Multivalent COBRA Hemagglutinin and Neuraminidase Influenza Vaccines Adjuvanted with TLR9 Agonist CpG 1018
Abstract
1. Introduction
2. Materials and Methods
2.1. Antigen Construction and Synthesis
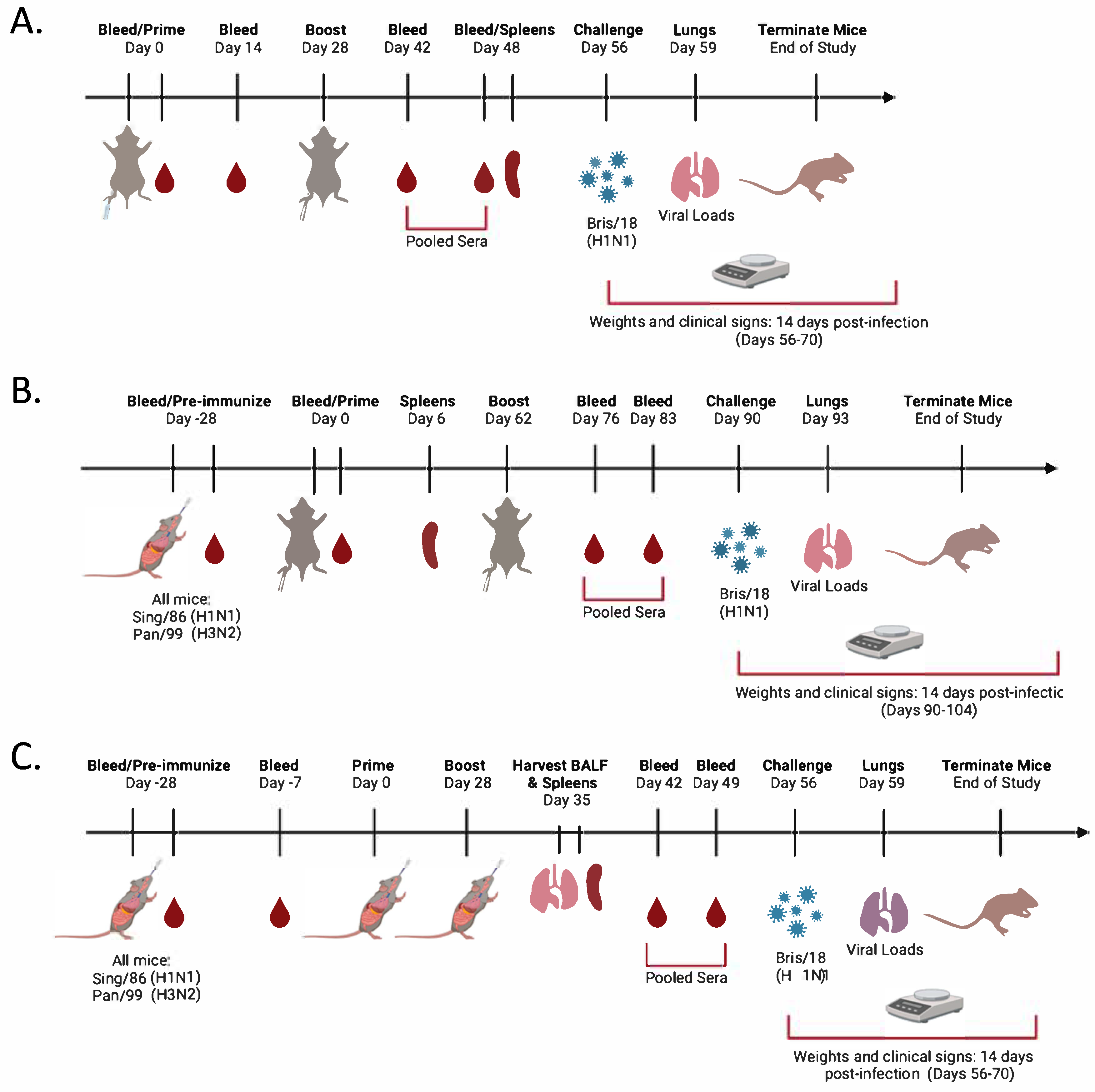
2.2. Vaccination and Infection
2.3. Enzyme-Linked Immunosorbant Assay (ELISA)
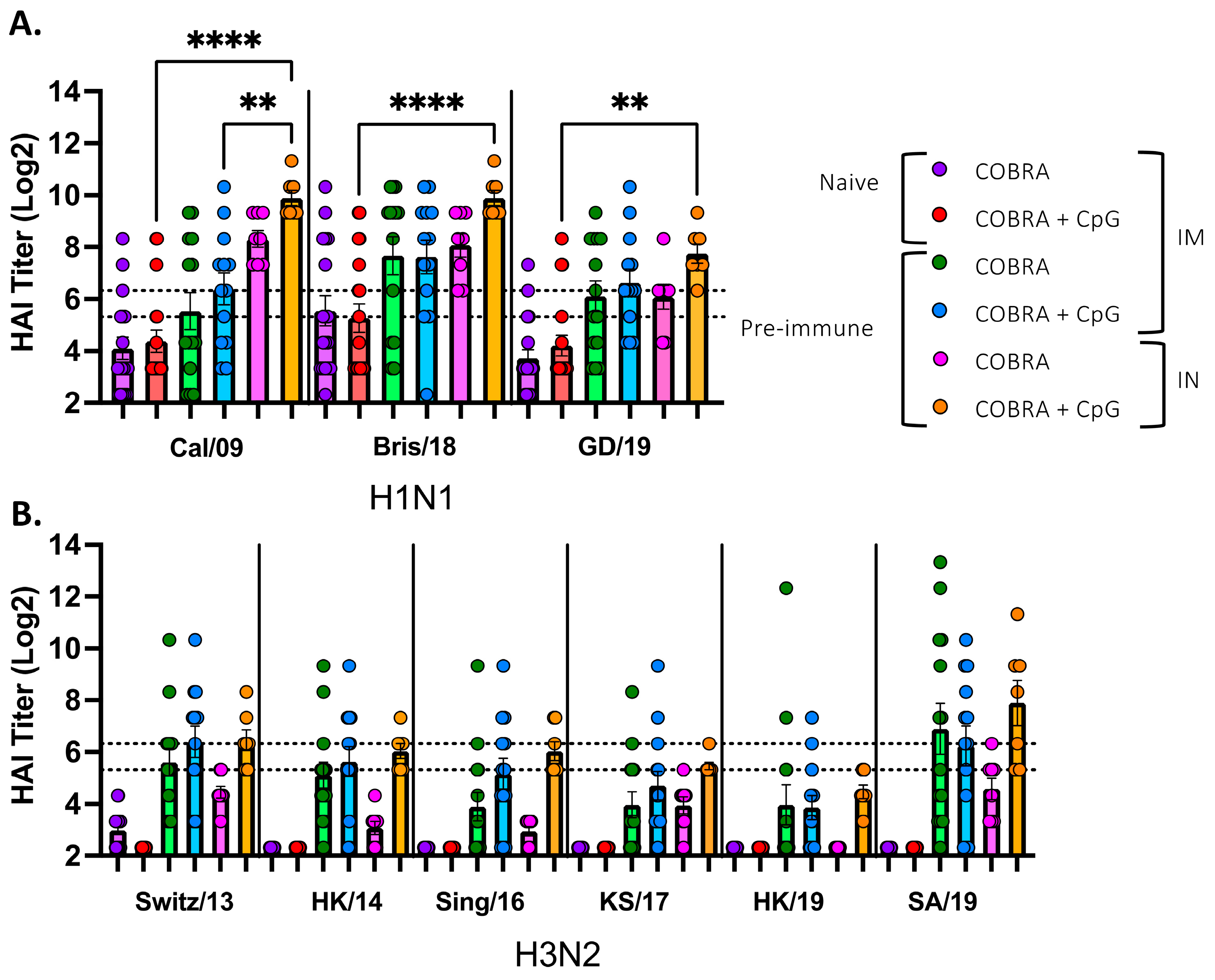
2.4. Hemagglutination Inhibition Assay (HAI)
2.5. Enzyme-Linked Lectin Assay (ELLA)
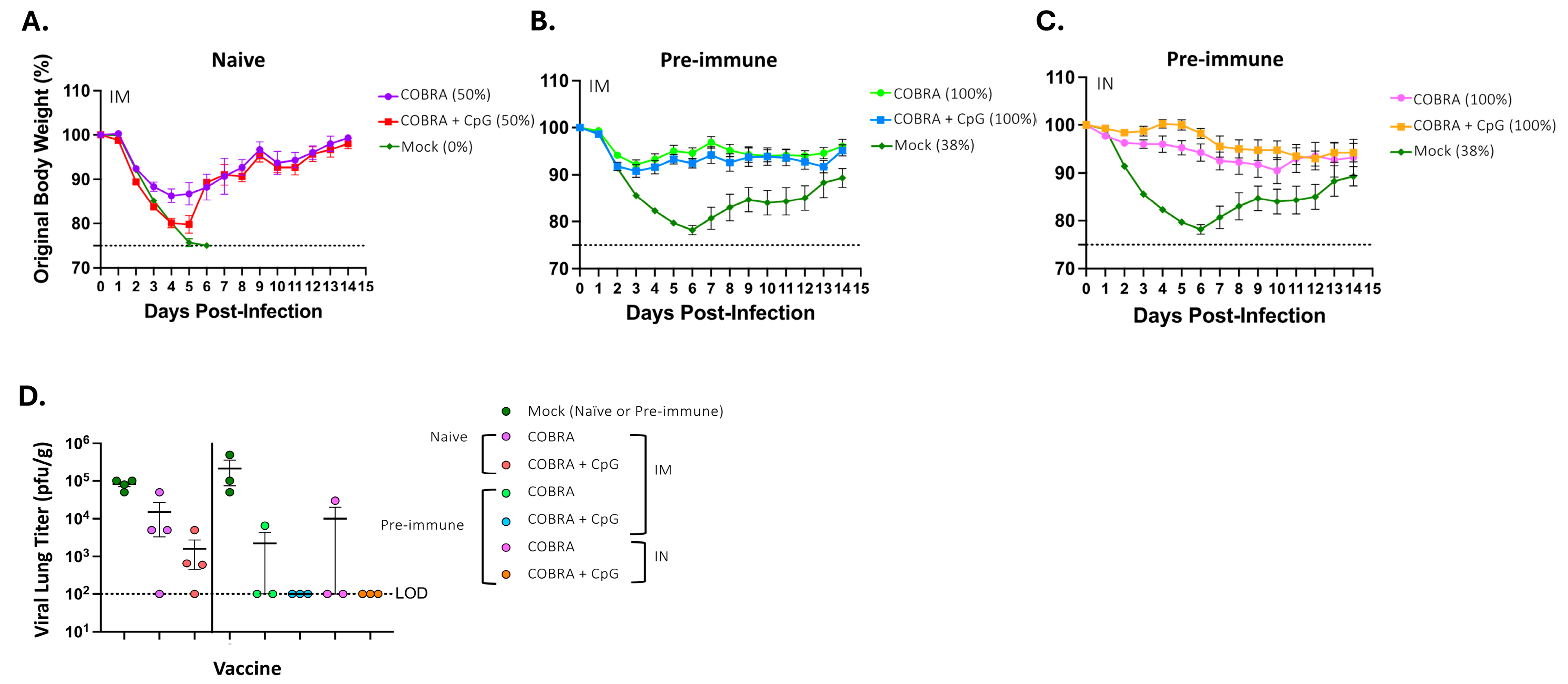
2.6. Lung Viral Titers
2.7. FluoroSpot Assay
Statistical Analysis
3. Results
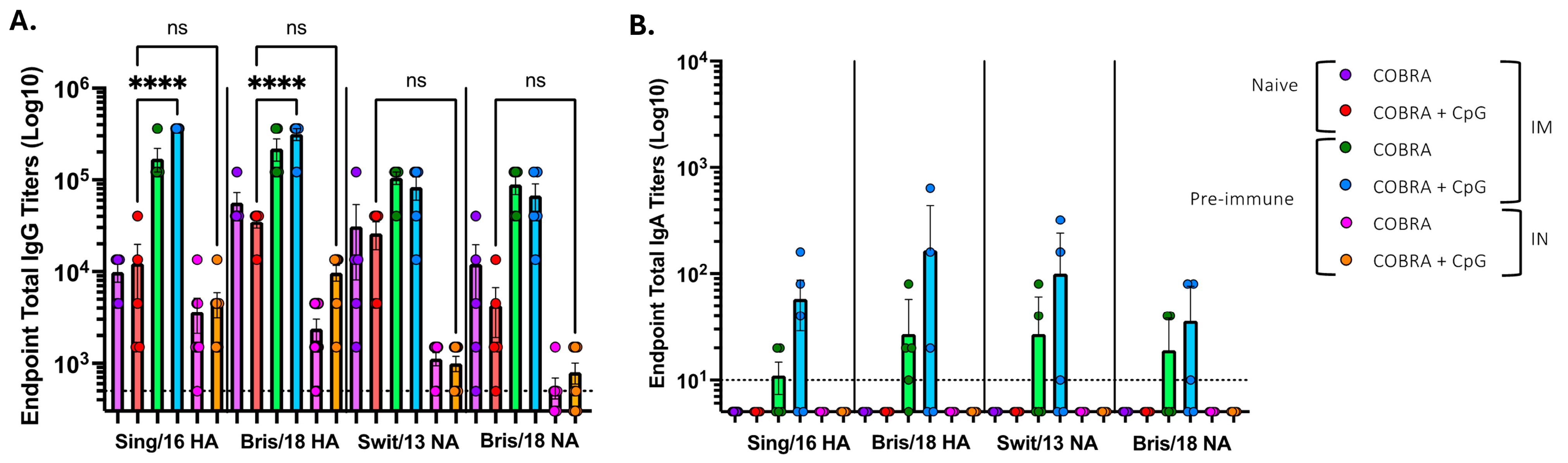
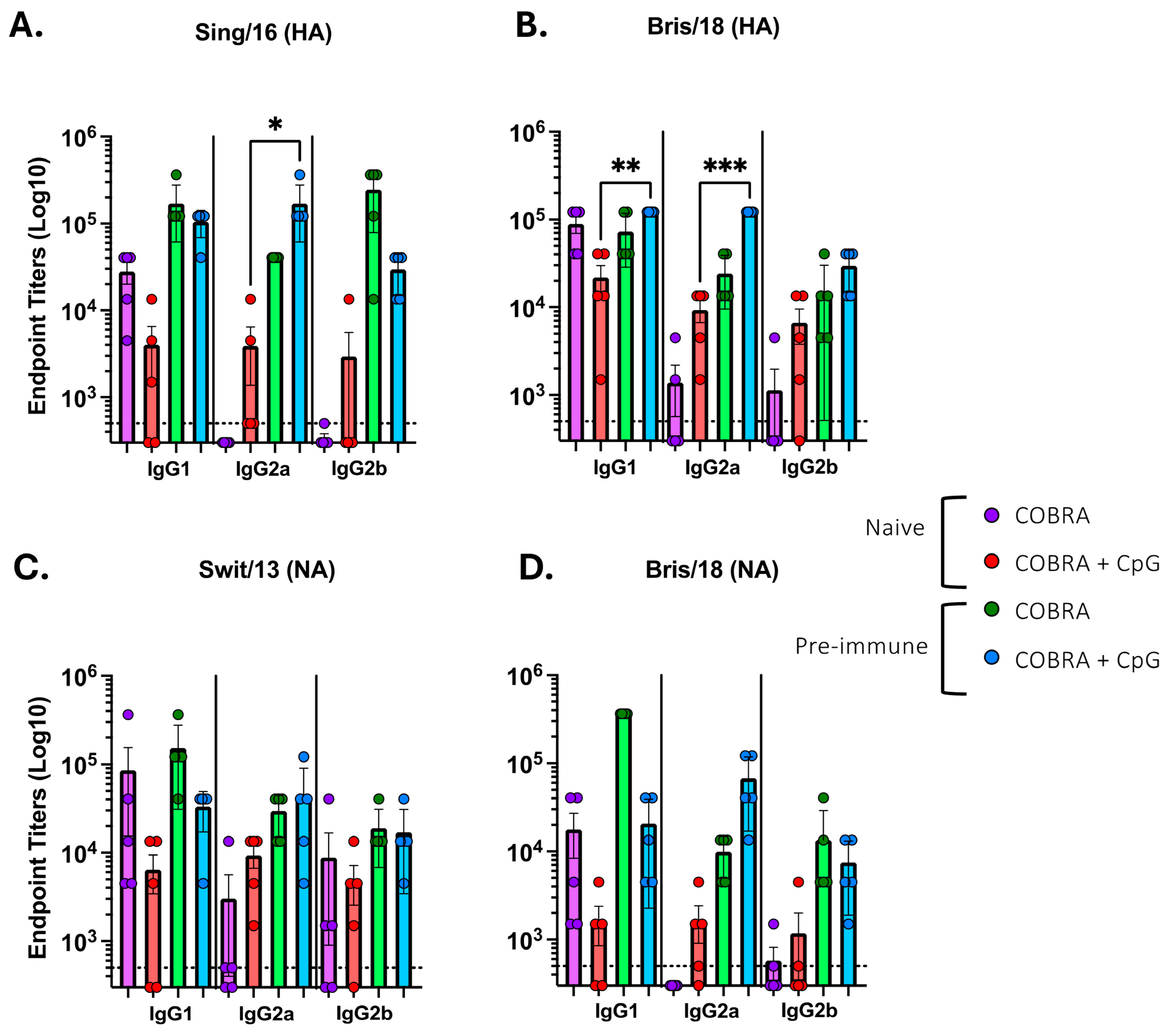
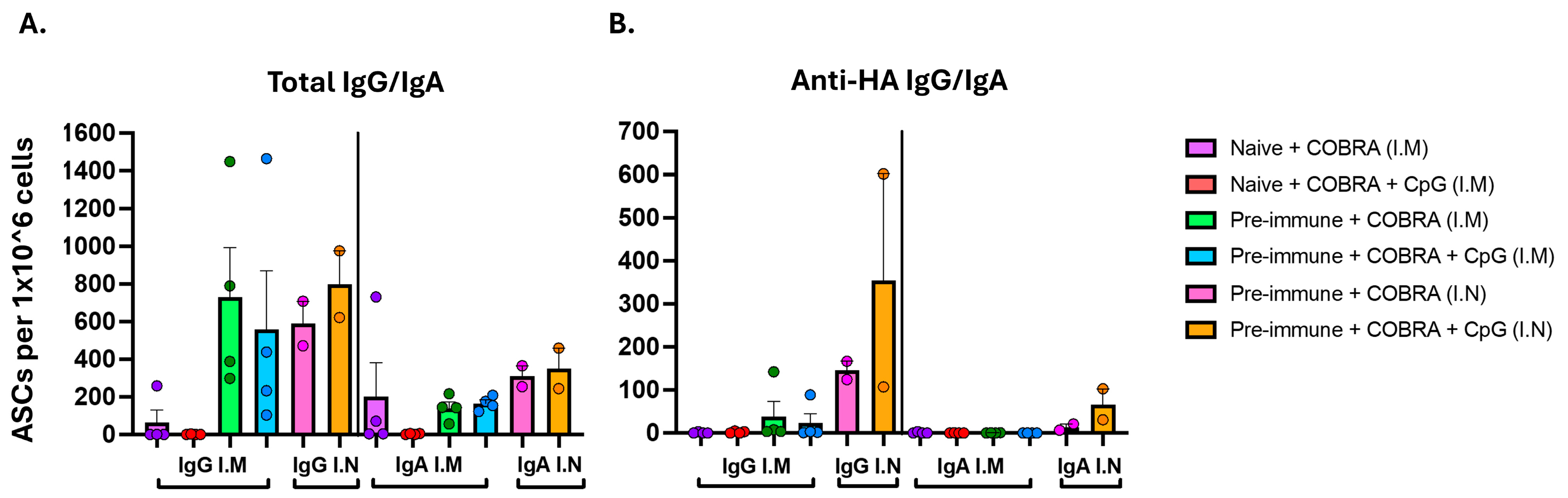
3.1. CpG 1018 Adjuvant Enhances Serum and BALF Anti-HA/NA Antibodies
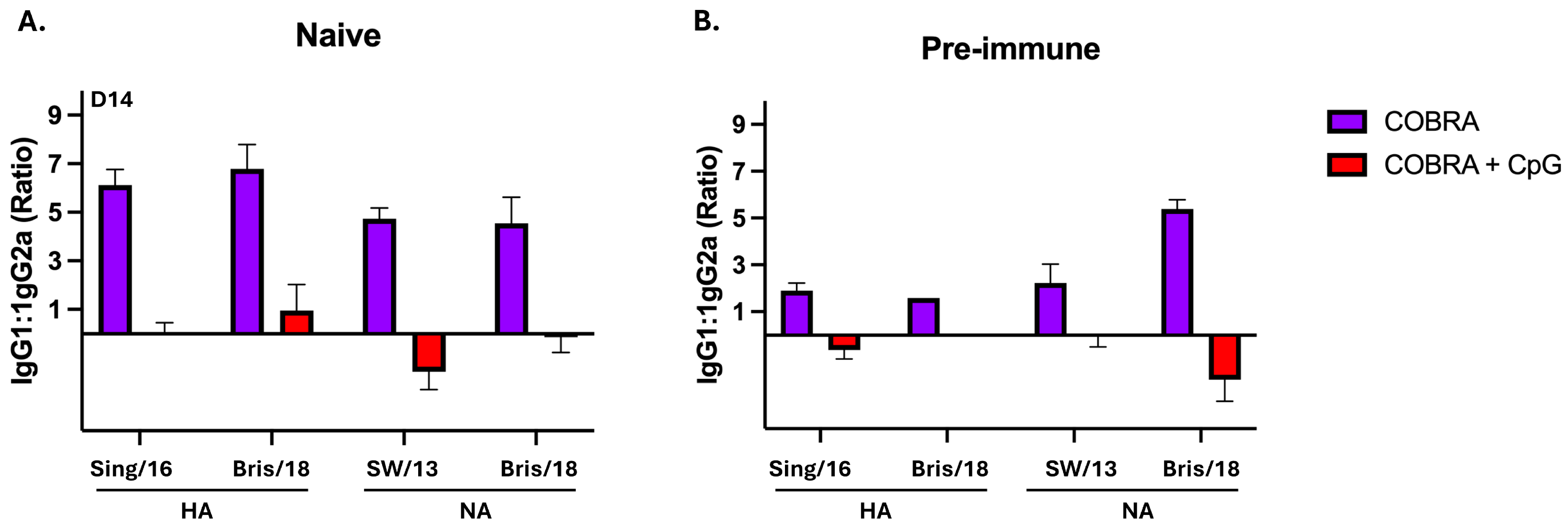
3.2. CpG 1018 Adjuvant Drives Production of IgG and IgA Antibody-Secreting Cells Following Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartley, J.M.; Cadar, A.N.; Martin, D.E. Better, Faster, Stronger: mRNA Vaccines Show Promise for Influenza Vaccination in Older Adults. Immunol. Invest. 2021, 50, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Tokars, J.I.; Olsen, S.J.; Reed, C. Seasonal Incidence of Symptomatic Influenza in the United States. Clin. Infect. Dis. 2018, 66, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Nafziger, A.N.; Pratt, D.S. Seasonal influenza vaccination and technologies. J. Clin. Pharmacol. 2014, 54, 719–731. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccin. Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Kazmin, D.; Napolitani, G.; Clutterbuck, E.A.; Pulendran, B.; Siegrist, C.A.; Pollard, A.J. AS03- and MF59-Adjuvanted Influenza Vaccines in Children. Front. Immunol. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Allen, J.D.; Medina, J.M.; Thomas, M.H.; Lynch, A.; Nelson, R.; Aguirre, J.; Ross, T.M. H3 hemagglutinin proteins optimized for 2018 to 2022 elicit neutralizing antibodies across panels of modern influenza A(H3N2) viruses. J. Immunol. 2025. [Google Scholar] [CrossRef]
- Allen, J.D.; Zhang, X.; Medina, J.M.; Thomas, M.H.; Lynch, A.; Nelson, R.; Aguirre, J.; Ross, T.M. Computationally Optimized Hemagglutinin Proteins Adjuvanted with Infectimune((R)) Generate Broadly Protective Antibody Responses in Mice and Ferrets. Vaccines 2024, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Franca, M.S.; Allen, J.D.; Shi, H.; Ross, T.M. Next Generation of Computationally Optimized Broadly Reactive HA Vaccines Elicited Cross-Reactive Immune Responses and Provided Protection against H1N1 Virus Infection. Vaccines 2021, 9, 793. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Ross, T.M. Multivalent H3 COBRA-based influenza vaccine elicits enhanced immune response in a pre-immune elderly ferret model. Vaccine 2025, 56, 127156. [Google Scholar] [CrossRef]
- Skarlupka, A.L.; Bebin-Blackwell, A.G.; Sumner, S.F.; Ross, T.M. Universal Influenza Virus Neuraminidase Vaccine Elicits Protective Immune Responses against Human Seasonal and Pre-pandemic Strains. J. Virol. 2021, 95, e0075921. [Google Scholar] [CrossRef] [PubMed]
- Soema, P.C.; Kompier, R.; Amorij, J.P.; Kersten, G.F. Current and next generation influenza vaccines: Formulation and production strategies. Eur. J. Pharm. Biopharm. 2015, 94, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, S.; Amanat, F.; Campbell, J.D.; Traquina, P.; Coffman, R.L.; Krammer, F. A CpG 1018 adjuvanted neuraminidase vaccine provides robust protection from influenza virus challenge in mice. NPJ Vaccines 2022, 7, 81. [Google Scholar] [CrossRef]
- Lai, C.Y.; Yu, G.Y.; Luo, Y.; Xiang, R.; Chuang, T.H. Immunostimulatory Activities of CpG-Oligodeoxynucleotides in Teleosts: Toll-Like Receptors 9 and 21. Front. Immunol. 2019, 10, 179. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X. CpG 1018 Is an Effective Adjuvant for Influenza Nucleoprotein. Vaccines 2023, 11, 649. [Google Scholar] [CrossRef]
- Rose, M.A.; Zielen, S.; Baumann, U. Mucosal immunity and nasal influenza vaccination. Expert Rev. Vaccines 2012, 11, 595–607. [Google Scholar] [CrossRef]
- Ecker, J.W.; Kirchenbaum, G.A.; Pierce, S.R.; Skarlupka, A.L.; Abreu, R.B.; Cooper, R.E.; Taylor-Mulneix, D.; Ross, T.M.; Sautto, G.A. High-Yield Expression and Purification of Recombinant Influenza Virus Proteins from Stably-Transfected Mammalian Cell Lines. Vaccines 2020, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortes-Garcia, G.; Vogel, T.U.; et al. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Adjuvanted influenza vaccines. Hum. Vaccin. Immunother. 2012, 8, 59–66. [Google Scholar] [CrossRef]
- Killingley, B.; Nguyen-Van-Tam, J. Routes of influenza transmission. Influenza Other Respir. Viruses 2013, 7, 42–51. [Google Scholar] [CrossRef]
- Wong, J.P.; Christopher, M.E.; Viswanathan, S.; Karpoff, N.; Dai, X.; Das, D.; Sun, L.Q.; Wang, M.; Salazar, A.M. Activation of toll-like receptor signaling pathway for protection against influenza virus infection. Vaccine 2009, 27, 3481–3483. [Google Scholar] [CrossRef] [PubMed]
- Schnare, M.; Holt, A.C.; Takeda, K.; Akira, S.; Medzhitov, R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr. Biol. 2000, 10, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Li, B.; Sun, X.; Pan, Q.; Zheng, Y.; Liu, J.; Zhao, Y.; Wang, J.; Liu, L.; et al. A novel CpG ODN compound adjuvant enhances immune response to spike subunit vaccines of porcine epidemic diarrhea virus. Front. Immunol. 2024, 15, 1336239. [Google Scholar] [CrossRef]
- Duan, S.; Thomas, P.G. Balancing Immune Protection and Immune Pathology by CD8(+) T-Cell Responses to Influenza Infection. Front. Immunol. 2016, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 49. [Google Scholar] [CrossRef]
- Janssens, Y.; Joye, J.; Waerlop, G.; Clement, F.; Leroux-Roels, G.; Leroux-Roels, I. The role of cell-mediated immunity against influenza and its implications for vaccine evaluation. Front. Immunol. 2022, 13, 959379. [Google Scholar] [CrossRef]
- Blaas, S.H.; Stieber-Gunckel, M.; Falk, W.; Obermeier, F.; Rogler, G. CpG-oligodeoxynucleotides stimulate immunoglobulin A secretion in intestinal mucosal B cells. Clin. Exp. Immunol. 2009, 155, 534–540. [Google Scholar] [CrossRef]
- Skountzou, I.; Koutsonanos, D.G.; Kim, J.H.; Powers, R.; Satyabhama, L.; Masseoud, F.; Weldon, W.C.; Martin Mdel, P.; Mittler, R.S.; Compans, R.; et al. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus. J. Immunol. 2010, 185, 1642–1649. [Google Scholar] [CrossRef]
- Siegrist, C.A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef]
- Henn, A.D.; Laski, M.; Yang, H.; Welle, S.; Qiu, X.; Miao, H.; Barry, C.T.; Wu, H.; Zand, M.S. Functionally Distinct Subpopulations of CpG-Activated Memory B Cells. Sci. Rep. 2012, 2, 345. [Google Scholar] [CrossRef]
- Powers, D.C.; McElhaney, J.E.; Florendo, O.A., Jr.; Manning, M.C.; Upshaw, C.M.; Bentley, D.W.; Wilkinson, B.E. Humoral and cellular immune responses following vaccination with purified recombinant hemagglutinin from influenza A (H3N2) virus. J. Infect. Dis. 1997, 175, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Nian, X.; Zhang, J.; Huang, S.; Duan, K.; Li, X.; Yang, X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics 2022, 14, 1983. [Google Scholar] [CrossRef]
- Gallichan, W.S.; Woolstencroft, R.N.; Guarasci, T.; McCluskie, M.J.; Davis, H.L.; Rosenthal, K.L. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 2001, 166, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Aljurayyan, A.N.; Sharma, R.; Upile, N.; Beer, H.; Vaughan, C.; Xie, C.; Achar, P.; Ahmed, M.S.; McNamara, P.S.; Gordon, S.B.; et al. A critical role of T follicular helper cells in human mucosal anti-influenza response that can be enhanced by immunological adjuvant CpG-DNA. Antiviral. Res. 2016, 132, 122–130. [Google Scholar] [CrossRef] [PubMed]
| Vaccine | Immune Status | Route | Bris/18 NA | Swit/13 NA |
|---|---|---|---|---|
| COBRA | Naïve | Intramuscular | 7.80 | 9.00 |
| COBRA + CpG | Naïve | Intramuscular | 3.50 | 2.20 |
| COBRA | Pre-immune | Intramuscular | 10.00 | 11.25 |
| COBRA + CpG | Pre-immune | Intramuscular | 8.75 | 11.00 |
| COBRA | Pre-immune | Intranasal | 8.20 | 10.10 |
| COBRA + CpG | Pre-immune | Intranasal | 9.00 | 9.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, P.L.; Lynch, A.; Ross, T.M. Multivalent COBRA Hemagglutinin and Neuraminidase Influenza Vaccines Adjuvanted with TLR9 Agonist CpG 1018. Vaccines 2025, 13, 662. https://doi.org/10.3390/vaccines13070662
Sanchez PL, Lynch A, Ross TM. Multivalent COBRA Hemagglutinin and Neuraminidase Influenza Vaccines Adjuvanted with TLR9 Agonist CpG 1018. Vaccines. 2025; 13(7):662. https://doi.org/10.3390/vaccines13070662
Chicago/Turabian StyleSanchez, Pedro L., Amanda Lynch, and Ted M. Ross. 2025. "Multivalent COBRA Hemagglutinin and Neuraminidase Influenza Vaccines Adjuvanted with TLR9 Agonist CpG 1018" Vaccines 13, no. 7: 662. https://doi.org/10.3390/vaccines13070662
APA StyleSanchez, P. L., Lynch, A., & Ross, T. M. (2025). Multivalent COBRA Hemagglutinin and Neuraminidase Influenza Vaccines Adjuvanted with TLR9 Agonist CpG 1018. Vaccines, 13(7), 662. https://doi.org/10.3390/vaccines13070662






