Hepatitis E Vaccination Preferences and Willingness-to-Pay Among Residents: A Discrete Choice Experiment Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
- (a)
- Permanent residency: Continuous residence ≥6 months in the sampled administrative village/community as of the survey date, irrespective of household registration status;
- (b)
- Age range: 15 to 66 years inclusive, verified through government-issued identification or household registration documents;
- (c)
- Informed consent: After trained investigators comprehensively explained the study purpose, procedures, potential benefits/risks (primarily time commitment and privacy safeguards), voluntary participation, right to withdraw unconditionally, and confidentiality measures in a clear, non-suggestive manner, participants provided written informed consent. For non-literate individuals, impartial witnesses attested to the complete disclosure process before participants affixed their thumbprint, with both investigator and witness countersigning.
- (a)
- Comprehension and communication barriers: Individuals with severe hearing, linguistic, or cognitive impairments (e.g., intellectual disability, advanced dementia) were excluded if investigators assessed them as incapable of comprehending survey content or engaging in basic communication during the consent process;
- (b)
- Psychiatric disorders: Clinically diagnosed patients with severe mental illnesses (e.g., schizophrenia, bipolar disorder in acutely symptomatic phase) exhibiting unstable conditions that could impair rational decision making or study understanding.
2.2. Study Design
2.2.1. Attribute and Level Identification
- (a)
- Post-marketing surveillance data from long-term, large-scale HEV vaccination programs (e.g., the China-licensed vaccine [13]) demonstrate an incidence rate orders of magnitude lower than common vaccines.
- (b)
- China’s established and rigorous Adverse Events Following Immunization (AEFI) surveillance system ensures robust safety monitoring, maintaining relatively high public trust.
- (a)
- Most participants clearly understood “efficacy,” “duration,” and “cost” attributes.
- (b)
- Regarding “vaccination location,” respondents widely reported minimal perceived differences in convenience (e.g., distance, time cost) across healthcare tiers (village clinics, township health centers, county hospitals) due to Shaanxi’s well-established primary healthcare network. Typical responses included the following: “Similar convenience regardless of site” or “Routine vaccination occurs at nearest local clinics.”
2.2.2. DCE Framework
2.2.3. Questionnaire Development and Piloting
- Instructions: Study background, objectives, and completion guidelines.
- Demographics: Age, gender, education, income, etc.
- Health status: Chronic liver disease history, prior vaccination experience.
- Vaccination intent: Willingness to receive HEV vaccination.
- DCE section: Presented choice sets requiring participants to select preferred alternatives.
2.3. Sample Size Estimation
- (a)
- Basic sample: 300 residents were sampled from each of the 10 sample counties, for a total of 3000 people.
- (b)
- Professional sample: An additional 300 healthcare workers were selected.
2.4. Data Collection
2.5. Data Analysis Methods
2.5.1. Descriptive Statistics
2.5.2. Mixed Logit Model
- (a)
- Random parameters: protective efficacy, duration of protection, and out-of-pocket cost;
- (b)
- Distributional assumption: All random parameters were assumed to follow a normal distribution;
- (c)
- Correlation structure: Random parameters were specified as mutually independent, resulting in a diagonal covariance matrix (implemented by omitting the “corr” option in Stata17.0’s “mixlogit” command);
- (d)
- Variance estimation: The model estimated the standard deviation and statistical significance of each random parameter.
2.5.3. WTP Analysis
2.6. Ethics
3. Results
3.1. Participant Characteristics
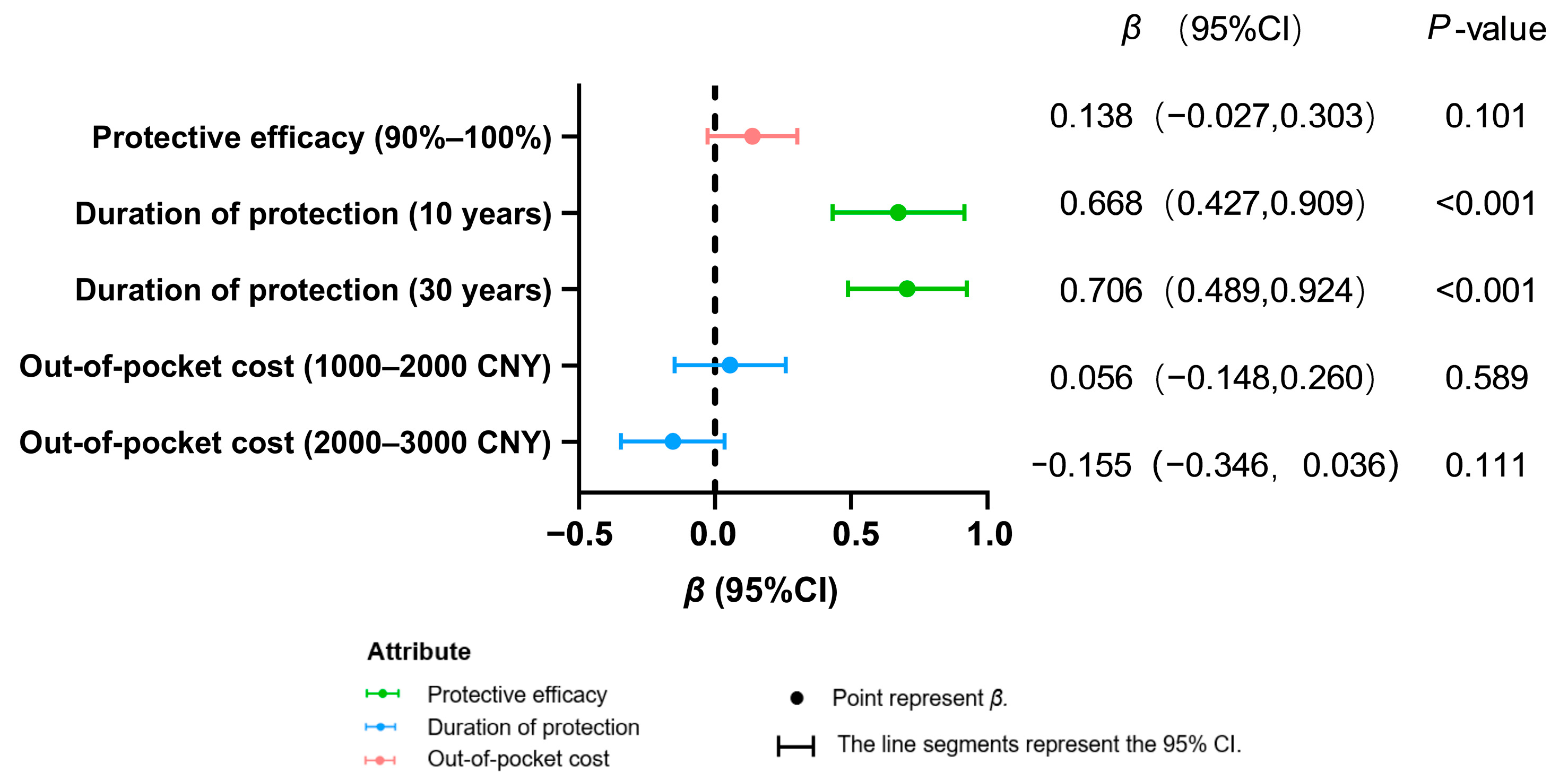
3.2. Attribute-Specific Influences on Vaccination Decisions
3.3. WTP Analysis for Hepatitis E Vaccine
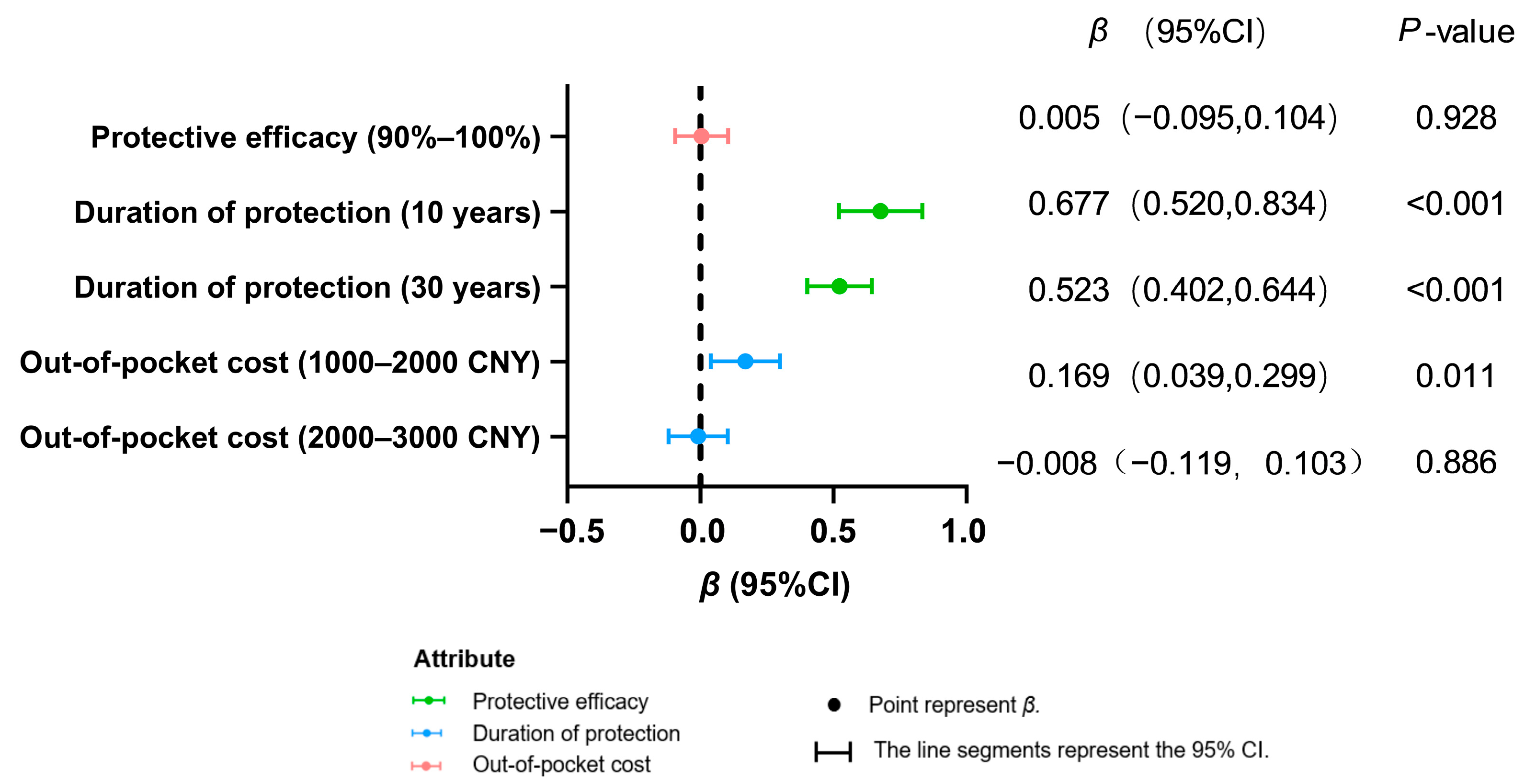
3.4. Subgroup Preference Heterogeneity
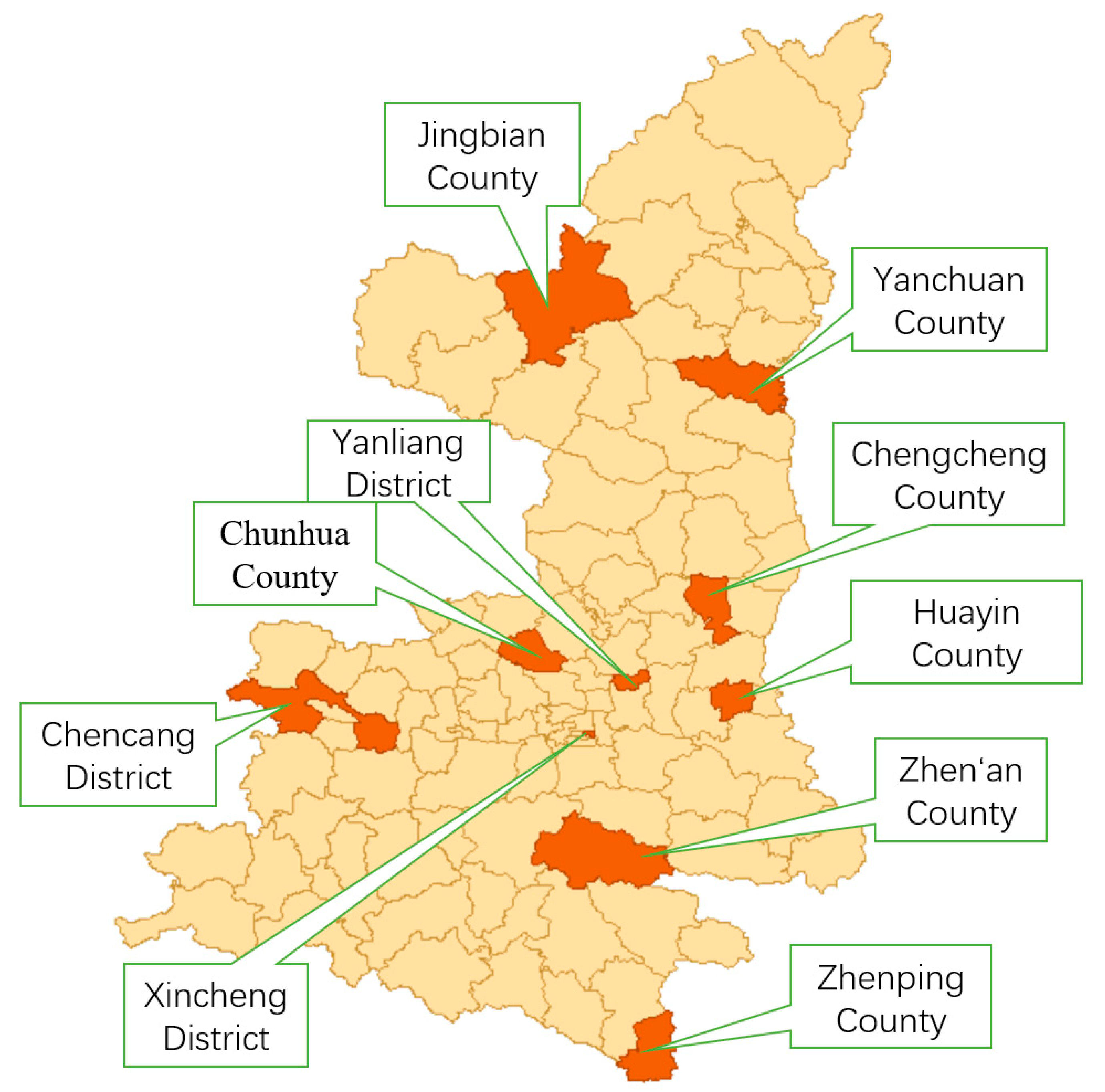
3.5. Residential Heterogeneity in Vaccine Preferences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, L.Z.; Zheng, M.H.; Li, S.W.; Xia, N.S. Epidemiological characteristics of hepatitis E and progress in vaccine development. J. Xiamen Univ. (Nat. Sci.) 2024, 63, 378–386. [Google Scholar]
- World Health Organization. Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-E (accessed on 11 March 2025).
- Songtanin, B.; Molehin, A.J.; Brittan, K.; Manatsathit, W.; Nugent, K. Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations. Viruses 2023, 15, 1389. [Google Scholar] [CrossRef]
- Pérez-Gracia, M.T.; Suay-García, B.; Mateos-Lindemann, M.L. Hepatitis E and pregnancy: Current state. Rev. Med. Virol. 2017, 27, e1929. [Google Scholar] [CrossRef]
- Dong, D.B.; Zou, S.Q.; Tang, S. Spatio-temporal epidemiological characterisation of viral hepatitis incidence in China, 2009–2019. Mod. Prev. Med. 2024, 51, 595–601. [Google Scholar]
- Kong, D.G.; Tang, W.F.; Chen, J.; Hu, Q. Attitude toward Hepatitis E vaccine and influence factors among urban residents in Wuhan. J. Public Health Prev. Med. 2018, 29, 41–44. [Google Scholar]
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.Y.; Zhou, T.T.; Wang, R.Z.; Si, Y.; Hu, W.J. Seroprevalence of Hepatitis E Antibody among a 1-60-year-old Population of Shaanxi Province in 2017. Chin. J. Vaccines Immun. 2022, 28, 15–18. [Google Scholar]
- Li, L.J. Expert consensus on the process of in-hospital screening and management of viral hepatitis E in China. J. Clin. Hepatol. 2023, 39, 785–794. [Google Scholar]
- Tang, Z.M.; Ge, S.X. Research progress in laboratory diagnosis of hepatitis E virus infection. J. Xiamen Univ. (Nat. Sci.) 2024, 63, 345–356. [Google Scholar]
- Almeida, P.H.; Matielo, C.E.L.; Curvelo, L.A.; Rocco, R.A.; Felga, G.; Della Guardia, B.; Boteon, Y.L. Update on the management and treatment of viral hepatitis. World J. Gastroenterol. 2021, 27, 3249–3261. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.F.; Huang, S.J.; Wu, T.; Hu, Y.M.; Wang, Z.Z.; Wang, H.; Jiang, H.M.; Wang, Y.J.; Yan, Q.; et al. Long-term efficacy of a hepatitis E vaccine. N. Engl. J. Med. 2015, 372, 914–922. [Google Scholar] [CrossRef]
- WHO. Hepatitis E vaccine: WHO position paper, May 2015—Recommendations. Vaccine 2016, 34, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, R.C.; Asilaza, V.K.; Gignoux, E.; Koyuncu, A.; Gitahi, P.; Nkemenang, P.; Duncker, J.; Antier, Z.; Haile, M.; Gakima, P.; et al. Vaccination coverage and adverse events following a reactive vaccination campaign against hepatitis E in Bentiu displaced persons camp, South Sudan. PLoS Neglected Trop. Dis. 2024, 18, e0011661. [Google Scholar] [CrossRef]
- Ciglenecki, I.; Rumunu, J.; Wamala, J.F.; Nkemenang, P.; Duncker, J.; Nesbitt, R.; Gignoux, E.; Newport, T.; Heile, M.; Jamet, C.; et al. The first reactive vaccination campaign against hepatitis E. Lancet Infect. Dis. 2022, 22, 1110–1111. [Google Scholar] [CrossRef]
- Yan, R.; Sun, X.D.; Li, Z.; Zhang, L.P.; Chen, H.H.; Wu, X.T.; Du, Y.; Jin, B.F.; Mei, K.W. Uptake of nine vaccines and influencing factors of vaccine hesitancy among adults in Minhang district, Shanghai. Chin. J. Viral Dis. 2023, 13, 278–285. [Google Scholar]
- Martinón-Torres, F.; de Miguel, Á.G.; Ruiz-Contreras, J.; Vallejo-Aparicio, L.A.; García, A.; Gonzalez-Inchausti, M.C.; de Gomensoro, E.; Kocaata, Z.; Gabás-Rivera, C.; Comellas, M.; et al. Societal Preferences for Meningococcal B Vaccination in Children: A Discrete Choice Experiment in Spain. Infect. Dis. Ther. 2023, 12, 157–175. [Google Scholar] [CrossRef]
- Chan, A.H.Y.; Tao, M.; Marsh, S.; Petousis-Harris, H. Vaccine decision making in New Zealand: A discrete choice experiment. BMC Public Health 2024, 24, 447. [Google Scholar] [CrossRef]
- Zhang, M.X.; Xu, S.F.; Xiong, H.Y. Selection preference study of 13-valent pneumococcal conjugate vaccine based on discrete choice experiment in Guizhou province. Health Dev. Policy Res. 2023, 26, 35–41. [Google Scholar]
- Michaels-Igbokwe, C.; MacDonald, S.; Currie, G.R. Individual Preferences for Child and Adolescent Vaccine Attributes: A Systematic Review of the Stated Preference Literature. Patient 2017, 10, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.W.; Guo, N.; Wang, J.; Wang, C.; Ma, H. The Application of Discrete Choice Experiment in the Study of Preferences and Demands for Vaccination. Health Dev. Policy Res. 2016, 35, 5–7. [Google Scholar]
- Yang, Y.Z.; Hou, X.; Jia, Y.C.; Kang, W.; Wen, Y.; Hao, L.X. Scoping review of factors influencing vaccination preferences based on discrete choice experiments. Chin. J. Vaccines Immun. 2023, 29, 698–707. [Google Scholar]
- Zhu, S.; Chang, J.; Fang, Y. Application of discrete choice experiments in immunization service researches. Chin. J. Vaccines Immun. 2017, 23, 235–240. [Google Scholar]
- de Bekker-Grob, E.W.; Donkers, B.; Jonker, M.F.; Stolk, E.A. Sample Size Requirements for Discrete-Choice Experiments in Healthcare: A Practical Guide. Patient 2015, 8, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, N.; Takian, A.; Yaseri, M.; Daroudi, R.; Ghanbari Motlagh, A.; Nahvijou, A.; Zendehdel, K. Mothers’ preferences and willingness-to-pay for human papillomavirus vaccines in Iran: A discrete choice experiment study. Prev. Med. Rep. 2021, 23, 101438. [Google Scholar] [CrossRef] [PubMed]
- Hofman, R.; de Bekker-Grob, E.W.; Richardus, J.H.; de Koning, H.J.; van Ballegooijen, M.; Korfage, I.J. Have preferences of girls changed almost 3 years after the much debated start of the HPV vaccination program in The Netherlands? A discrete choice experiment. PLoS ONE 2014, 9, e104772. [Google Scholar] [CrossRef]
- Marshall, H.S.; Chen, G.; Clarke, M.; Ratcliffe, J. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: A Discrete Choice Experiment. Vaccine 2016, 34, 671–677. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Tian, G.; Feng, B.; Jia, X.; He, Z.; Liu, T.; Zhao, X.; Huang, M.; Yu, W.; et al. Understanding influencing attributes of COVID-19 vaccine preference and willingness-to-pay among Chinese and American middle-aged and elderly adults: A discrete choice experiment and propensity score matching study. Front. Public Health 2023, 11, 1067218. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, X.; Wang, J.H.; Wu, Q.H.; Shan, L.H.; Gao, L.J.; Chen, G.Y.; Chen, R.H.; Pan, L.; Xu, J.; et al. Self-paid direct medical expense and economic risk among hospitalized chronic disease patients in China: 2013. Chin. J. Public Health 2021, 37, 618–622. [Google Scholar]
- Sarker, A.R.; Ali, S.M.Z.; Ahmed, M.; Chowdhury, S.M.Z.I.; Ali, N. Out-of-pocket payment for healthcare among urban citizens in Dhaka, Bangladesh. PLoS ONE 2022, 17, e0262900. [Google Scholar] [CrossRef]
- Huang, A.D.; Xu, X.; Tang, L.; Huang, L.F.; Zhang, X.; Zhou, Y.; Zhang, Q.; Zhou, Z.M.; Wang, Y.; Wang, X.Q.; et al. Parental preferences for childhood vaccination regimens with diphtheria, tetanus, and acellular pertussis; hepatitis B; inactivated poliovirus; and Haemophilus influenzae type b containing vaccines: A discrete choice experiment in selected cities. Chin. J. Vaccines Immun. 2024, 30, 127–133. [Google Scholar]
- Carpio, C.E.; Coman, I.A.; Sarasty, O.; García, M. COVID-19 Vaccine Demand and Financial Incentives. Appl. Health Econ. Health Policy 2021, 19, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Böhme, S.; Sabat, I.; Brinkmann, C.; Attema, A.E.; Stargardt, T.; Schreyögg, J.; Brouwer, W. Jumping the Queue:Willingness to Pay for Faster Access to COVID-19 Vaccines in Seven European Countries. Pharmacoeconomics 2023, 41, 1389–1402. [Google Scholar] [CrossRef]
- Wang, H.B.; Yang, J.; Hu, C.F.; Zhou, L.F.; Lu, B.J.; Xu, M. Influencing factors of human papilloma virus vaccination in the female population in Liuzhou. J. China Med. Univ. 2025, 54, 185–189. [Google Scholar] [CrossRef]
- Qian, H.K.; Ji, W.Y.; Suo, L.D. Investigation Report and Analysis Knowledge, attitudes and vaccination status regarding HPV vaccines among female healthcare workers at vaccination clinics in Beijing city: A survey report. Chin. J. Public Health 2024, 40, 1487–1492. [Google Scholar]
- Guo, F.; Guo, Y.L. Influencing factors of cognitive level of human papillomavirus vaccination in women of childbearing age. Med. J. Chin. People’s Health 2025, 37, 7–10. [Google Scholar]
- Wassie, G.T.; Ambelie, Y.A.; Adebabay, T.; Yeshiwas, A.G.; Fenta, E.T.; Abebe, E.C.; Wassie, G.T.; Adella, G.A.; Anley, D.T. COVID-19 vaccine uptake and its associated factors among adult population in Dangila district, Awi Zone, Northwest Ethiopia: A mixed method study. PLoS ONE 2024, 19, e0302531. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.T.; Lu, M.X.; Dou, Q.H.; Wang, C.S.; Xiao, Z.P.; Wang, Y.; Zhang, M.Y.; Ji, Y.F.; Bai, Y.R.; Zhang, X.X.; et al. Analysis of hepatitis B vaccination rates and influencing factors for timely first dose among low-birth-weight newborns in Henan Province. Mod. Dis. Control Prev. 2025, 36, 9–13. [Google Scholar]
- Xu, Q.Q.; Huang, Z.S.; Meng, X.; Li, S.L. Epidemiological characteristics of varicella and the coverage of varicella vaccine among population aged 1–17 from 2005 to 2021 in Zibo City. Prog. Microbiol. Immunol. 2025, 53, 67–72. [Google Scholar]
- Li, M.C.; Tao, J.; Zhang, R.; Wu, Y.M.; Hee, Z.K.; Wu, C. Factors influencing influenza vaccination coverage among kindergarten and primary school children in Zhejiang Province, 2023. Shanghai J. Prev. Med. 2024, 37, 23–28. [Google Scholar]
| Variable Category | Variable Name | Coding/Levels | Rationale for Inclusion |
|---|---|---|---|
| Demographic Characteristics | Gender | 1 = Male 2 = Female | Control for gender-related preference differences |
| Occupation | 1 = Health workers 2 = Non-health workers | Control for professional background influence | |
| Education Level | 1 = Below college 2 = Over College | Control for knowledge disparity | |
| Economic Factors | Annual Income | 1 = Below 50,000 CNY 2 = Over 50,000 CNY | Control for payment capacity differences |
| Vaccine Attributes | Protective Efficacy | 1 = 80–90% 2 = 90–100% | Key effectiveness evaluation metric |
| Duration of Protection | 1 = 5 years 2 = 10 years 3 = 30 years | Key durability assessment metric | |
| Out-of-Pocket Cost | 1 = 0–1000 CNY 2 = 1000–2000 CNY 3 = 2000–3000 CNY | Key cost evaluation metric |
| Variable | Cluster | Value |
|---|---|---|
| Age (years) | Mean (SD) | 38.11(13.83) |
| Gender (%) | Male | 1549(48.42) |
| Female | 1650(51.58) | |
| Region (%) | Rural | 2536(79.27) |
| Urban | 663(20.73) | |
| Educational level (%) | Primary and lower | 466(14.57) |
| Junior high school | 998(31.20) | |
| High school/technical secondary school | 743(23.23) | |
| University/professional training college | 965(30.17) | |
| Postgraduate and above | 27(0.84) | |
| Annual income (%) | <10,000 CNY | 625(19.54) |
| 10,000–30,000 CNY | 1114(34.82) | |
| 30,000–50,000 CNY | 905(28.29) | |
| 50,000–100,000 CNY | 398(12.44) | |
| >100,000 CNY | 157(4.91) | |
| Occupation (%) | Health workers | 324(10.13) |
| Students | 450(14.07) | |
| Educational and public service practitioners | 282(8.82) | |
| Industrial/commercial workers and production practitioners | 2103(65.74) | |
| Retirees | 40(1.25) | |
| Familial hepatitis history (%) | Yes | 64(2.00) |
| No | 2533(79.18) | |
| Not clear | 602(18.82) | |
| Personal hepatitis history (%) | Yes | 17(0.53) |
| No | 2679(83.74) | |
| Not clear | 503(15.72) |
| Model | Log-Likelihood | AIC | BIC |
|---|---|---|---|
| Conditional logit model (Model 1) | −6217.917 | 12,445.83 | 12,484.90 |
| Mixed logit model (Model 2) | −6158.312 | 12,370.62 | 12,581.56 |
| Attributes and Levels | Coefficient (SE) | p-Value | 95% CI |
|---|---|---|---|
| Protective efficacy | |||
| 80–90% (Ref) | |||
| 90–100% | 0.006 (0.021) | 0.768 | (−0.036, 0.048) |
| Duration of protection | |||
| 5 years (Ref) | |||
| 10 years | 0.407 (0.031) | <0.001 | (0.346, 0.467) |
| 30 years | 0.288 (0.029) | <0.001 | (0.232, 0.345) |
| Out-of-pocket cost | |||
| 0–1000 CNY (Ref) | |||
| 1000–2000 CNY | 0.007 (0.031) | 0.811 | (−0.053, 0.067) |
| 2000–3000 CNY | −0.121 (0.029) | <0.001 | (−0.178, −0.064) |
| Mean | SD | ||||
|---|---|---|---|---|---|
| Attributes and Levels | Coefficient | SE | Coefficient | SE | |
| Protective efficacy | |||||
| 80–90% (Ref) | |||||
| 90–100% | −0.028 | 0.045 | 0.601 ** | 0.061 | |
| Duration of protection | |||||
| 5 years (Ref) | |||||
| 10 years | 0.456 ** | 0.049 | 0.650 ** | 0.088 | |
| 30 years | 0.253 ** | 0.042 | 0.277 * | 0.141 | |
| Out-of-pocket cost | |||||
| 0–1000 CNY (Ref) | |||||
| 1000–2000 CNY | 0.040 | 0.036 | −0.002 | 0.142 | |
| 2000–3000 CNY | −0.179 ** | 0.051 | 0.506 ** | 0.087 | |
| Interaction terms | |||||
| Covariates × Protective efficacy | |||||
| Female × 90–100% | 0.018 | 0.061 | -- | -- | |
| Educational level of over college × 90–100% | −0.039 | 0.064 | -- | -- | |
| Annual income of over 50,000 CNY × 90–100% | 0.145 | 0.084 | -- | -- | |
| Covariates × Duration of protection | |||||
| Educational level of over college × 10 years | 0.013 | 0.092 | -- | -- | |
| Educational level of over college × 30 years | 0.083 | 0.081 | -- | -- | |
| Health workers × 10 years | 0.224 | 0.134 | -- | -- | |
| Health workers × 30 years | 0.397 ** | 0.122 | -- | -- | |
| Covariates × Out-of-pocket cost | |||||
| Female × 2000–3000 CNY | 0.114 | 0.068 | -- | -- | |
| Annual income of over 50,000 CNY × 2000–3000 CNY | −0.107 | 0.089 | -- | -- | |
| Attributes | Willingness-to-Pay (WTP, CNY) | 95% CI for WTP | |
|---|---|---|---|
| Lower | Upper | ||
| Protective efficacy | |||
| 80–90%→90–100% | 0.102 | −0.695 | 0.900 |
| Duration of protection | |||
| 5 years→10 years | 7.077 ** | 3.511 | 10.643 |
| 5 years→30 years | 4.840 ** | 2.280 | 7.399 |
| Attributes and Levels | Rural (n = 2536) | Urban (n = 663) | |||
|---|---|---|---|---|---|
| Coefficient (SE) | p-Value | Coefficient (SE) | p-Value | ||
| ASC | −19.243 (451.073) | 0.966 | −18.990 (663.338) | 0.977 | |
| Protective efficacy | |||||
| 80–90% (Ref) | |||||
| 90–100% | −0.010 (0.030) | 0.739 | 0.106 (0.077) | 0.168 | |
| Duration of protection | |||||
| 5 years (Ref) | |||||
| 10 years | 0.449(0.044) | <0.001 | 0.722 (0.203) | <0.001 | |
| 30 years | 0.271 (0.037) | <0.001 | 0.707(0.103) | <0.001 | |
| Out-of-pocket cost | |||||
| 0–1000 CNY (Ref) | |||||
| 1000–2000 CNY | 0.036(0.038) | 0.345 | 0.042 (0.091) | 0.644 | |
| 2000–3000 CNY | −0.064 (0.037) | 0.086 | −0.511 (0.097) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, C.; Zou, Z.; Hu, W.; Zhang, D.; Zhao, S.; Zhang, S.; Wu, Q.; Zhang, L. Hepatitis E Vaccination Preferences and Willingness-to-Pay Among Residents: A Discrete Choice Experiment Analysis. Vaccines 2025, 13, 906. https://doi.org/10.3390/vaccines13090906
Chen Y, Zhang C, Zou Z, Hu W, Zhang D, Zhao S, Zhang S, Wu Q, Zhang L. Hepatitis E Vaccination Preferences and Willingness-to-Pay Among Residents: A Discrete Choice Experiment Analysis. Vaccines. 2025; 13(9):906. https://doi.org/10.3390/vaccines13090906
Chicago/Turabian StyleChen, Yuanqiong, Chao Zhang, Zhuoru Zou, Weijun Hu, Dan Zhang, Sidi Zhao, Shaobai Zhang, Qian Wu, and Lei Zhang. 2025. "Hepatitis E Vaccination Preferences and Willingness-to-Pay Among Residents: A Discrete Choice Experiment Analysis" Vaccines 13, no. 9: 906. https://doi.org/10.3390/vaccines13090906
APA StyleChen, Y., Zhang, C., Zou, Z., Hu, W., Zhang, D., Zhao, S., Zhang, S., Wu, Q., & Zhang, L. (2025). Hepatitis E Vaccination Preferences and Willingness-to-Pay Among Residents: A Discrete Choice Experiment Analysis. Vaccines, 13(9), 906. https://doi.org/10.3390/vaccines13090906






