The Application of Single-Cell Technologies for Vaccine Development Against Viral Infections
Abstract
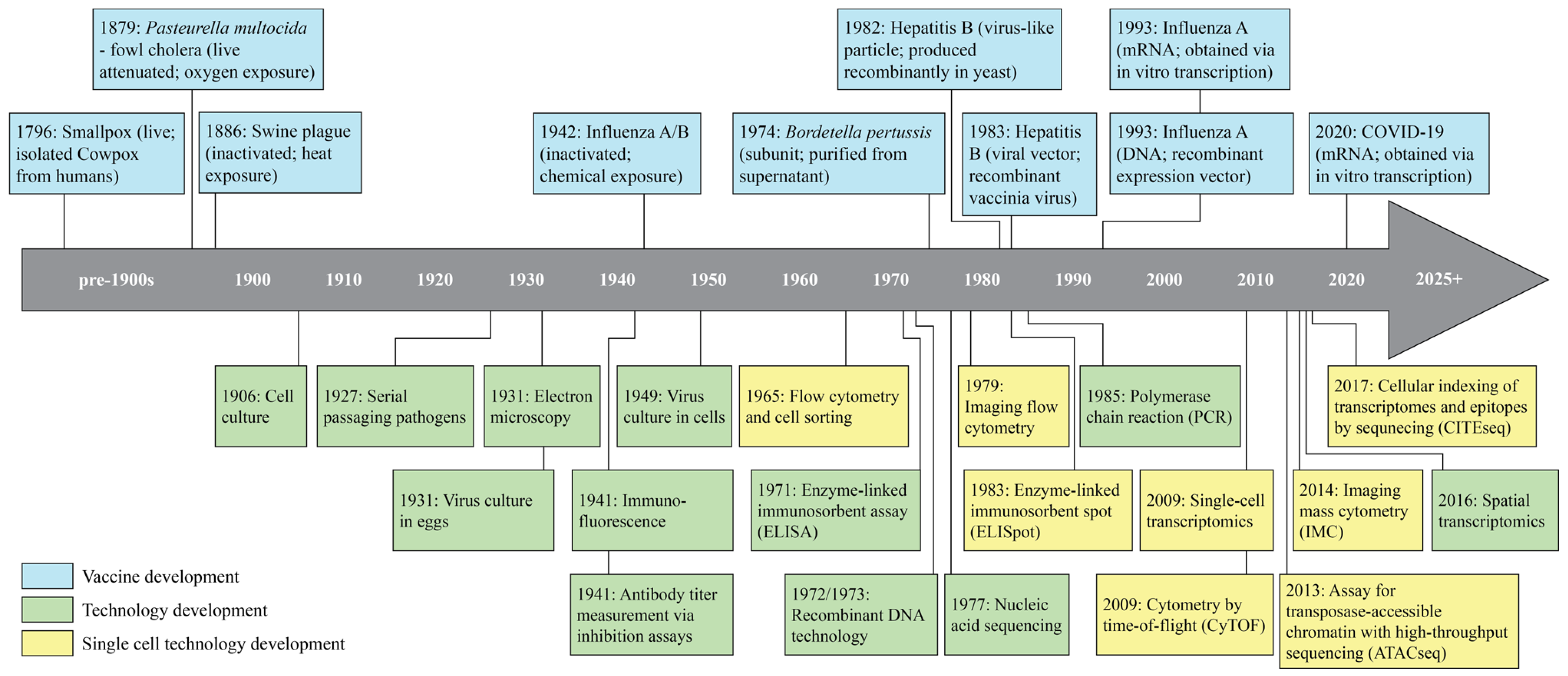
1. Introduction
2. Single-Cell Technologies
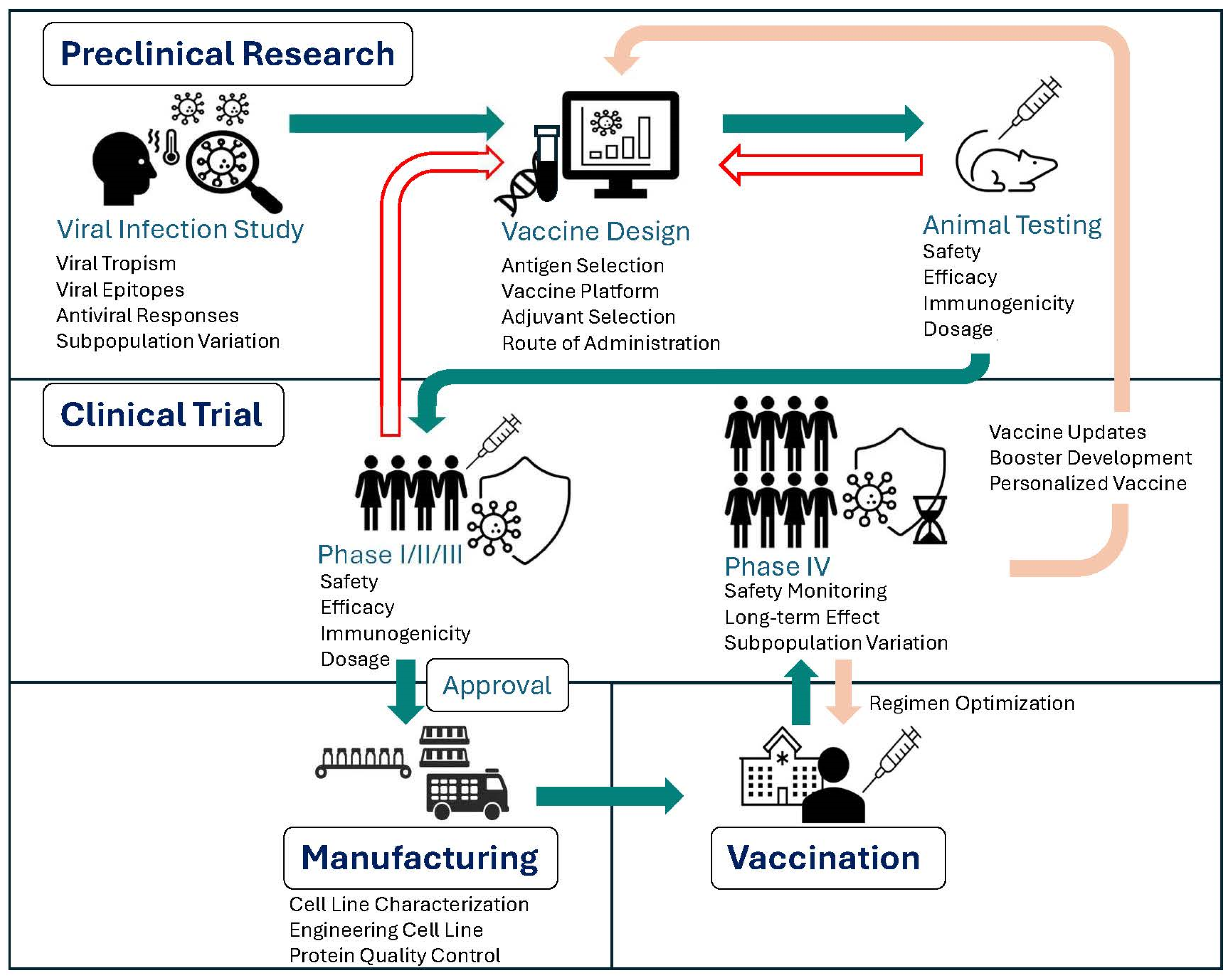
3. Harnessing Single-Cell Technologies to Advance Vaccine Development
3.1. Informing Vaccine Development via Analyses of Immune Response to Viral Infection
3.1.1. Determining Viral Tropism
3.1.2. Identifying Viral Epitopes
3.1.3. Comparative Analyses of Responders vs. Non-Responders
3.1.4. Analyzing Subpopulation Variation
3.2. Evaluating Vaccine Efficacy and Safety via Immunogenicity Analysis
3.2.1. Discovering CoPs
3.2.2. Characterizing Immunogenicity of Vaccine Components
3.2.3. Characterizing Immune Dysregulation Underlying Adverse Events (AEs)
3.2.4. Evaluating Vaccine Responses in Immunocompromised Populations
3.3. Refine Vaccine Manufacturing
4. Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaccines and Immunization. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed on 26 March 2025).
- Health and Economic Benefits of Routine Childhood Immunizations in the Era of the Vaccines for Children Program—United States, 1994–2023. Available online: https://www.cdc.gov/mmwr/volumes/73/wr/mm7331a2.htm#:~:text=This%20calculation%20means%20that%20every,a%20savings%20of%20approximately%20%2411 (accessed on 26 March 2025).
- A Brief History of Vaccines. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/a-brief-history-of-vaccination (accessed on 26 March 2025).
- Global Polio Vaccination. Available online: https://www.cdc.gov/global-polio-vaccination/about/index.html (accessed on 26 March 2025).
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. BUMC Proc. 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.-M.; Legout, S. Louis Pasteur: Between Myth and Reality. Biomolecules 2022, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, L. Sur le choléra des poules. Étude des conditions de la non-récidive de la maladie et de quelques autres de ses caractères. Recl. De Médecine Vétérinaire 1880, 57, 422–432. [Google Scholar] [CrossRef]
- Salmon, D.E. On a New Method of Producing Immunity from Contagious Diseases. Proc. Biol. Soc. Wash. 1886, 3, 29–33. [Google Scholar]
- Moro, L.G.; Guarnier, L.P.; Azevedo, M.F.; Fracasso, J.A.R.; Lucio, M.A.; Castro, M.V.d.; Dias, M.L.; Lívero, F.A.d.R.; Ribeiro-Paes, J.T. A Brief History of Cell Culture: From Harrison to Organs-on-a-Chip. Cells 2024, 13, 2068. [Google Scholar] [CrossRef]
- Harrison, R.G. Observations on the living developing nerve fiber. Proc. Soc. Exp. Biol. Med. 1906, 4, 140–143. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Calmette, A. La Vaccination Preventive Contre La Tuberculose Par Le “BCG”. Libr. De L’Academie De Med. 1927, 18, 43–54. [Google Scholar]
- Shampo, M.A.; Kyle, R.A. Ernst Ruska—Inventor of the Electron Microscope. Mayo Clin. Proc. 1997, 72, 148. [Google Scholar] [CrossRef]
- Woodruff, A.M. The Susceptibility of the Chorio-Allantoic Membrane of Chick Embryos to Infection with the Fowl-Pox Virus. Am. J. Pathol. 1931, 7, 209–222. [Google Scholar]
- Coons, A.H.; Creech, H.J.; Jones, R.N. Immunological Properties of an Antibody Containing a Fluorescent Group. Proc. Soc. Exp. Biol. Med. 1941, 47, 200–202. [Google Scholar] [CrossRef]
- Hirst, G.K. The quantitative determination of influenza virus and antibodies by means of red cell agglutination. J. Exp. Med. 1942, 75, 49–64. [Google Scholar] [CrossRef] [PubMed]
- History of the Influenza Vaccine. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/history-of-influenza-vaccination (accessed on 26 March 2025).
- Salk, J.E.; Pearson, H.E.; Brown, P.N.; Smyth, C.J.; Francis, T. Immunization against influenza with observations during an epidemic of influenza A one year after vaccination. Am. J. Epidemiol. 1945, 42, 307–322. [Google Scholar] [CrossRef]
- Enders, J.F.; Weller, T.H.; Robbins, F.C. Cultivation of the Lansing Strain of Poliomyelitis Virus in Cultures of Various Human Embryonic Tissues. Science 1949, 109, 85–87. [Google Scholar] [CrossRef]
- Fulwyler, M.J. Electronic Separation of Biological Cells by Volume. Science 1965, 150, 910–911. [Google Scholar] [CrossRef]
- Robinson, J.P. Flow Cytometry: Past and Future. Biotechniques 2022, 72, 159–169. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Jackson, D.; Symons, R.; Berg, P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: Circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc. Natl. Acad. Sci. USA 1972, 69, 2904–2909. [Google Scholar] [CrossRef]
- Cohen, S.; Chang, A.; Boyer, H.; Helling, R. Construction of biologically functional bacterial plasmids in vitro. Proc. Natl. Acad. Sci. USA 1973, 70, 3240–3244. [Google Scholar] [CrossRef]
- Sato, Y.; Arai, H.; Suzuki, K. Leukocytosis-Promoting Factor of Bordetella pertussis III. Its identity with Protective Antigen. Infect. Immun. 1974, 9, 801–810. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M. First recombinant DNA vaccine for HBV. Nat. Res. 2020, 13, 3–9. [Google Scholar]
- Valenzuela, P.; Medina, A.; Rutter, W.J.; Ammerer, G.; Hall, B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 1982, 298, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Mackett, M.; Moss, B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature 1983, 302, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, J.; Holt, P. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J. Immunol. Methods 1983, 57, 301–309. [Google Scholar] [CrossRef]
- Czerkinsky, C.; Nilsson, L.; Nygren, H.; Ouchterlony, O.; Tarkowski, A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 1983, 65, 109–121. [Google Scholar] [CrossRef]
- Kaunitz, J.D. The Discovery of PCR: ProCuRement of Divine Power. Dig. Dis. Sci. 2015, 60, 2230–2231. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Donnelly, J.J.; Parker, S.E.; Rhodes, G.H.; Felgner, P.L.; Dwarki, V.J.; Gromkowski, S.H.; Deck, R.R.; DeWitt, C.M.; Friedman, A.; et al. Heterologous Protection Against Influenza by Injection of DNA Encoding a Viral Protein. Science 1993, 259, 1745–1749. [Google Scholar] [CrossRef]
- Tang, F.; Barbacioru, C.; Wang, Y.; Nordman, E.; Lee, C.; Xu, N.; Wang, X.; Bodeau, J.; Tuch, B.B.; Siddiqui, A.; et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 2009, 6, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Cosma, A.; Nolan, G.; Gaudilliere, B. Mass Cytometry: The Time to Settle Down. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2017, 91, 12–13. [Google Scholar] [CrossRef]
- Bandura, D.R. Mass Cytometry: Technique for Real Time Single Cell Multitarget Immunoassay Based on Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Anal. Chem. 2009, 81, 6813–6822. [Google Scholar] [CrossRef]
- Buenrostro, J.D.; Giresi, P.G.; Zaba, L.C.; Chang, H.Y.; Greenleaf, W.J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 2013, 10, 1213–1218. [Google Scholar] [CrossRef]
- Chang, Q.; Ornatsky, O.I.; Siddiqui, I.; Loboda, A.; Baranov, V.I.; Hedley, D.W. Imaging Mass Cytometry. Cytom. Part A 2017, 91, 160–169. [Google Scholar] [CrossRef]
- Giesen, C.; Wang, H.A.O.; Schapiro, D.; Zivanovic, N.; Jacobs, A.; Hattendorf, B.; Schüffler, P.J.; Grolimund, D.; Buhmann, J.M.; Brandt, S.; et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 2014, 11, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Stoeckius, M.; Hafemeister, C.; Stephenson, W.; Houck-Loomis, B.; Chattopadhyay, P.K.; Swerdlow, H.; Satija, R.; Smibert, P. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 2017, 14, 865–868. [Google Scholar] [CrossRef]
- FDA Approves First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 26 March 2025).
- Ståhl, P.L.; Salmén, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef]
- Kay, D.; Cambier, J.L.; Wheeless, L.L., Jr. Imaging in flow. J. Histochem. Cytochem. 1979, 27, 329–334. [Google Scholar] [CrossRef]
- Smith, W.; Andrewes, C.H.; Laidlaw, P.P. A virus obtained from influenza patients. Lancet 1933, 222, 66–68. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Gaston Ramon’s Big Four. Toxins 2024, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.M. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J. Exp. Med. 1945, 82, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.C.D. Vaccine applications of flow cytometry. Methods 2012, 57, 383–391. [Google Scholar] [CrossRef]
- Wolf, J.J.; Wang, L.; Wang, F. Application of PCR technology in vaccine product development. Expert Rev. Vaccines 2007, 6, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Hu, J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef]
- Cuevas, J.M.; Geller, R.; Garijo, R.; López-Aldeguer, J.; Sanjuán, R. Extremely High Mutation Rate of HIV-1 In Vivo. PLoS Biol. 2015, 13, e1002251. [Google Scholar] [CrossRef] [PubMed]
- Tisthammer, K.H.; Solis, C.; Orcales, F.; Nzerem, M.; Winstead, R.; Dong, W.; Joy, J.B.; Pennings, P.S. Assessing in vivo mutation frequencies and creating a high-resolution genome-wide map of fitness costs of Hepatitis C virus. PLoS Genet. 2022, 18, e1010179. [Google Scholar] [CrossRef]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.-L. Evolution of Influenza A Virus by Mutation and Re-Assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef]
- Aristizábal, B. Innate Immune System—Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Devenish, L.P.; Mhlanga, M.M.; Negishi, Y. Immune Regulation in Time and Space: The Role of Local- and Long-Range Genomic Interactions in Regulating Immune Responses. Front. Immunol. 2021, 12, 662565. [Google Scholar] [CrossRef]
- Lefkowitz, R.B.; Miller, C.M.; Martinez-Caballero, J.D.; Ramos, I. Epigenetic Control of Innate Immunity: Consequences of Acute Respiratory Virus Infection. Viruses 2024, 16, 197. [Google Scholar] [CrossRef]
- Silmon De Monerri, N.C.; Kim, K. Pathogens Hijack the Epigenome: A New Twist on Host-Pathogen Interactions. Am. J. Pathol. 2014, 184, 897. [Google Scholar] [CrossRef]
- Weerakoon, H.; Mohamed, A.; Wong, Y.; Chen, J.; Senadheera, B.; Haigh, O.; Watkins, T.S.; Kazakoff, S.; Mukhopadhyay, P.; Mulvenna, J.; et al. Integrative temporal multi-omics reveals uncoupling of transcriptome and proteome during human T cell activation. npj Syst. Biol. Appl. 2024, 10, 1–13. [Google Scholar] [CrossRef]
- Salgado-Albarrán, M.; Navarro-Delgado, E.I.; Del Moral-Morales, A.; Alcaraz, N.; Baumbach, J.; González-Barrios, R.; Soto-Reyes, E. Comparative transcriptome analysis reveals key epigenetic targets in SARS-CoV-2 infection. npj Syst. Biol. Appl. 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Cano, R.L.E.; Lopera, H.D.E. Introduction to T and B lymphocytes; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Kim, H.; Ahn, H.S.; Hwang, N.; Huh, Y.; Bu, S.; Seo, K.J.; Kwon, S.H.; Lee, H.K.; Kim, J.w.; Yoon, B.K.; et al. Epigenomic landscape exhibits interferon signaling suppression in the patient of myocarditis after BNT162b2 vaccination. Sci. Rep. 2023, 13, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Yang, M.; Peng, D.; Yu, J.; Liu, Y.; Lv, J.; Chen, L.; Peng, X. scBridge embraces cell heterogeneity in single-cell RNA-seq and ATAC-seq data integration. Nat. Commun. 2023, 14, 6045. [Google Scholar] [CrossRef]
- Zhang, X.; Marand, A.P.; Yan, H.; Schmitz, R.J. Massive-scale single-cell chromatin accessibility sequencing using combinatorial fluidic indexing. Genome Biol. 2024, 25, 90. [Google Scholar] [CrossRef]
- Mazan-Mamczarz, K.; Ha, J.; De, S.; Sen, P. Single-Cell Analysis of the Transcriptome and Epigenome. Methods Mol. Biol. 2022, 2399, 21–60. [Google Scholar]
- Sinha, S.; Satpathy, A.T.; Zhou, W.; Ji, H.; Stratton, J.A.; Jaffer, A.; Bahlis, N.; Morrissy, S.; Biernaskie, J.A. Profiling Chromatin Accessibility at Single-Cell Resolution. Genom. Proteom. Bioinform. 2021, 19, 172–190. [Google Scholar] [CrossRef]
- Shi, P.; Nie, Y.; Yang, J.; Zhang, W.; Tang, Z.; Xu, J. Fundamental and practical approaches for single-cell ATAC-seq analysis. Abiotech 2022, 3, 212. [Google Scholar] [CrossRef]
- Ferreira, I.; Lee, C.; Foster, W.; Abdullahi, A.; Dratva, L.; Tuong, Z.; Stewart, B.; Ferdinand, J.; Guillaume, S.; Potts, M.; et al. Atypical B cells and impaired SARS-CoV-2 neutralization following heterologous vaccination in the elderly. Cell Rep. 2023, 42, 112991. [Google Scholar] [CrossRef]
- Waickman, A.T.; Victor, K.; Li, T.; Hatch, K.; Rutvisuttinunt, W.; Medin, C.; Gabriel, B.; Jarman, R.G.; Friberg, H.; Currier, J.R. Dissecting the heterogeneity of DENV vaccine-elicited cellular immunity using single-cell RNA sequencing and metabolic profiling. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Huang, W.; Wang, D.; Yao, Y.F. Understanding the pathogenesis of infectious diseases by single-cell RNA sequencing. Microb. Cell 2021, 8, 208. [Google Scholar] [CrossRef]
- Tzani, I.; Herrmann, N.; Carillo, S.; Spargo, C.A.; Hagan, R.; Barron, N.; Bones, J.; Dillmore, W.S.; Clarke, C. Tracing production instability in a clonally derived CHO cell line using single-cell transcriptomics. Biotechnol. Bioeng. 2021, 118, 2016–2030. [Google Scholar] [CrossRef]
- Svensson, V.; Vento-Tormo, R.; Teichmann, S.A. Exponential scaling of single-cell RNA-seq in the past decade. Nat. Protoc. 2018, 13, 599–604. [Google Scholar] [CrossRef]
- What Fraction of mRNA Transcripts Are Captured Per Cell? Available online: https://kb.10xgenomics.com/hc/en-us/articles/360001539051-What-fraction-of-mRNA-transcripts-are-captured-per-cell (accessed on 28 May 2025).
- Single-Cell RNA Sequencing (scRNA-Seq) Protocols: A Comparative Guide. Available online: https://www.bgee.org/support/scRNA-seq-protocols-comparison (accessed on 10 April 2025).
- Ding, J.; Adiconis, X.; Simmons, S.K.; Kowalczyk, M.S.; Hession, C.C.; Marjanovic, N.D.; Hughes, T.K.; Wadsworth, M.H.; Burks, T.; Nguyen, L.T.; et al. Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nat. Biotechnol. 2020, 38, 737. [Google Scholar] [CrossRef]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Noé, A.; Cargill, T.N.; Nielsen, C.M.; Russell, A.J.C.; Barnes, E. The Application of Single-Cell RNA Sequencing in Vaccinology. J. Immunol. Res. 2020, 2020, 8624963. [Google Scholar] [CrossRef]
- Silvano, M.; Virgolini, N.; Correia, R.; Clarke, C.; Isidro, I.; Alves, P.; Roldão, A. Dissecting insect cell heterogeneity during influenza VLP production using single-cell transcriptomics. Front. Bioeng. Biotechnol. 2023, 11, 1143255. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Z.; Zhou, W.; Jin, X.; Xu, C.; Luo, M.; Ma, K.; Cao, H.; Huang, Y.; Lin, X.; et al. Identification of potential vaccine targets for COVID-19 by combining single-cell and bulk TCR sequencing. Clin. Transl. Med. 2021, 11, e430. [Google Scholar] [CrossRef]
- Irac, S.E.; Soon, M.S.F.; Borcherding, N.; Tuong, Z.K. Single-cell immune repertoire analysis. Nat. Methods 2024, 21, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yang, Y.; Chen, L.; Wu, M.; Zhou, S. B-cell receptor repertoire sequencing: Deeper digging into the mechanisms and clinical aspects of immune-mediated diseases. iScience 2022, 25, 105002. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Voigt, A.; Leng, X.; Rodriguez, A.A.; Nguyen, C.Q. A current and future perspective on T cell receptor repertoire profiling. Front. Genet. 2023, 14, 1159109. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.; Wilfong, E.M.; Voss, K.; Barone, S.M.; Shiakolas, A.R.; Raju, N.; Roe, C.E.; Suryadevara, N.; Walker, L.M.; Wall, S.C.; et al. Single-cell profiling of the antigen-specific response to BNT162b2 SARS-CoV-2 RNA vaccine. Nat. Commun. 2022, 13, 1–20. [Google Scholar] [CrossRef]
- Hill, B.D.; Zak, A.J.; Raja, S.; Bugada, L.F.; Rizvi, S.M.; Roslan, S.B.; Nguyen, H.N.; Chen, J.; Jiang, H.; Ono, A.; et al. iGATE analysis improves the interpretability of single-cell immune landscape of influenza infection. JCI Insight 2024, 9, e172140. [Google Scholar] [CrossRef]
- Hogg, K.; Thomas, J.; Ashford, D.; Cartwright, J.; Coldwell, R.; Weston, D.; Pillmoor, J.; Surry, D.; O’Toole, P. Quantification of proteins by flow cytometry: Quantification of human hepatic transporter P-gp and OATP1B1 using flow cytometry and mass spectrometry. Methods 2015, 82, 38–46. [Google Scholar] [CrossRef]
- Boettler, T.; Csernalabics, B.; Salié, H.; Luxenburger, H.; Wischer, L.; Salimi Alizei, E.; Zoldan, K.; Krimmel, L.; Bronsert, P.; Schwabenland, M.; et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J. Hepatol. 2022, 77, 653–659. [Google Scholar] [CrossRef]
- Picot, J.; Guerin, C.L.; Le Van Kim, C.; Boulanger, C.M. Flow cytometry: Retrospective, fundamentals and recent instrumentation. Cytotechnology 2012, 64, 109. [Google Scholar] [CrossRef]
- Nettey, L.; Giles, A.J.; Chattopadhyay, P.K. OMIP-050: A 28-color/30-parameter Fluorescence Flow Cytometry Panel to Enumerate and Characterize Cells Expressing a Wide Array of Immune Checkpoint Molecules. Cytom. Part A 2018, 93, 1094–1096. [Google Scholar] [CrossRef]
- Achieving Reliable Results with Multiparameter Flow Cytometry. Available online: https://www.biocompare.com/Editorial-Articles/593617-Achieving-Reliable-Results-with-Multiparameter-Flow-Cytometry/ (accessed on 28 May 2025).
- Brestoff, J.R. Full spectrum flow cytometry in the clinical laboratory. Int. J. Lab. Hematol. 2023, 45, 44–49. [Google Scholar] [CrossRef]
- Park, L.M.; Lannigan, J.; Jaimes, M.C. OMIP-069: Forty-Color Full Spectrum Flow Cytometry Panel for Deep Immunophenotyping of Major Cell Subsets in Human Peripheral Blood. Cytom. Part A 2020, 97, 1044–1051. [Google Scholar] [CrossRef]
- Ashhurst, T.M.; Smith, A.L.; King, N.J.C. High-Dimensional Fluorescence Cytometry. Curr. Protoc. Immunol. 2017, 119, 5.8.1–5.8.38. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.M.; Greenplate, A.R.; Ihrie, R.A.; Irish, J.M. Beyond the Message: Advantages of Snapshot Proteomics with Single-Cell Mass Cytometry in Solid Tumors. FEBS J. 2019, 286, 1523. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.G.; Glass, D.R.; Juntilla, M.; Hartmann, F.J.; Oak, J.S.; Fernandez-Pol, S.; Ohgami, R.S.; Bendall, S.C. Multiplexed single-cell morphometry for hematopathology diagnostics. Nat. Med. 2020, 26, 408–417. [Google Scholar] [CrossRef]
- Bendall, S.C.; Simonds, E.F.; Qiu, P.; Amir, E.A.D.; Krutzik, P.O.; Finck, R.; Bruggner, R.V.; Melamed, R.; Trejo, A.; Ornatsky, O.I.; et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011, 332, 687–696. [Google Scholar] [CrossRef]
- Iyer, A.; Hamers, A.A.J.; Pillai, A.B. CyTOF® for the Masses. Front. Immunol. 2022, 13, 815828. [Google Scholar] [CrossRef]
- Hartmann, F.J.; Bendall, S.C. Immune monitoring using mass cytometry and related high-dimensional imaging approaches. Nat. Reviews. Rheumatol. 2019, 16, 87–99. [Google Scholar] [CrossRef]
- Naderi-Azad, S.; Croitoru, D.; Khalili, S.; Eder, L.; Piguet, V. Research Techniques Made Simple: Experimental Methodology for Imaging Mass Cytometry. J. Investig. Dermatol. 2021, 141, 467–473.e1. [Google Scholar] [CrossRef]
- Røgenes, H.; Finne, K.; Winge, I.; Akslen, L.A.; Östman, A.; Milosevic, V. Development of 42 marker panel for in-depth study of cancer associated fibroblast niches in breast cancer using imaging mass cytometry. Front. Immunol. 2024, 15, 1325191. [Google Scholar] [CrossRef]
- Stephenson, E.; Reynolds, G.; Botting, R.A.; Calero-Nieto, F.J.; Morgan, M.D.; Tuong, Z.K.; Bach, K.; Sungnak, W.; Worlock, K.B.; Yoshida, M.; et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat. Med. 2021, 27, 904–916. [Google Scholar] [CrossRef]
- Song, H.W.; Martin, J.; Shi, X.; Tyznik, A.J. Key Considerations on CITE-Seq for Single-Cell Multiomics. Proteomics 2025, e202400011. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M.; Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Franceschi, C.; Blomberg, B.B.; Pirazzini, C.; Ravaioli, F.; Gentilini, D.; Di Blasio, A.M.; Garagnani, P.; Frasca, D.; Bacalini, M.G. Responders and non-responders to influenza vaccination: A DNA methylation approach on blood cells. Exp. Gerontol. 2018, 105, 94. [Google Scholar] [CrossRef]
- Fu, H.; Pickering, H.; Rubbi, L.; Ross, T.M.; Reed, E.F.; Pellegrini, M. Longitudinal analysis of influenza vaccination implicates regulation of RIG-I signaling by DNA methylation. Sci. Rep. 2024, 14, 1455. [Google Scholar] [CrossRef]
- Cao, L.; Luo, Y.; Guo, X.; Liu, S.; Li, S.; Li, J.; Zhang, Z.; Zhao, Y.; Zhang, Q.; Gao, F.; et al. SAFA facilitates chromatin opening of immune genes through interacting with anti-viral host RNAs. PLoS Pathog. 2022, 18, e1010599. [Google Scholar] [CrossRef]
- Wen, L.; Li, G.; Huang, T.; Geng, W.; Pei, H.; Yang, J.; Zhu, M.; Zhang, P.; Hou, R.; Tian, G.; et al. Single-cell technologies: From research to application. Innov. 2022, 3, 100342. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Chen, L.; Zhang, D.; Zhao, P.; Chen, Z.; Qin, E.-Q.; Gao, Y.; Davis, M.M.; Yang, P.; You, M.; et al. Single-cell epigenomic landscape of peripheral immune cells reveals establishment of trained immunity in individuals convalescing from COVID-19. Nat. Cell Biol. 2021, 23, 620–630. [Google Scholar] [CrossRef]
- Akimov, V.E.; Tychinin, D.I.; Antonova, O.A.; Shaymardanov, A.M.; Voronina, M.D.; Deinichenko, K.A.; Fateev, O.D.; Yudin, V.S.; Yudin, S.M.; Mukhin, V.E.; et al. Remodeling of the chromatin landscape in peripheral blood cells in patients with severe Delta COVID-19. Front. Immunol. 2024, 15, 1415317. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, B.; Ling, Y.; Guo, M.; Qin, B.; Ren, X.; Wang, C.; Yang, H.; Chen, L.; Liao, Y.; et al. Frontiers|Epigenetic Landscapes of Single-Cell Chromatin Accessibility and Transcriptomic Immune Profiles of T Cells in COVID-19 Patients. Front. Immunol. 2021, 12, 625881. [Google Scholar]
- Lee, D.; Choi, S.Y.; Shin, S.-I.; An, H.; Choi, B.-S.; Park, J. Multi-Omics Single-Cell Analysis Reveals Key Regulators of HIV-1 Persistence and Aberrant Host Immune Responses in Early Infection. Microbiol. Infect. Dis. 2025, 14, RP104856. [Google Scholar]
- Byrne, A.; Le, D.; Sereti, K.; Menon, H.; Vaidya, S.; Patel, N.; Lund, J.; Xavier-Magalhães, A.; Shi, M.; Liang, Y.; et al. Single-cell long-read targeted sequencing reveals transcriptional variation in ovarian cancer. Nat. Commun. 2024, 15, 6916. [Google Scholar] [CrossRef]
- Pokhilko, A.; Handel, A.; Curion, F.; Volpato, V.; Whiteley, E.; Bøstrand, S.; Newey, S.; Akerman, C.; Webber, C.; Clark, M.; et al. Targeted single-cell RNA sequencing of transcription factors enhances the identification of cell types and trajectories—PubMed. Genome Res. 2021, 31, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Method of the Year: Spatially resolved transcriptomics. Nat. Methods 2021, 18, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.K.; Guo, M.G.; Ji, A.L.; Khavari, P.A. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet. 2021, 22, 627–644. [Google Scholar] [CrossRef]
- Chen, T.; You, L.; Hardillo, J.; Chien, M. Spatial Transcriptomic Technologies. Cells 2023, 12, 2042. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Z.; Jiao, Y.; Yu, K.; Bhatara, S.; Yang, X.; Natarajan, S.; Zhang, J.; Pan, Q.; Easton, J.; et al. Spotiphy enables single-cell spatial whole transcriptomics across an entire section. Nat. Methods 2025, 22, 724–736. [Google Scholar] [CrossRef]
- Bruker Announces Advancements in Transcriptomics and Spatial Biology at AGBT 2025|RNA-Seq Blog. Available online: https://www.rna-seqblog.com/bruker-announces-advancements-in-transcriptomics-and-spatial-biology-at-agbt-2025/ (accessed on 10 April 2025).
- Vahid, M.R.; Brown, E.L.; Steen, C.B.; Zhang, W.; Jeon, H.S.; Kang, M.; Gentles, A.J.; Newman, A.M. High-resolution alignment of single-cell and spatial transcriptomes with CytoSPACE. Nat. Biotechnol. 2023, 41, 1543–1548. [Google Scholar] [CrossRef]
- Fulcher, J.M.; Markillie, L.M.; Mitchell, H.D.; Williams, S.M.; Engbrecht, K.M.; Degnan, D.J.; Bramer, L.M.; Moore, R.J.; Chrisler, W.B.; Cantlon-Bruce, J.; et al. Parallel measurement of transcriptomes and proteomes from same single cells using nanodroplet splitting. Nat. Commun. 2024, 15, 1–13. [Google Scholar] [CrossRef]
- Gong, H.; Wang, X.; Liu, B.; Boutet, S.; Holcomb, I.; Dakshinamoorthy, G.; Ooi, A.; Sanada, C.; Sun, G.; Ramakrishnan, R. Single-cell protein-mRNA correlation analysis enabled by multiplexed dual-analyte co-detection. Sci. Rep. 2017, 7, 2776. [Google Scholar] [CrossRef]
- Albayrak, C.; Jordi, C.A.; Zechner, C.; Lin, J.; Bichsel, C.A.; Khammash, M.; Tay, S. Digital Quantification of Proteins and mRNA in Single Mammalian Cells. Mol. Cell 2016, 61, 914–924. [Google Scholar] [CrossRef]
- McSharry, J.J. Analysis of virus-infected cells by flow cytometry. Methods 2000, 21, 249–257. [Google Scholar] [CrossRef]
- Warren, C.J.; Barbachano-Guerrero, A.; Huey, D.; Yang, Q.; Worden-Sapper, E.R.; Kuhn, J.H.; Sawyer, S.L. Quantification of virus-infected cells using RNA FISH-Flow. STAR Protoc. 2023, 4, 102291. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef]
- Olsen, L.R.; Leipold, M.D.; Pedersen, C.B.; Maecker, H.T. The anatomy of single cell mass cytometry data. Cytom. Part A 2019, 95, 156–172. [Google Scholar] [CrossRef]
- Bennett, H.M.; Stephenson, W.; Rose, C.M.; Darmanis, S.; Bennett, H.M.; Stephenson, W.; Rose, C.M.; Darmanis, S. Single-cell proteomics enabled by next-generation sequencing or mass spectrometry. Nat. Methods 2023, 20, 363–374. [Google Scholar] [CrossRef]
- Budnik, B.; Levy, E.; Harmange, G.; Slavov, N. SCoPE-MS: Mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol. 2018, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Specht, H.; Emmott, E.; Petelski, A.A.; Huffman, R.G.; Perlman, D.H.; Serra, M.; Kharchenko, P.; Koller, A.; Slavov, N. Single-cell proteomic and transcriptomic analysis of macrophage heterogeneity using SCoPE2. Genome Biol. 2021, 22, 50. [Google Scholar] [CrossRef]
- Zhu, Y.; Piehowski, P.D.; Zhao, R.; Chen, J.; Shen, Y.; Moore, R.J.; Shukla, A.K.; Petyuk, V.A.; Campbell-Thompson, M.; Mathews, C.E.; et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10–100 mammalian cells. Nat. Commun. 2018, 9, 882. [Google Scholar] [CrossRef]
- Woo, J.; Williams, S.M.; Markillie, L.M.; Feng, S.; Tsai, C.-F.; Aguilera-Vazquez, V.; Sontag, R.L.; Moore, R.J.; Hu, D.; Mehta, H.S.; et al. High-throughput and high-efficiency sample preparation for single-cell proteomics using a nested nanowell chip. Nat. Commun. 2021, 12, 6246. [Google Scholar] [CrossRef]
- Mansuri, M.S.; Williams, K.; Nairn, A.C. Uncovering biology by single-cell proteomics. Commun. Biol. 2023, 6, 381. [Google Scholar] [CrossRef]
- Lee, S.; Vu, H.M.; Lee, J.H.; Lim, H.; Kim, M.S. Advances in Mass Spectrometry-Based Single Cell Analysis. Biology 2023, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Evrony, G.D.; Hinch, A.G.; Luo, C. Applications of Single-cell DNA Sequencing. Annu. Rev. Genom. Hum. Genet. 2021, 22, 171. [Google Scholar] [CrossRef]
- Bouzidi, M.S.; Dossani, Z.Y.; Benedetto, C.D.; Raymond, K.A.; Desai, S.; Chavez, L.R.; Betancur, P.; Pillai, S.K. High-resolution Inference of Multiplexed Anti-HIV Gene Editing using Single-Cell Targeted DNA Sequencing. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ryan, D.G.; Peace, C.G.; Hooftman, A. Basic Mechanisms of Immunometabolites in Shaping the Immune Response. J. Innate Immun. 2023, 15, 925. [Google Scholar] [CrossRef]
- Dolatmoradi, M.; Samarah, L.Z.; Vertes, A. Single-Cell Metabolomics by Mass Spectrometry: Opportunities and Challenges. Anal. Sens. 2022, 2, e202100032. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, M.; Kumar, S.; Prasad, M. Single Cell Metabolomics: A Future Tool to Unmask Cellular Heterogeneity and Virus-Host Interaction in Context of Emerging Viral Diseases. Front. Microbiol. 2020, 11, 1152. [Google Scholar] [CrossRef]
- Dimitriu, M.A.; Lazar-Contes, I.; Roszkowski, M.; Mansuy, I.M. Single-Cell Multiomics Techniques: From Conception to Applications. Front. Cell Dev. Biol. 2022, 10, 854317. [Google Scholar] [CrossRef]
- Baysoy, A.; Bai, Z.; Satija, R.; Fan, R. The technological landscape and applications of single-cell multi-omics. Nat. Rev. Mol. Cell Biol. 2023, 24, 695–713. [Google Scholar] [CrossRef]
- Lee, J.; Hyeon, D.Y.; Hwang, D. Single-cell multiomics: Technologies and data analysis methods. Exp. Mol. Med. 2020, 52, 1428–1442. [Google Scholar] [CrossRef]
- Mosaheb, M.M.; Brown, M.C.; Dobrikova, E.Y.; Dobrikov, M.I.; Gromeier, M. Harnessing Virus Tropism for Dendritic Cells for Vaccine Design. Curr. Opin. Virol. 2020, 44, 73–80. [Google Scholar] [CrossRef]
- Mosaheb, M.M.; Dobrikova, E.Y.; Brown, M.C.; Yang, Y.; Cable, J.; Okada, H.; Nair, S.K.; Bigner, D.D.; Ashley, D.M.; Gromeier, M.; et al. Genetically stable poliovirus vectors activate dendritic cells and prime antitumor CD8 T cell immunity. Nat. Commun. 2020, 11, 524. [Google Scholar] [CrossRef]
- Suder, E.; Furuyama, W.; Feldmann, H.; Marzi, A.; Wit, E.d. The vesicular stomatitis virus-based Ebola virus vaccine: From concept to clinical trials. Hum. Vaccines Immunother. 2018, 14, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Nichols, R.; Tussey, L.; Scappaticci, K.; Pullano, T.G.; Whiteman, M.D.; Vasilakis, N.; Rossi, S.L.; Campos, R.K.; Azar, S.R.; et al. Recombinant vesicular stomatitis vaccine against Nipah virus has a favorable safety profile: Model for assessment of live vaccines with neurotropic potential. PLoS Pathog. 2022, 18, e1010658. [Google Scholar] [CrossRef]
- McCall, L.-I. Frontiers|Quo vadis? Central Rules of Pathogen and Disease Tropism. Front. Cell. Infect. Microbiol. 2021, 11, 640987. [Google Scholar] [CrossRef] [PubMed]
- Riggs, J.B.; Medina, E.M.; Perrenoud, L.J.; Bonilla, D.L.; Clambey, E.T.; van Dyk, L.F.; Berg, L.J. Frontiers|Optimized Detection of Acute MHV68 Infection With a Reporter System Identifies Large Peritoneal Macrophages as a Dominant Target of Primary Infection. Front. Microbiol. 2021, 12, 656979. [Google Scholar] [CrossRef]
- Traum, D.; Wang, Y.J.; Schwarz, K.B.; Schug, J.; Wong, D.K.; Janssen, H.L.; Terrault, N.A.; Khalili, M.; Wahed, A.S.; Murray, K.F.; et al. Highly multiplexed 2-dimensional imaging mass cytometry analysis of HBV-infected liver. JCI Insight 2021, 6, e146883. [Google Scholar] [CrossRef]
- Rendeiro, A.F.; Ravichandran, H.; Bram, Y.; Chandar, V.; Kim, J.; Meydan, C.; Park, J.; Foox, J.; Hether, T.; Warren, S.; et al. The spatial landscape of lung pathology during COVID-19 progression. Nature 2021, 593, 564–569. [Google Scholar] [CrossRef]
- Ghazalpour, A.; Bennett, B.; Petyuk, V.A.; Orozco, L.; Hagopian, R.; Mungrue, I.N.; Farber, C.R.; Sinsheimer, J.; Kang, H.M.; Furlotte, N.; et al. Comparative Analysis of Proteome and Transcriptome Variation in Mouse. PLoS Genet. 2011, 7, e1001393. [Google Scholar] [CrossRef]
- Mullan, K.A.; de Vrij, N.; Valkiers, S.; Meysman, P. Frontiers|Current annotation strategies for T cell phenotyping of single-cell RNA-seq data. Front. Immunol. 2023, 14, 1306169. [Google Scholar] [CrossRef]
- Steuerman, Y.; Cohen, M.; Peshes-Yaloz, N.; Valadarsky, L.; Cohn, O.; David, E.; Frishberg, A.; Mayo, L.; Bacharach, E.; Amit, I.; et al. Dissection of Influenza Infection In Vivo by Single-Cell RNA Sequencing. Cell Syst. 2018, 6, 679–691.e4. [Google Scholar] [CrossRef]
- Yang, L.e.; Xiong, J.; Liu, Y.; Liu, Y.; Wang, X.; Si, Y.; Zhu, B.; Chen, H.; Cao, S.; Ye, J.; et al. Single-cell RNA sequencing reveals the immune features and viral tropism in the central nervous system of mice infected with Japanese encephalitis virus. J. Neuroinflamm. 2024, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, X.; Zhang, H.; Zhao, G.-D.; Yang, H.; Qiu, J.; Meng, S.; Wu, P.; Tao, L.; Wang, Q.; et al. Single-Cell Transcriptome Identifies the Renal Cell Type Tropism of Human BK Polyomavirus. Int. J. Mol. Sci. 2023, 24, 1330. [Google Scholar] [CrossRef]
- Liukang, C.; Zhao, J.; Tian, J.; Huang, M.; Liang, R.; Zhao, Y.; Zhang, G. Deciphering infected cell types, hub gene networks and cell-cell communication in infectious bronchitis virus via single-cell RNA sequencing. PLoS Pathog. 2024, 20, e1012232. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M. Cellular Tropism of SARS-CoV-2 across Human Tissues and Age-related Expression of ACE2 and TMPRSS2 in Immune-inflammatory Stromal Cells. Aging Dis. 2021, 12, 718–725. [Google Scholar] [CrossRef]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’Souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef]
- Oyarzún, P.; Kobe, B. Recombinant and epitope-based vaccines on the road to the market and implications for vaccine design and production. Hum. Vaccines Immunother. 2015, 12, 763–767. [Google Scholar] [CrossRef]
- Marchese, A.M.; Beyhaghi, H.; Orenstein, W.A. With established safe and effective use, protein vaccines offer another choice against COVID-19. Vaccine 2022, 40, 6567–6569. [Google Scholar] [CrossRef]
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol. J. 2019, 14, e1800376. [Google Scholar] [CrossRef]
- Kwong, P.D.; Mascola, J.R. HIV-1 Vaccines Based on Antibody Identification, B Cell Ontogeny, and Epitope Structure. Immunity 2018, 48, 855–871. [Google Scholar] [CrossRef]
- Kar, T.; Narsaria, U.; Basak, S.; Deb, D.; Castiglione, F.; Mueller, D.M.; Srivastava, A.P.; Kar, T.; Narsaria, U.; Basak, S.; et al. A candidate multi-epitope vaccine against SARS-CoV-2. Sci. Rep. 2020, 10, 10895. [Google Scholar] [CrossRef]
- Gottlieb, T.; Ben-Yedidia, T. Epitope-based approaches to a universal influenza vaccine. J. Autoimmun. 2014, 54, 15–20. [Google Scholar] [CrossRef]
- Burton, D.R.; Poignard, P.; Stanfield, R.L.; Wilson, I.A. Broadly Neutralizing Antibodies Present New Prospects to Counter Highly Antigenically Diverse Viruses. Science 2012, 337, 183–186. [Google Scholar] [CrossRef]
- Corti, D.; Lanzavecchia, A.; Corti, D.; Lanzavecchia, A. Broadly Neutralizing Antiviral Antibodies. Annu. Rev. Immunol. 2013, 31, 705–742. [Google Scholar] [CrossRef]
- Zost, S.J.; Wu, N.C.; Hensley, S.E.; Wilson, I.A. Immunodominance and Antigenic Variation of Influenza Virus Hemagglutinin: Implications for Design of Universal Vaccine Immunogens. J. Infect. Dis. 2019, 219, S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Irving, A.T. Frontiers | Massively-multiplexed epitope mapping techniques for viral antigen discovery. Front. Immunol. 2023, 14, 1192385. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Guelen, L.; Hulsik, D.L.; Ermakov, G.; Hsieh, E.J.; Kreijtz, J.; Stammen-Vogelzangs, J.; Lodewijks, I.; Bertens, A.; Bramer, A.; et al. Epitope mapping of monoclonal antibodies: A comprehensive comparison of different technologies. mAbs 2023, 15, 2285285. [Google Scholar] [CrossRef]
- Smith, M.; Bugada, L.; Wen, F. Rapid microsphere-assisted peptide screening (MAPS) of promiscuous MHCII-binding peptides in Zika virus envelope protein. AIChE J. Am. Inst. Chem. Eng. 2020, 66, e16697. [Google Scholar] [CrossRef]
- Wen, F.; Sethi, D.; Wucherpfennig, K.; Zhao, H. Cell surface display of functional human MHC class II proteins: Yeast display versus insect cell display. Protein Eng. Des. Sel. PEDS 2011, 24, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Smith, M.; Zhao, H. Construction and Screening of an Antigen-Derived Peptide Library Displayed on Yeast Cell Surface for CD4+ T Cell Epitope Identification. Methods Mol. Biol. 2019, 2024, 213–234. [Google Scholar]
- Pira, G.L.; Ivaldi, F.; Moretti, P.; Manca, F. High Throughput T Epitope Mapping and Vaccine Development. J. Biomed. Biotechnol. 2010, 2010, 325720. [Google Scholar]
- Phage Display Antibody Discovery. Available online: https://www.criver.com/products-services/discovery-services/antibody-discovery-services/phage-display?region=3701 (accessed on 23 June 2025).
- Ahmed, R.K.S.; Maeurer, M.J. T-Cell Epitope Mapping. Ep. Mapp. Protoc. 2009, 524, 427–438. [Google Scholar]
- Walker, L.M.; Shiakolas, A.R.; Venkat, R.; Liu, Z.A.; Wall, S.; Raju, N.; Pilewski, K.A.; Setliff, I.; Murji, A.A.; Gillespie, R.; et al. High-Throughput B Cell Epitope Determination by Next-Generation Sequencing. Front. Immunol. 2022, 13, 855772. [Google Scholar] [CrossRef]
- Sela-Culang, I.; Ashkenazi, S.; Peters, B.; Ofran, Y. PEASE: Predicting B-cell epitopes utilizing antibody sequence. Bioinformatics 2015, 31, 1313–1315. [Google Scholar] [CrossRef]
- Setliff, I.; Shiakolas, A.R.; Pilewski, K.A.; Murji, A.A.; Mapengo, R.E.; Janowska, K.; Richardson, S.; Oosthuysen, C.; Raju, N.; Ronsard, L. High-Throughput Mapping of B Cell Receptor Sequences to Antigen Specificity. Cell 2019, 179, 1636–1646.e1615. [Google Scholar] [CrossRef]
- Shiakolas, A.R.; Kramer, K.J.; Johnson, N.V.; Wall, S.C.; Suryadevara, N.; Wrapp, D.; Periasamy, S.; Pilewski, K.A.; Raju, N.; Nargi, R.; et al. Efficient discovery of SARS-CoV-2-neutralizing antibodies via B cell receptor sequencing and ligand blocking. Nat. Biotechnol. 2022, 40, 1270–1275. [Google Scholar] [CrossRef]
- Zepp, F. Principles of vaccine design—Lessons from nature. Vaccine 2010, 28, C14–C24. [Google Scholar] [CrossRef]
- Howard, F.H.N.; Kwan, A.; Winder, N.; Mughal, A.; Collado-Rojas, C.; Muthana, M. Understanding Immune Responses to Viruses—Do Underlying Th1/Th2 Cell Biases Predict Outcome? Viruses 2022, 14, 1493. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X.; Zhao, T.; Cai, Y.; Jiang, Y.; et al. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.-G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Koff, W.C.; Burton, D.R.; Johnson, P.R.; Walker, B.D.; King, C.R.; Nabel, G.J.; Ahmed, R.; Bhan, M.K.; Plotkin, S.A. Accelerating Next-Generation Vaccine Development for Global Disease Prevention. Science 2013, 340, 1232910. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Soni, D.; Brook, B.; Nanishi, E.; Dowling, D.J. Precision Vaccine Development: Cues from Natural Immunity. Front. Immunol. 2022, 12, 662218. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Vazquez Guillamet, C.; Day, A.; Borcherding, N.; Vazquez Guillamet, R.; Choreño-Parra, J.A.; House, S.L.; O’Halloran, J.A.; Zúñiga, J.; Ellebedy, A.H.; et al. Comprehensive Immunologic Evaluation of Bronchoalveolar Lavage Samples from Human Patients with Moderate and Severe Seasonal Influenza and Severe COVID-19. J. Immunol. 2021, 207, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Bost, P.; Giladi, A.; Liu, Y.; Bendjelal, Y.; Xu, G.; David, E.; Blecher-Gonen, R.; Cohen, M.; Medaglia, C.; Li, H.; et al. Host-Viral Infection Maps Reveal Signatures of Severe COVID-19 Patients. Cell 2020, 181, 1475–1488.e12. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Montin, D.; Santilli, V.; Beni, A.; Costagliola, G.; Martire, B.; Mastrototaro, M.F.; Ottaviano, G.; Rizzo, C.; Sgrulletti, M.; Miraglia Del Giudice, M.; et al. Frontiers | Towards personalized vaccines. Front. Immunol. 2024, 15, 1436108. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef]
- Cope, A.; Friec, G.L.; Cardone, J.; Kemper, C. The Th1 life cycle: Molecular control of IFN-γ to IL-10 switching. Trends Immunol. 2011, 32, 278–286. [Google Scholar] [CrossRef]
- Guo, S.A.; Bowyer, G.S.; Ferdinand, J.R.; Maes, M.; Tuong, Z.K.; Gillman, E.; Liao, M.; Lindeboom, R.G.H.; Yoshida, M.; Worlock, K.; et al. Obesity Is Associated with Attenuated Tissue Immunity in COVID-19. Am. J. Respir. Crit. Care Med. 2023, 207, 566–576. [Google Scholar] [CrossRef]
- van der Weerd, K.; Dik, W.A.; Schrijver, B.; Schweitzer, D.H.; Langerak, A.W.; Drexhage, H.A.; Kiewiet, R.M.; van Aken, M.O.; van Huisstede, A.; van Dongen, J.J.M.; et al. Morbidly Obese Human Subjects Have Increased Peripheral Blood CD4+ T Cells with Skewing Toward a Treg- and Th2-Dominated Phenotype. Diabetes 2012, 61, 401–408. [Google Scholar] [CrossRef]
- Paich, H.A.; Sheridan, P.A.; Handy, J.; Karlsson, E.A.; Schultz-Cherry, S.; Hudgens, M.G.; Noah, T.L.; Weir, S.S.; Beck, M.A. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 Influenza a virus. Obesity 2013, 21, 2377–2386. [Google Scholar] [CrossRef]
- Higdon, M.; Wahl, B.; Jones, C.; Rosen, J.; Truelove, S.; Baidya, A.; Nande, A.; ShamaeiZadeh, P.; Walter, K.; Feikin, D.; et al. A Systematic Review of Coronavirus Disease 2019 Vaccine Efficacy and Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Disease. Open Forum Infect. Dis. 2022, 9, ofac138. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, A.; Radzikowski, A. Efficacy, effectiveness, immunogenicity—Are not the same in vaccinology. World J. Gastroenterol. 2013, 19, 7217–7218. [Google Scholar] [CrossRef]
- Mahanty, S.; Prigent, A.; Garraud, O.; Mahanty, S.; Prigent, A.; Garraud, O. Immunogenicity of infectious pathogens and vaccine antigens. BMC Immunol. 2015, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- King, D.F.; Groves, H.; Weller, C.; Jones, I.; Cramer, J.P.; Gilbert, P.B.; Goldblatt, D.; Gruber, M.F.; Kampmann, B.; Maïga, D.; et al. Realising the potential of correlates of protection for vaccine development, licensure and use: Short summary. Npj Vaccines 2024, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Reber, A.; Katz, J. Immunological assessment of influenza vaccines and immune correlates of protection. Expert Rev. Vaccines 2013, 12, 519. [Google Scholar] [CrossRef]
- WHO. Measles vaccines: WHO position paper—April 2017. Wkly. Epidemiol. Rec. 2017, 92, 205–228. [Google Scholar]
- WHO. Recommendations for inactivated rabies vaccine for human use produced in cell substrates and embryonated eggs. WHO Tech. Rep. Ser. 2007, 941, 83–132. [Google Scholar]
- WHO. Recommendations to assure the quality, safety and efficacy of poliomyelitis vaccines (oral, live, attenuated). WHO Tech. Rep. Ser. 2014, 98, 49–140. [Google Scholar]
- WHO. Hepatitis A vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2022, 97, 493–512. [Google Scholar]
- WHO. Recommendations to Assure the Quality, Safety and Efficacy of Recombinant Hepatitis B Vaccines. WHO Tech. Rep. Ser. 2013, 978, 189–240. [Google Scholar]
- Plotkin, S.A. Recent updates on correlates of vaccine-induced protection. Front. Immunol. 2023, 13, 1081107. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Chen, H.; Rahil, Z.; Bidoki, N.H.; Jiang, S.; Bjornson, Z.; Kolhatkar, N.S.; Martinez, C.J.; Gaudillière, B.; Hedou, J.; et al. Human influenza virus challenge identifies cellular correlates of protection for oral vaccination. Cell Host Microbe 2021, 29, 1828–1837.e1825. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; Azeredo, E.L.d.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; Silva, A.D.D.; Silva, A.M.d.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Tang, W.W.; Eddy, W.E.; King, K.; Sanchez, M.C.; Shresta, S. CD8+ T Cells Can Mediate Short-Term Protection against Heterotypic Dengue Virus Reinfection in Mice. J. Virol. 2015, 89, 6494–6505. [Google Scholar] [CrossRef]
- Yauch, L.E.; Zellweger, R.l.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A Protective Role for Dengue Virus-Specific CD8+ T Cells. J. Immunol. 2009, 182, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Babor, M.; Lane, J.; Seumois, G.; Liang, S.; Goonawardhana, N.S.; Silva, A.D.D.; Phillips, E.J.; Mallal, S.A.; Antunes, R.d.S.; et al. Dengue-specific CD8+ T cell subsets display specialized transcriptomic and TCR profiles. J. Clin. Investig. 2019, 129, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, R.I.; Martínez-Pérez, A.; Escarrega, E.A.; Singh, T.; Zambrana, J.V.; Balmaseda, Á.; Harris, E.; Weiskopf, D. Frequency of dengue virus–specific T cells is related to infection outcome in endemic settings. JCI Insight 2025, 10, e179771. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Miller, R.; Eddy, W.E.; White, L.J.; Johnston, R.E.; Shresta, S. Role of Humoral versus Cellular Responses Induced by a Protective Dengue Vaccine Candidate. PLoS Pathog. 2013, 9, e1003723. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Grifoni, A.; Sette, A.; Weiskopf, D. Frontiers | Human T Cell Response to Dengue Virus Infection. Front. Immunol. 2019, 10, 2125. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Carmen, J.M.; Shrivastava, S.; Lu, Z.; Anderson, A.; Morrison, E.B.; Sankhala, R.S.; Chen, W.-H.; Chang, W.C.; Bolton, J.S.; Matyas, G.R.; et al. SARS-CoV-2 ferritin nanoparticle vaccine induces robust innate immune activity driving polyfunctional spike-specific T cell responses. npj Vaccines 2021, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, F.; Donato, M.; Kuo, A.; Ashuach, T.; Gupta, S.; Li, C.; Dvorak, M.; Foecke, M.H.; Chang, S.E.; Hagan, T.; et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell 2021, 184, 3915–3935.e3921. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, J.H.; Kim, M.; Lee, Y.; Hwang, Y.-H.; Park, M.; Li, C.H.; Lee, T.; Lee, J.-A.; Kim, Y.-M.; et al. Innate immune responses against mRNA vaccine promote cellular immunity through IFN-β at the injection site. Nat. Commun. 2024, 15, 7226. [Google Scholar] [CrossRef]
- Swaminathan, G.; Thoryk, E.; Cox, K.; Meschino, S.; Dubey, S.; Vora, K.; Celano, R.; Gindy, M.; Casimiro, D.; Bett, A. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine 2016, 34, 110–119. [Google Scholar] [CrossRef]
- Ni, H.; Capodici, J.; Cannon, G.; Communi, D.; Boeynaems, J.; Karikó, K.; Weissman, D. Extracellular mRNA induces dendritic cell activation by stimulating tumor necrosis factor-alpha secretion and signaling through a nucleotide receptor. J. Biol. Chem. 2002, 277, 12689–12696. [Google Scholar] [CrossRef]
- Mullins, D.; Sheasley, S.; Ream, R.; Bullock, T.; Fu, Y.; Engelhard, V. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 2003, 198, 1023–1034. [Google Scholar] [CrossRef]
- Lambert, P.H.; Laurent, P.E. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine 2008, 26, 3197–3208. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Bonduelle, O.; Duffy, D.; Parizot, C.; Verrier, B.; Combadière, B. Original Encounter with Antigen Determines Antigen-Presenting Cell Imprinting of the Quality of the Immune Response in Mice. PLoS ONE 2009, 4, e8159. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Tchitchek, N.; Joly, C.; Pozo, A.R.; Stimmer, L.; Langlois, S.; Hocini, H.; Gosse, L.; Pejoski, D.; Cosma, A.; et al. Vaccine Inoculation Route Modulates Early Immunity and Consequently Antigen-Specific Immune Response. Front. Immunol. 2021, 12, 645210. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US—December 14, 2020–January 18, 2021. JAMA 2021, 325, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Ahmad Cheema, H.; Ahmed Khan, M.H.; Shahid, H.; Saad Ali, M.; Hassan, U.; Wahaj Murad, M.; Aemaz Ur Rehman, M.; Farooq, H. Development of myocarditis and pericarditis after COVID-19 vaccination in adult population: A systematic review. Ann. Med. Surg. 2022, 76, 103486. [Google Scholar] [CrossRef] [PubMed]
- Trontzas, I.; Kyriakoulis, K.; Vathiotis, I.; Syrigos, A.; Kounadis, G.; Siasiakou, S.; Poulakou, G. Vaccine-Related Autoimmune Hepatitis: Emerging Association with SARS-CoV-2 Vaccination or Coincidence? Vaccines 2022, 10, 2073. [Google Scholar] [CrossRef] [PubMed]
- Yaamika, H.; Muralidas, D.; Elumalai, K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J. Taibah Univ. Med. Sci. 2023, 18, 1646–1661. [Google Scholar] [CrossRef]
- Syed, A.S.; Sultana, S.; Begum, A.; Nadeem, K.; Ara, J.; Askarey, S.H.; Siddiqui, A.A.; Anwar, A.; Hashmi, A.A. Severity of Adverse Effects of Sinovac COVID-19 Vaccine in Postmenopausal Women: A Multicenter Experience From Pakistan. Cureus 2023, 15, e46682. [Google Scholar] [CrossRef]
- Takano, T.; Morikawa, M.; Adachi, Y.; Kabasawa, K.; Sax, N.; Moriyama, S.; Sun, L.; Isogawa, M.; Nishiyama, A.; Onodera, T.; et al. Distinct immune cell dynamics correlate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. Cell Rep. Med. 2022, 3, 100631. [Google Scholar] [CrossRef]
- Yoon, B.; Oh, T.; Bu, S.; Seo, K.; Kwon, S.; Lee, J.; Kim, Y.; Kim, J.; Ahn, H.; Fang, S. The Peripheral Immune Landscape in a Patient with Myocarditis after the Administration of BNT162b2 mRNA Vaccine. Mol. Cells 2022, 45, 738–748. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, H.; Luo, L.; Zhang, L.; Xiong, A.; Wang, J.; Wang, Q.; Liu, Y.; Liu, S.; Xiong, Y.; et al. Single cell characteristics of patients with vaccine-related adverse reactions following inactivated COVID-19 vaccination. Hum. Vaccines Immunother. 2023, 19, 2246542. [Google Scholar] [CrossRef]
- Whitaker, J.; Ovsyannikova, I.; Poland, G. Adversomics: A new paradigm for vaccine safety and design. Expert Rev. Vaccines 2015, 14, 935–947. [Google Scholar] [CrossRef]
- Trøseid, M.; Hentzien, M.; Ader, F.; Cardoso, S.W.; Arribas, J.R.; Molina, J.-M.; Mueller, N.; Hites, M.; Bonnet, F.; Manuel, O.; et al. Immunocompromised patients have been neglected in COVID-19 trials: A call for action. Clin. Microbiol. Infect. 2022, 28, 1182–1183. [Google Scholar] [CrossRef]
- Gillard, P.; Nakhle, S.; Brimhall, D.; Henry, O.; Mesaros, N. Enhancing vaccine clinical trials participation among elderly: Challenges and strategies—PubMed. Trials 2025, 26, 38. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R.; Hou, Y.; Chen, M.; et al. Insights into vaccines for elderly individuals: From the impacts of immunosenescence to delivery strategies. npj Vaccines 2024, 9, 77. [Google Scholar] [CrossRef]
- Fung, M.; Babik, J. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, 340–350. [Google Scholar] [CrossRef]
- Rüthrich, M.M.; Giesen, N.; Mellinghoff, S.C.; Rieger, C.T.; von Lilienfeld-Toal, M. Cellular Immune Response after Vaccination in Patients with Cancer—Review on Past and Present Experiences. Vaccines 2022, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Tranter, E.; Frentsch, M.; Hütter-Krönke, M.; Vuong, G.; Busch, D.; Loyal, L.; Henze, L.; Rosnev, S.; Blau, I.; Thiel, A.; et al. Comparable CD8+ T-cell responses to SARS-CoV-2 vaccination in single-cell transcriptomics of recently allogeneic transplanted patients and healthy individuals. J. Med. Virol. 2024, 96, e29539. [Google Scholar] [CrossRef] [PubMed]
- Alimam, S.; Ann Timms, J.; Harrison, C.N.; Dillon, R.; Mare, T.; DeLavallade, H.; Radia, D.; Woodley, C.; Francis, Y.; Sanchez, K.; et al. Altered immune response to the annual influenza A vaccine in patients with myeloproliferative neoplasms. Br. J. Haematol. 2021, 193, 150–154. [Google Scholar] [CrossRef]
- Hu, M.; Oliveira, A.; Fang, Z.; Feng, Y.; Miranda, M.; Kowli, S.; Arunachalam, P.; Vasudevan, G.; Hui, H.; Grifoni, A.; et al. Altered baseline immunological state and impaired immune response to SARS-CoV-2 mRNA vaccination in lung transplant recipients. Cell Rep. Med. 2025, 6, 102050. [Google Scholar] [CrossRef]
- Haberman, R.H.; Herati, R.; Simon, D.; Samanovic, M.; Blank, R.B.; Tuen, M.; Koralov, S.B.; Atreya, R.; Tascilar, K.; Allen, J.R.; et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann. Rheum. Dis. 2021, 80, 1339–1344. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X.; et al. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef]
- Gerelkhuu, Z.; Park, S.; Lee, K.H.; Kim, Y.C.; Kwon, S.J.; Song, K.-H.; Kim, E.S.; Song, Y.G.; Park, Y.S.; Ahn, J.Y.; et al. Overcoming the age-dependent SARS-CoV-2 vaccine response through hybrid immunity: Analysis of humoral and cellular immunity with mass cytometry profiling. Immun. Ageing 2024, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Gomez, P.L.; Robinson, J.M. Vaccine Manufacturing. Plotkin’s Vaccines 2017, 51–60.e1. [Google Scholar] [CrossRef]
- Nettleship, J.E.; Assenberg, R.; Diprose, J.M.; Rahman-Huq, N.; Owens, R.J. Recent advances in the production of proteins in insect and mammalian cells for structural biology. J. Struct. Biol. 2010, 172, 55–65. [Google Scholar] [CrossRef]
- Sanden, S.M.G.v.d.; Wu, W.; Dybdahl-Sissoko, N.; Weldon, W.C.; Brooks, P.; O’Donnell, J.; Jones, L.P.; Brown, C.; Tompkins, S.M.; Oberste, M.S.; et al. Engineering Enhanced Vaccine Cell Lines To Eradicate Vaccine-Preventable Diseases: The Polio End Game. J. Virol. 2015, 90, 1694–1704. [Google Scholar] [CrossRef]
- Long, G.; Pan, X.; Kormelink, R.; Vlak, J.M. Functional Entry of Baculovirus into Insect and Mammalian Cells Is Dependent on Clathrin-Mediated Endocytosis. J. Virol. 2006, 80, 8830–8833. [Google Scholar] [CrossRef]
- Paz-Cortés, E.; Pastor, A.; Salinas-Marín, R.; Ramírez, O.; Palomares, L. Molecular characterization of the effects of heat shock on the infection cycle progression and productivity of the baculovirus expression vector system. PLoS ONE 2025, 20, e0320917. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, Y.; Mo, Y.; Gao, X.; Miao, M.; Yu, W. Acetylation of citrate synthase inhibits Bombyx mori nucleopolyhedrovirus propagation by affecting energy metabolism. Microb. Pathog. 2022, 173, 105890. [Google Scholar] [CrossRef]
- Virgolini, N.; Silvano, M.; Hagan, R.; Correia, R.; Alves, P.M.; Clarke, C.; Roldão, A.; Isidro, I.A. Impact of dual-baculovirus infection on the Sf9 insect cell transcriptome during rAAV production using single-cell RNA-seq. Biotechnol. Bioeng. 2023, 120, 2588–2600. [Google Scholar] [CrossRef]
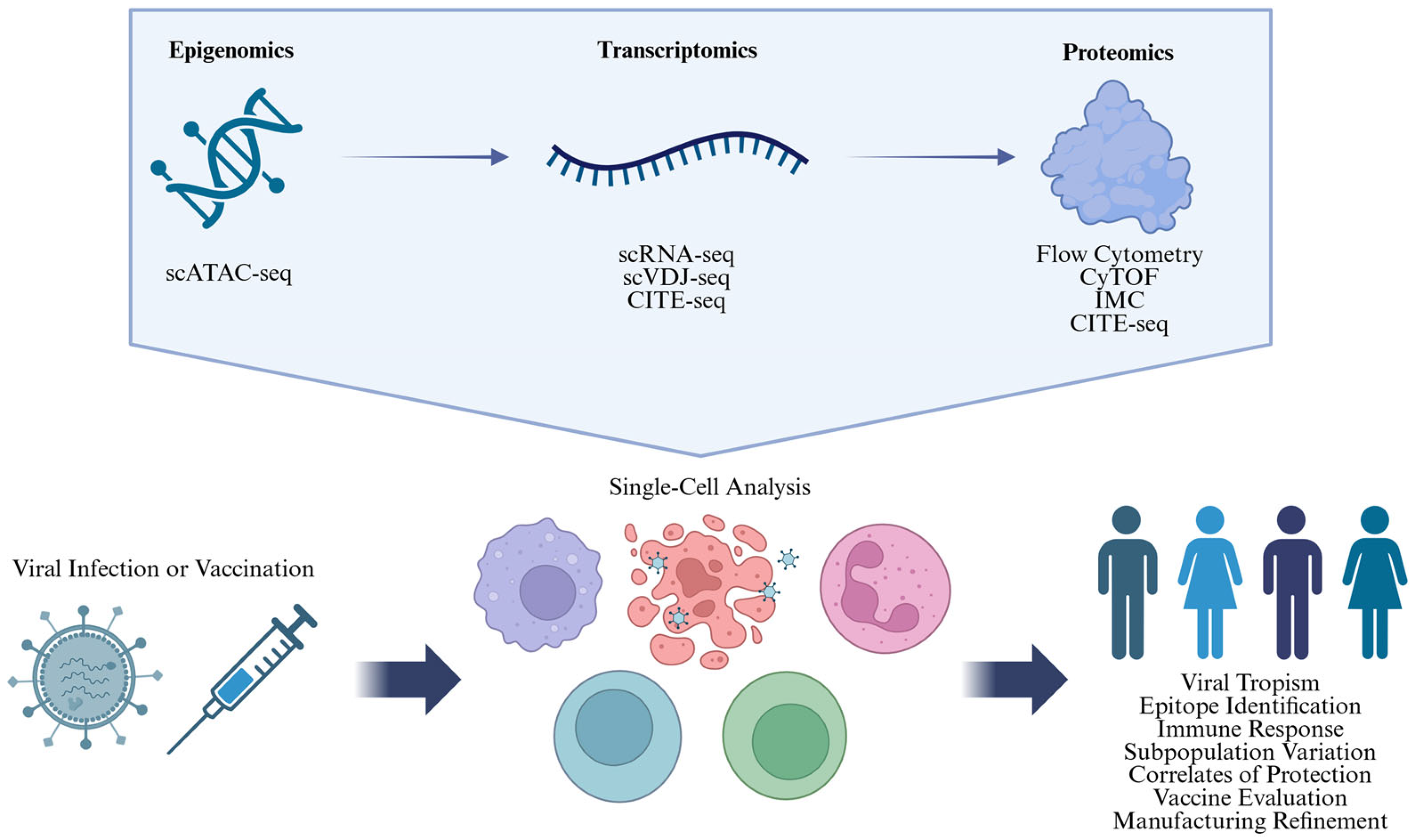
| Technology | Insights | Example Applications | Throughput and Multiplexity | Review Papers | |
|---|---|---|---|---|---|
| Epigenomic | scATAC-seq | Chromatin accessibility | Identify chromatin alterations induced by infection and vaccination [62] Characterize regulatory elements controlling immune responses [62] Discover epigenetically distinct immune cell types and novel subsets [63] | ~102–~105 cells/experiment [64] ~2500–73,000 reads/cell [65] | [66,67] |
| Transcriptomic | scRNA-seq | Transcript diversity | Identify immune cell subsets and infer effector states, detect rare cellular subsets [68] Identify infection or vaccine induced transcript expression signatures [69] Simultaneously detect host and pathogen RNA [70] Characterize cell line heterogeneity [71] | ~102–~105 cells/experiment [72] 5–30% transcriptome coverage [73] 1000–14,000 genes/cell [74,75] 1–3 days/experiment [74] | [76,77] |
| scVDJ-seq (scBCR-seq and scTCR-seq) | B/T-cell receptor clonotype diversity | Characterize B/T-cell receptor clonotypes heterogeneity, specific to infection or vaccination [78] Identify antigen-specific dominant B/T-cell receptor sequences associated with protection and deduce immunodominant antigens [79] Track B/T-cell differentiation and trace lineage (combined with scRNA-seq) [69] | [80,81,82] | ||
| Proteomic | Flow cytometry | Fluorescence-based protein expression measurement in suspended cells | Identify immune cell subsets and functional states Measure proliferation, detect cytokine and antibody production [83] Detect infected cells, intracellular pathogens, and apoptosis [84] Flow cytometry: Characterize protein expression levels of cell lines [85] IMC: Track immune cells interaction, infiltration and tissue remodeling [86] | ~105 cells/s [87] Conventional: 5–10 markers, up to 28 [88,89] Spectral: 20–30 markers, up to 40 [90,91] | [90,92] |
| CyTOF | Metal-tag-based protein expression measurement in suspended cells | ~1000–2000 cells/s [93] 30–40+ markers [94,95] | [96,97] | ||
| IMC | Metal-tag-based protein expression measurement and localization in tissue | 1–5 µm/pixel [96] 20–200 Hz [97] 30–40+ markers [41,98,99] | [40,97] | ||
| Transcriptomic + Proteomic | CITE-seq | Integrated transcript diversity and surface protein expression | Identify immune cell subsets and functional states with enhanced cell type resolution Detect activation markers and cytokine production [100] Detect immune dysregulation (e.g., hyperactivation or suppression) | ~103 cells/experiment [42] ~102 proteins + transcriptomic data [42] | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.N.; Vanderzee, I.O.; Wen, F. The Application of Single-Cell Technologies for Vaccine Development Against Viral Infections. Vaccines 2025, 13, 687. https://doi.org/10.3390/vaccines13070687
Nguyen HN, Vanderzee IO, Wen F. The Application of Single-Cell Technologies for Vaccine Development Against Viral Infections. Vaccines. 2025; 13(7):687. https://doi.org/10.3390/vaccines13070687
Chicago/Turabian StyleNguyen, Hong Nhi, Isabel O. Vanderzee, and Fei Wen. 2025. "The Application of Single-Cell Technologies for Vaccine Development Against Viral Infections" Vaccines 13, no. 7: 687. https://doi.org/10.3390/vaccines13070687
APA StyleNguyen, H. N., Vanderzee, I. O., & Wen, F. (2025). The Application of Single-Cell Technologies for Vaccine Development Against Viral Infections. Vaccines, 13(7), 687. https://doi.org/10.3390/vaccines13070687






