Seroprevalence of RSV IgG Antibodies Across Age Groups in Poland After the COVID-19 Pandemic: Data from the 2023/2024 Epidemic Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of Anti-RSV IgG Antibodies
2.3. Statistical Analyses
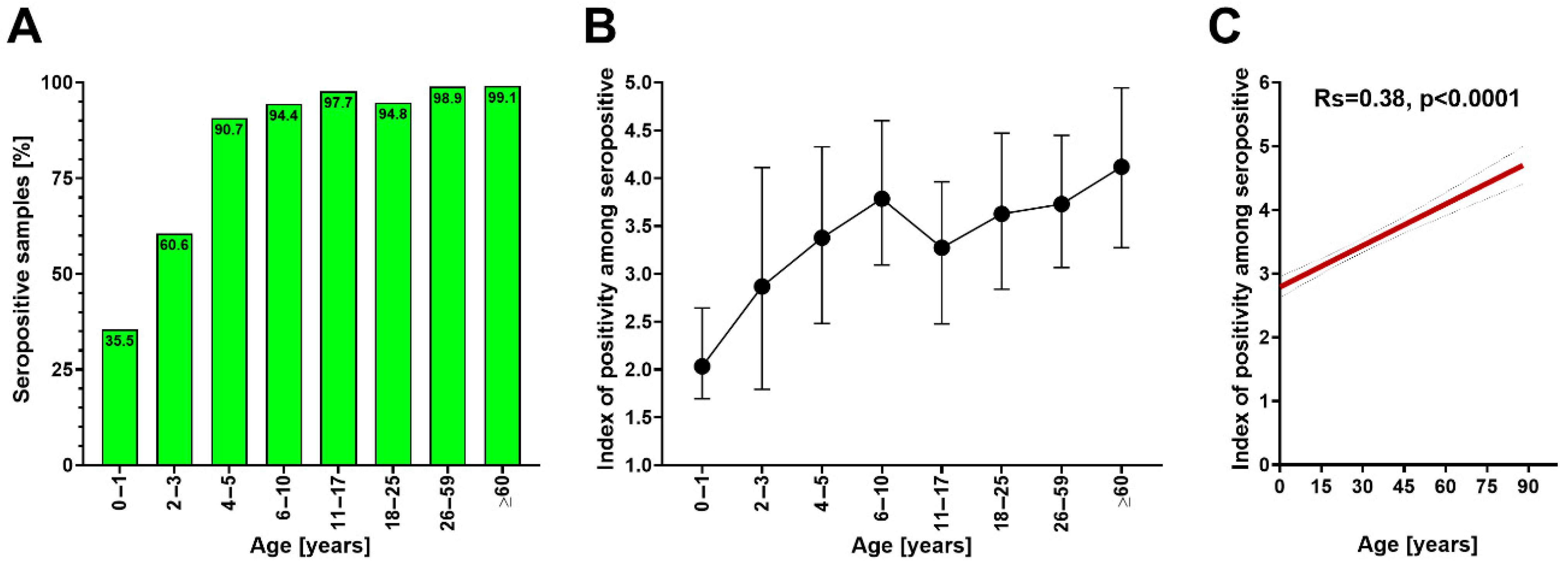
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaler, J.; Hussain, A.; Patel, K.; Hernandez, T.; Ray, S. Respiratory Syncytial Virus: A Comprehensive Review of Transmission, Pathophysiology, and Manifestation. Cureus 2023, 15, e36342. [Google Scholar] [CrossRef]
- Johannesen, C.K.; Gideonse, D.; Osei-Yeboah, R.; Lehtonen, T.; Jollivet, O.; Cohen, R.A.; Urchueguía-Fornes, A.; Herrero-Silvestre, M.; López-Lacort, M.; Kramer, R.; et al. Estimation of Respiratory Syncytial Virus-Associated Hospital Admissions in Five European Countries: A Modelling Study. Lancet Reg. Health Eur. 2025, 51, 101227. [Google Scholar] [CrossRef]
- Trifonov, G.; Büscher, E.; Fistera, D.; Kill, C.; Risse, J.; Taube, C.; Todt, D.; Dittmer, U.; Elsner, C. Disease Burden of RSV Infection in Adult Patients in Comparison to Influenza Virus Infection. J. Med. Virol. 2025, 97, e70373. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Wee, L.E.; Lim, J.T.; Ho, R.W.L.; Chiew, C.J.; Young, B.; Venkatachalam, I.; Sim, J.X.Y.; Cheong, H.Y.; Ng, T.Y.; Yung, C.-F.; et al. Severity of Respiratory Syncytial Virus versus SARS-CoV-2 Omicron and Influenza Infection amongst Hospitalized Singaporean Adults: A National Cohort Study. Lancet Reg. Health West. Pac. 2025, 55, 101494. [Google Scholar] [CrossRef]
- Surie, D.; Yuengling, K.A.; DeCuir, J.; Zhu, Y.; Lauring, A.S.; Gaglani, M.; Ghamande, S.; Peltan, I.D.; Brown, S.M.; Ginde, A.A.; et al. Severity of Respiratory Syncytial Virus vs. COVID-19 and Influenza among Hospitalized US Adults. JAMA Netw. Open 2024, 7, e244954. [Google Scholar] [CrossRef]
- Mazela, J.; Jackowska, T.; Czech, M.; Helwich, E.; Martyn, O.; Aleksiejuk, P.; Smaga, A.; Glazewska, J.; Wysocki, J. Epidemiology of Respiratory Syncytial Virus Hospitalizations in Poland: An Analysis from 2015 to 2023 Covering the Entire Polish Population of Children Aged under Five Years. Viruses 2024, 16, 704. [Google Scholar] [CrossRef]
- Preparing for RSV Immunisation and Surveillance in Europe. Available online: https://www.ihi.europa.eu/projects-results/project-factsheets/promise (accessed on 2 July 2025).
- Osei-Yeboah, R.; Spreeuwenberg, P.; Del Riccio, M.; Fischer, T.K.; Egeskov-Cavling, A.M.; Bøås, H.; van Boven, M.; Wang, X.; Lehtonen, T.; Bangert, M.; et al. Estimation of the Number of Respiratory Syncytial Virus-Associated Hospitalizations in Adults in the European Union. J. Infect. Dis. 2023, 228, 1539–1548. [Google Scholar] [CrossRef]
- Szymański, K.; Poznańska, A.; Kondratiuk, K.; Hallmann, E.; Łuniewska, K.; Masny, A.; Brydak, L.B. Circulation of Respiratory Syncytial Virus (RSV) in Poland between Seasons of 2009/2010 and 2022/2023 Based on SENTINEL System. Microorganisms 2025, 13, 140. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Viñeta Paramo, M.; Reicherz, F.; Lavoie, P.M. Why Has the Epidemiology of RSV Changed during the COVID-19 Pandemic? eClinicalMedicine 2023, 61, 102089. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.W.; Dürrwald, R.; Reiche, J.; Rose, N.; Scherag, A.; Weis, S.; CoNAN study group. Impact of the COVID-19 Pandemic on Influenza and Respiratory Syncytial Virus Antibody Titres in the Community: A Prospective Cohort Study in Neustadt, Thuringia, Germany. Eur. Respir. J. 2022, 60, 2200947. [Google Scholar] [CrossRef]
- van Summeren, J.; Meijer, A.; Aspelund, G.; Casalegno, J.S.; Erna, G.; Hoang, U.; Lina, B.; de Lusignan, S.; Teirlinck, A.C.; Thors, V.; et al. Low Levels of Respiratory Syncytial Virus Activity in Europe during the 2020/21 Season: What Can We Expect in the Coming Summer and Autumn/Winter? Euro Surveill. 2021, 26, 2100639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, T.; Guo, L.; Sun, S.; Miao, Y.; Yung, C.F.; Tomlinson, J.; Stolyarov, K.; Shchomak, Z.; Poovorawan, Y.; et al. Characterising the Asynchronous Resurgence of Common Respiratory Viruses Following the COVID-19 Pandemic. Nat. Commun. 2025, 16, 1610. [Google Scholar] [CrossRef] [PubMed]
- Del Riccio, M.; Caini, S.; Bonaccorsi, G.; Lorini, C.; Paget, J.; van der Velden, K.; Meijer, A.; Haag, M.; McGovern, I.; Zanobini, P. Global Analysis of Respiratory Viral Circulation and Timing of Epidemics in the Pre-COVID-19 and COVID-19 Pandemic Eras, Based on Data from the Global Influenza Surveillance and Response System (GISRS). Int. J. Infect. Dis. 2024, 144, 107052. [Google Scholar] [CrossRef]
- Lastrucci, V.; Pacifici, M.; Puglia, M.; Alderotti, G.; Berti, E.; Bonaccorsi, G.; Moriondo, M.; Resti, M.; Peroni, D.; Martini, M.; et al. Recent Trends in Hospitalizations for Respiratory Syncytial Virus after the COVID-19 Pandemic and before Routine Immunization: Seasonality and Severity Updates from the 2023/2024 Season in Tuscany, Italy. Int. J. Infect. Dis. 2025, 154, 107879. [Google Scholar] [CrossRef]
- Meyer Sauteur, P.M.; Plebani, M.; Trück, J.; Wagner, N.; Agyeman, P.K.A.; RSV EpiCH investigators. Ongoing Disruption of RSV Epidemiology in Children in Switzerland. Lancet Reg. Health Eur. 2024, 45, 101050. [Google Scholar] [CrossRef]
- Leija-Martínez, J.J.; Cadena-Mota, S.; González-Ortiz, A.M.; Muñoz-Escalante, J.C.; Mata-Moreno, G.; Hernández-Sánchez, P.G.; Vega-Morúa, M.; Noyola, D.E. Respiratory Syncytial Virus and Other Respiratory Viruses in Hospitalized Infants during the 2023-2024 Winter Season in Mexico. Viruses 2024, 16, 1917. [Google Scholar] [CrossRef]
- Rzymski, P.; Pleśniak, R.; Piekarska, A.; Sznajder, D.; Moniuszko-Malinowska, A.; Tomasiewicz, K.; Skwara, P.; Zarębska-Michaluk, D.; Turzańska, K.; Piasecki, M.; et al. Tracking Clinical Severity of Influenza in Adult Hospitalized Patients in 2024: Data from the FluTer Registry in Poland. Vaccine 2025, 61, 127443. [Google Scholar] [CrossRef]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N. Engl. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Gwenzi, W. Respiratory Syncytial Virus Immunoprophylaxis: Novel Opportunities and a Call for Equity. J. Med. Virol. 2024, 96, e29453. [Google Scholar] [CrossRef]
- Nham, E.; Jang, A.-Y.; Hyun, H.; Yoon, J.G.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Ahn, K.B.; Ji, H.J.; Seo, H.S.; et al. Age-Stratified Seroprevalence of Respiratory Syncytial Virus: Analysis Using Prefusion F and G Protein Antibodies. Vaccines 2024, 12, 513. [Google Scholar] [CrossRef]
- Pasittungkul, S.; Thongpan, I.; Vichaiwattana, P.; Thongmee, T.; Klinfueng, S.; Suntronwong, N.; Wanlapakorn, N.; Vongpunsawad, S.; Poovorawan, Y. High Seroprevalence of Antibodies against Human Respiratory Syncytial Virus and Evidence of Respiratory Syncytial Virus Reinfection in Young Children in Thailand. Int. J. Infect. Dis. 2022, 125, 177–183. [Google Scholar] [CrossRef]
- Andeweg, S.P.; Schepp, R.M.; van de Kassteele, J.; Mollema, L.; Berbers, G.A.M.; van Boven, M. Population-Based Serology Reveals Risk Factors for RSV Infection in Children Younger than 5 Years. Sci. Rep. 2021, 11, 8953. [Google Scholar] [CrossRef]
- Sastre, P.; Ruiz, T.; Schildgen, O.; Schildgen, V.; Vela, C.; Rueda, P. Seroprevalence of Human Respiratory Syncytial Virus and Human Metapneumovirus in Healthy Population Analyzed by Recombinant Fusion Protein-Based Enzyme Linked Immunosorbent Assay. Virol. J. 2012, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Kondratiuk, K.; Hallmann, E.; Szymański, K.; Łuniewska, K.; Poznańska, A.; Brydak, L.B. Prevalence of Circulating Antibodies against Hemagglutinin of Influenza Viruses in Epidemic Season 2021/2022 in Poland. Acta Biochim. Pol. 2024, 71, 12289. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, M.; Wang, Y.; Yu, H. 916. The Transfer of Maternal Antibodies and Dynamics of Maternal and Natural Infection-Induced Antibodies against RSV in Children: A Longitudinal Cohort Study. Open Forum Infect. Dis. 2023, 10, ofad500.961. [Google Scholar] [CrossRef]
- Coindy, E.L.; Efstathiou, C.; Talwar, S.; Moureau, A.; Vernhes, C.; Openshaw, P.J.M.; Thwaites, R.S.; PROMISE investigators. Antibody-Mediated Protection against Respiratory Syncytial Virus in Children. Eur. Respir. Rev. 2024, 33, 240106. [Google Scholar] [CrossRef]
- Mazur, N.I.; Horsley, N.M.; Englund, J.A.; Nederend, M.; Magaret, A.; Kumar, A.; Jacobino, S.R.; de Haan, C.A.M.; Khatry, S.K.; LeClerq, S.C.; et al. Breast Milk Prefusion F Immunoglobulin G as a Correlate of Protection against Respiratory Syncytial Virus Acute Respiratory Illness. J. Infect. Dis. 2019, 219, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Losonsky, G.A.; Theodore, C.M.; Peri, B.; Fishaut, M.; Rothberg, R.M.; Ogra, P.L. 942 Immunology of Breast Milk: Origin of Antibodies to Respiratory Syncytial Virus (RSV) and Bovine Serum Albumin (BSA) in the Lactating Breast. Pediatr. Res. 1981, 15, 599. [Google Scholar] [CrossRef][Green Version]
- Christiaansen, A.F.; Syed, M.A.; Ten Eyck, P.P.; Hartwig, S.M.; Durairaj, L.; Kamath, S.S.; Varga, S.M. Altered Treg and Cytokine Responses in RSV-Infected Infants. Pediatr. Res. 2016, 80, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Turi, K.N.; Shankar, J.; Anderson, L.J.; Rajan, D.; Gaston, K.; Gebretsadik, T.; Das, S.R.; Stone, C.; Larkin, E.K.; Rosas-Salazar, C.; et al. Infant Viral Respiratory Infection Nasal Immune-Response Patterns and Their Association with Subsequent Childhood Recurrent Wheeze. Am. J. Respir. Crit. Care Med. 2018, 198, 1064–1073. [Google Scholar] [CrossRef]
- Pérez Marc, G.; Vizzotti, C.; Fell, D.B.; Di Nunzio, L.; Olszevicki, S.; Mankiewicz, S.W.; Braem, V.; Rearte, R.; Atwell, J.E.; Bianchi, A.; et al. Real-World Effectiveness of RSVpreF Vaccination during Pregnancy against RSV-Associated Lower Respiratory Tract Disease Leading to Hospitalisation in Infants during the 2024 RSV Season in Argentina (BERNI Study): A Multicentre, Retrospective, Test-Negative, Case-Control Study. Lancet Infect. Dis. 2025. [Google Scholar] [CrossRef]
- Carbajal, R.; Boelle, P.-Y.; Pham, A.; Chazette, Y.; Schellenberger, M.; Weil, C.; Colas, A.-S.; Lecarpentier, T.; Schnuriger, A.; Guedj, R.; et al. Real-World Effectiveness of Nirsevimab Immunisation against Bronchiolitis in Infants: A Case-Control Study in Paris, France. Lancet Child Adolesc. Health 2024, 8, 730–739. [Google Scholar] [CrossRef]
- Berbers, G.; Mollema, L.; van der Klis, F.; den Hartog, G.; Schepp, R. Antibody Responses to Respiratory Syncytial Virus: A Cross-Sectional Serosurveillance Study in the Dutch Population Focusing on Infants Younger than 2 Years. J. Infect. Dis. 2021, 224, 269–278. [Google Scholar] [CrossRef]
- Bhattarakosol, P.; Pancharoen, C.; Mungmee, V.; Thammaborvorn, R.; Semboonlor, L. Seroprevalence of Anti-RSV IgG in Thai Children Aged 6 Months to 5 Years. Asian Pac. J. Allergy Immunol. 2003, 21, 269–271. [Google Scholar]
- Teodoro, L.I.; Ovsyannikova, I.G.; Grill, D.E.; Poland, G.A.; Kennedy, R.B. Seroprevalence of RSV Antibodies in a Contemporary (2022–2023) Cohort of Adults. Int. J. Infect. Dis. 2025, 107964. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Kulkarni, R.; Malshe, N.; Palkar, S.; Lalwani, S.; Mishra, A.C. Seroepidemiology of Respiratory Syncytial Virus in Western India with Special Reference to Appropriate Age for Infant Vaccination. J. Med. Virol. 2019, 91, 1566–1570. [Google Scholar] [CrossRef]
- Terrosi, C.; Di Genova, G.; Martorelli, B.; Valentini, M.; Cusi, M.G. Humoral Immunity to Respiratory Syncytial Virus in Young and Elderly Adults. Epidemiol. Infect. 2009, 137, 1684–1686. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Walsh, E.E.; Long, C.E.; Schnabel, K.C. Immunity to and Frequency of Reinfection with Respiratory Syncytial Virus. J. Infect. Dis. 1991, 163, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Kutsaya, A.; Teros-Jaakkola, T.; Kakkola, L.; Toivonen, L.; Peltola, V.; Waris, M.; Julkunen, I. Prospective Clinical and Serological Follow-up in Early Childhood Reveals a High Rate of Subclinical RSV Infection and a Relatively High Reinfection Rate within the First 3 Years of Life. Epidemiol. Infect. 2016, 144, 1622–1633. [Google Scholar] [CrossRef]
- Patel, N.; Tian, J.-H.; Flores, R.; Jacobson, K.; Walker, M.; Portnoff, A.; Gueber-Xabier, M.; Massare, M.J.; Glenn, G.; Ellingsworth, L.; et al. Flexible RSV Prefusogenic Fusion Glycoprotein Exposes Multiple Neutralizing Epitopes That May Collectively Contribute to Protective Immunity. Vaccines 2020, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E. Relationship of Serum Antibody to Risk of Respiratory Syncytial Virus Infection in Elderly Adults. J. Infect. Dis. 1998, 177, 463–466. [Google Scholar] [CrossRef]
- Parsons, E.L.; Kim, J.S.; Malloy, A.M.W. Development of Innate and Adaptive Immunity to RSV in Young Children. Cell. Immunol. 2024, 399–400, 104824. [Google Scholar] [CrossRef]
- Havers, F.P.; Whitaker, M.; Melgar, M.; Chatwani, B.; Chai, S.J.; Alden, N.B.; Meek, J.; Openo, K.P.; Ryan, P.A.; Kim, S.; et al. Characteristics and Outcomes among Adults Aged ≥60 Years Hospitalized with Laboratory-Confirmed Respiratory Syncytial Virus—RSV-NET, 12 States, July 2022–June 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1075–1082. [Google Scholar] [CrossRef]
- Miller, J.L.; Niewiesk, S. Review of Impaired Immune Parameters in RSV Infections in the Elderly. Virology 2025, 603, 110395. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Hamid, S.; Pham, H.; Derado, G.; Taylor, C.; Sekkarie, A.; Loustalot, F.; Lundeen, E.; Kirley, P.D.; Austin, E.; et al. P-715. Chronic Conditions as Risk Factors for Respiratory Syncytial Virus-Associated Hospitalization among Community-Dwelling Adults Aged ≥50 Years, 2017 to 2018. Open Forum Infect. Dis. 2025, 12, ofae631.911. [Google Scholar] [CrossRef]
- Fry, S.E.; Terebuh, P.; Kaelber, D.C.; Xu, R.; Davis, P.B. Effectiveness and Safety of Respiratory Syncytial Virus Vaccine for US Adults Aged 60 Years or Older. JAMA Netw. Open 2025, 8, e258322. [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an MRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Bajema, K.L.; Yan, L.; Li, Y.; Argraves, S.; Rajeevan, N.; Fox, A.; Vergun, R.; Berry, K.; Bui, D.; Huang, Y.; et al. Respiratory Syncytial Virus Vaccine Effectiveness among US Veterans, September, 2023 to March, 2024: A Target Trial Emulation Study. Lancet Infect. Dis. 2025, 25, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Heppe-Montero, M.; Walter, S.; Hernández-Barrera, V.; Gil-Prieto, R.; Gil-de-Miguel, Á. Burden of Respiratory Syncytial Virus-Associated Lower Respiratory Infections in Children in Spain from 2012 to 2018. BMC Infect. Dis. 2022, 22, 315. [Google Scholar] [CrossRef]
- Ursin, R.L.; Klein, S.L. Sex Differences in Respiratory Viral Pathogenesis and Treatments. Annu. Rev. Virol. 2021, 8, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Regis, E.; Fontanella, S.; Lin, L.; Howard, R.; Haider, S.; Curtin, J.A.; Edwards, M.R.; Rattray, M.; Simpson, A.; Custovic, A.; et al. Sex Differences in Innate Anti-Viral Immune Responses to Respiratory Viruses and in Their Clinical Outcomes in a Birth Cohort Study. Sci. Rep. 2021, 11, 23741. [Google Scholar] [CrossRef]
- Rzymski, P.; Pokorska-Śpiewak, M.; Jackowska, T.; Kuchar, E.; Nitsch-Osuch, A.; Pawłowska, M.; Babicki, M.; Jaroszewicz, J.; Szenborn, L.; Wysocki, J.; et al. Key Considerations during the Transition from the Acute Phase of the COVID-19 Pandemic: A Narrative Review. Vaccines 2023, 11, 1502. [Google Scholar] [CrossRef]
| Age Group | n | Seroprevalence [%] | Index of Positivity Among Seropositive | ||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| 0–1 | 45 | 36 | 37 | 2.4 (1.7–2.6) | 2.0 (1.7–2.4) |
| p = 0.60 | p = 0.96 | ||||
| 2–3 | 33 | 70 | 55 | 3.2 (2.1–4.3) | 2.1 (1.3–3.1) |
| p = 0.16 | p = 0.15 | ||||
| 4–5 | 43 | 89 | 94 | 2.9 (3.2–3.8) | 3.4 (2.5–4.4) |
| p = 0.57 | p = 0.36 | ||||
| 6–10 | 89 | 89 | 97 | 3.5 (2.7–4.4) | 3.7 (3.1–4.2) |
| p = 0.27 | p = 0.69 | ||||
| 11–17 | 132 | 98 | 98 | 3.4 (2.4–4.2) | 3.2 (2.5–4.0) |
| p = 71 | p = 0.77 | ||||
| 18–25 | 58 | 97 | 93 | 3.6 (2.7–4.3) | 3.6 (2.9–5.0) |
| p = 0.45 | p = 0.23 | ||||
| 26–59 | 188 | 100 | 98 | 3.9 (3.4–4.6) | 3.6 (2.8–4.4) |
| p = 0.34 | p = 0.09 | ||||
| ≥60 | 112 | 98 | 100 | 4.5 (3.6–5.0) | 4.0 (3.1–4.6) |
| p = 0.49 | p = 0.04 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poniedziałek, B.; Majewska, W.; Kondratiuk, K.; Masny, A.; Poznańska, A.; Szymański, K.; Łuniewska, K.; Czajkowska, E.; Mańkowski, B.; Brydak, L.B.; et al. Seroprevalence of RSV IgG Antibodies Across Age Groups in Poland After the COVID-19 Pandemic: Data from the 2023/2024 Epidemic Season. Vaccines 2025, 13, 741. https://doi.org/10.3390/vaccines13070741
Poniedziałek B, Majewska W, Kondratiuk K, Masny A, Poznańska A, Szymański K, Łuniewska K, Czajkowska E, Mańkowski B, Brydak LB, et al. Seroprevalence of RSV IgG Antibodies Across Age Groups in Poland After the COVID-19 Pandemic: Data from the 2023/2024 Epidemic Season. Vaccines. 2025; 13(7):741. https://doi.org/10.3390/vaccines13070741
Chicago/Turabian StylePoniedziałek, Barbara, Wiktoria Majewska, Katarzyna Kondratiuk, Aleksander Masny, Anna Poznańska, Karol Szymański, Katarzyna Łuniewska, Emilia Czajkowska, Bartosz Mańkowski, Lidia B. Brydak, and et al. 2025. "Seroprevalence of RSV IgG Antibodies Across Age Groups in Poland After the COVID-19 Pandemic: Data from the 2023/2024 Epidemic Season" Vaccines 13, no. 7: 741. https://doi.org/10.3390/vaccines13070741
APA StylePoniedziałek, B., Majewska, W., Kondratiuk, K., Masny, A., Poznańska, A., Szymański, K., Łuniewska, K., Czajkowska, E., Mańkowski, B., Brydak, L. B., Tomasiewicz, K., Flisiak, R., & Rzymski, P. (2025). Seroprevalence of RSV IgG Antibodies Across Age Groups in Poland After the COVID-19 Pandemic: Data from the 2023/2024 Epidemic Season. Vaccines, 13(7), 741. https://doi.org/10.3390/vaccines13070741









