malignancies. Vaccines 2023, 11, 1612. Vaccines 2025, 13, 913. https://doi.org/10.3390/vaccines13090913
Hernández-Pedro N, Arroyo-Hernández M, Barrios-Bernal P, Romero-Nuñez E, Sosa-Hernandez VA, Ávila-Ríos S, Maravillas-Montero JL, Pérez-Padilla R, de Miguel-Perez D, Rolfo C, et al. Correction: Hernández-Pedro et al. Impact of Tyrosine Kinase Inhibitors on the Immune Response to SARS-CoV-2 Vaccination in Patients with Thoracic Malignancies. Vaccines 2023, 11, 1612. Vaccines. 2025; 13(9):913. https://doi.org/10.3390/vaccines13090913
Chicago/Turabian StyleHernández-Pedro, Norma, Marisol Arroyo-Hernández, Pedro Barrios-Bernal, Eunice Romero-Nuñez, Victor A. Sosa-Hernandez, Santiago Ávila-Ríos, José Luis Maravillas-Montero, Rogelio Pérez-Padilla, Diego de Miguel-Perez, Christian Rolfo, and et al. 2025. "Correction: Hernández-Pedro et al. Impact of Tyrosine Kinase Inhibitors on the Immune Response to SARS-CoV-2 Vaccination in Patients with Thoracic Malignancies. Vaccines 2023, 11, 1612" Vaccines 13, no. 9: 913. https://doi.org/10.3390/vaccines13090913
APA StyleHernández-Pedro, N., Arroyo-Hernández, M., Barrios-Bernal, P., Romero-Nuñez, E., Sosa-Hernandez, V. A., Ávila-Ríos, S., Maravillas-Montero, J. L., Pérez-Padilla, R., de Miguel-Perez, D., Rolfo, C., & Arrieta, O. (2025). Correction: Hernández-Pedro et al. Impact of Tyrosine Kinase Inhibitors on the Immune Response to SARS-CoV-2 Vaccination in Patients with Thoracic Malignancies. Vaccines 2023, 11, 1612. Vaccines, 13(9), 913. https://doi.org/10.3390/vaccines13090913






