Engineering and Evaluation of a Live-Attenuated Vaccine Candidate with Enhanced Type 1 Fimbriae Expression to Optimize Protection Against Salmonella Typhimurium
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Construction of the Fim-Inducible S. Typhimurium Auxotrophic Derivative
2.3. Comparison of FimH Amino Acid Sequences
2.4. RNA Isolation and qRT-PCR
2.5. Transmission Electron Microscopy (TEM)
2.6. Yeast Agglutination Assay
2.7. D-Mannose Binding and Blocking Assays
2.8. Bacterial Adhesion to HT-29 Human Colorectal Cells and Inhibition Assays
2.9. Mouse Immunization, Sampling and Challenge Experiments
2.10. ELISA Assays
2.11. Statistical Analyses
3. Results
3.1. Construction and Characterization of a Double Auxotrophic Derivative Expressing Type 1 Fimbriae in S. Typhimurium
3.2. Safety Profile and Humoral Immune Responses to Vaccination
3.3. Protective Efficacy in Vaccinated BALB/c Mice Against Virulent Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Im, J.; Lee, J.S.; Jeon, H.J.; Mogeni, O.D.; Kim, J.H.; Rakotozandrindrainy, R.; Baker, S.; Marks, F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum. Vaccines Immunother. 2019, 15, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.N.; Humphrey, S.; Salisbury, A.M.; Mikoleit, J.; Hinton, J.C.; Gordon, M.A.; Wigley, P. Invasive non-typhoidal Salmonella typhimurium ST313 are not host-restricted and have an invasive phenotype in experimentally infected chickens. PLoS Neglected Trop. Dis. 2013, 7, e2487. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Siddique, A.; Wang, Z.; Zhou, H.; Huang, L.; Jia, C.; Wang, B.; Ed-Dra, A.; Teng, L.; Li, Y.; Yue, M. The Evolution of Vaccines Development Across Salmonella Serovars Among Animal Hosts: A Systematic Review. Vaccines 2024, 12, 1067. [Google Scholar] [CrossRef]
- Martin, L.B.; Tack, B.; Marchello, C.S.; Sikorski, M.J.; Owusu-Dabo, E.; Nyirenda, T.; Mogasale, V.; Crump, J.A. Vaccine value profile for invasive non-typhoidal Salmonella disease. Vaccine 2024, 42, S101–S124. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Tsolis, R.M.; Heffron, F. Fimbrial adhesins of Salmonella typhimurium. Role in bacterial interactions with epithelial cells. Adv. Exp. Med. Biol. 1997, 412, 149–158. [Google Scholar]
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 2009, 5, 580–592. [Google Scholar] [CrossRef]
- Kolenda, R.; Ugorski, M.; Grzymajlo, K. Everything You Always Wanted to Know About Salmonella Type 1 Fimbriae, but Were Afraid to Ask. Front. Microbiol. 2019, 10, 1017. [Google Scholar] [CrossRef]
- Ashkar, A.A.; Mossman, K.L.; Coombes, B.K.; Gyles, C.L.; Mackenzie, R. FimH adhesin of type 1 fimbriae is a potent inducer of innate antimicrobial responses which requires TLR4 and type 1 interferon signalling. PLoS Pathog. 2008, 4, e1000233. [Google Scholar] [CrossRef]
- Mossman, K.L.; Mian, M.F.; Lauzon, N.M.; Gyles, C.L.; Lichty, B.; Mackenzie, R.; Gill, N.; Ashkar, A.A. Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand. J. Immunol. 2008, 181, 6702–6706. [Google Scholar] [CrossRef] [PubMed]
- Uchiya, K.I.; Kamimura, Y.; Jusakon, A.; Nikai, T. Salmonella Fimbrial Protein FimH Is Involved in Expression of Proinflammatory Cytokines in a Toll-Like Receptor 4-Dependent Manner. Infect. Immun. 2019, 87, e00881-18. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, L.J.; Kraehenbuhl, J.P. The role of M cells in mucosal immunity. Cell. Mol. Life Sci. 2000, 57, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Kawano, K.; Nochi, T.; Pontes, G.S.; Fukuda, S.; Ebisawa, M.; Kadokura, K.; Tobe, T.; Fujimura, Y.; Kawano, S.; et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature 2009, 462, 226–230. [Google Scholar] [CrossRef]
- Langermann, S.; Ballou, W.R., Jr. Vaccination utilizing the FimCH complex as a strategy to prevent Escherichia coli urinary tract infections. J. Infect. Dis. 2001, 183 (Suppl. 1), S84–S86. [Google Scholar] [CrossRef]
- De Buck, J.; Van Immerseel, F.; Haesebrouck, F.; Ducatelle, R. Protection of laying hens against Salmonella Enteritidis by immunization with type 1 fimbriae. Vet. Microbiol. 2005, 105, 93–101. [Google Scholar] [CrossRef]
- Asadi Karam, M.R.; Oloomi, M.; Mahdavi, M.; Habibi, M.; Bouzari, S. Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine 2013, 31, 1210–1216. [Google Scholar] [CrossRef]
- Musa, H.H.; Zhang, W.J.; Lv, J.; Duan, X.L.; Yang, Y.; Zhu, C.H.; Li, H.F.; Chen, K.W.; Meng, X.; Zhu, G.Q. The molecular adjuvant mC3d enhances the immunogenicity of FimA from type I fimbriae of Salmonella enterica serovar Enteritidis. J. Microbiol. Immunol. Infect. 2014, 47, 57–62. [Google Scholar] [CrossRef]
- Duan, Q.; Pang, S.; Wu, W.; Jiang, B.; Zhang, W.; Liu, S.; Wang, X.; Pan, Z.; Zhu, G. A multivalent vaccine candidate targeting enterotoxigenic Escherichia coli fimbriae for broadly protecting against porcine post-weaning diarrhea. Vet. Res. 2020, 51, 93. [Google Scholar] [CrossRef]
- Ramezanalizadeh, F.; Owlia, P.; Rasooli, I. Type I pili, CsuA/B and FimA induce a protective immune response against Acinetobacter baumannii. Vaccine 2020, 38, 5436–5446. [Google Scholar] [CrossRef]
- Zandi, M.; Fallah Mehrabadi, J.; Mahdavi, M.; Irani, S. Construction and development of FimH lectin domain for rising immune response after injection by uropathogenic E. coli. Hum. Antibodies 2020, 28, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, G.R.; Hughey, H.; Rosenberger, L.; Martin, S.M.; Shapiro, A.M.; D’Antonio, E.; Krejci, K.G.; Shore, N.; Peterson, J.; Lukes, A.S.; et al. Safety and immunogenicity of an adjuvanted Escherichia coli adhesin vaccine in healthy women with and without histories of recurrent urinary tract infections: Results from a first-in-human phase 1 study. Hum. Vaccines Immunother. 2021, 17, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Karan, S.; Garg, L.C.; Choudhury, D.; Dixit, A. Recombinant FimH, a fimbrial tip adhesin of Vibrio parahaemolyticus, elicits mixed T helper cell response and confers protection against Vibrio parahaemolyticus challenge in murine model. Mol. Immunol. 2021, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Meng, Y.; Xu, L.; Tian, Y.; Lu, H.; Xie, J.; Ma, R.; Li, M.; Li, B. KbvR mutant of Klebsiella pneumoniae affects the synthesis of type 1 fimbriae and provides protection to mice as a live attenuated vaccine. Vet. Res. 2022, 53, 97. [Google Scholar] [CrossRef]
- Dai, P.; Wu, H.; Ding, G.; Fan, J.; Li, Y.; Li, S.; Bao, E.; Li, Y.; Gao, X.; Li, H.; et al. Recombinant Salmonella gallinarum (S. gallinarum) Vaccine Candidate Expressing Avian Pathogenic Escherichia coli Type I Fimbriae Provides Protections Against APEC O78 and O161 Serogroups and S. gallinarum Infection. Vaccines 2023, 11, 1778. [Google Scholar] [CrossRef]
- Vilander, A.C.; Shelton, K.; LaVoy, A.; Dean, G.A. Expression of E. coli FimH Enhances Trafficking of an Orally Delivered Lactobacillus acidophilus Vaccine to Immune Inductive Sites via Antigen-Presenting Cells. Vaccines 2023, 11, 1162. [Google Scholar] [CrossRef]
- Chorro, L.; Ciolino, T.; Torres, C.L.; Illenberger, A.; Aglione, J.; Corts, P.; Lypowy, J.; Ponce, C.; La Porte, A.; Burt, D.; et al. A cynomolgus monkey E. coli urinary tract infection model confirms efficacy of new FimH vaccine candidates. Infect. Immun. 2024, 92, e0016924. [Google Scholar] [CrossRef]
- Tong, X.; Cao, Z.; Cheng, S.; Zhang, B.; Li, X.; Kastelic, J.P.; Xu, C.; Han, B.; Gao, J. Immunoprotective efficacy of 3 Klebsiella pneumoniae type I fimbriae proteins in a murine model. Vet. Microbiol. 2024, 297, 110197. [Google Scholar] [CrossRef]
- Assoni, L.; Ciaparin, I.; Trentini, M.M.; Baboghlian, J.; Rodrigo, G.; Ferreira, B.V.; Pereira, J.A.; Martinez, C.; Ferraz, L.; Girardello, R.; et al. Protection Against Pneumonia Induced by Vaccination with Fimbriae Subunits from Klebsiella pneumoniae. Vaccines 2025, 13, 303. [Google Scholar] [CrossRef]
- Sang, S.; Yu, R.; Mao, Y.; Zhai, Y.; Cao, C.; Li, K.; Guan, Y.; Tao, H.; Liu, C.; Wang, Y. Surface Display of Type 1 Fimbriae on Shigella flexneri Induces Antigen-Specific Immune Response via Oral Route. Vaccines 2025, 13, 280. [Google Scholar] [CrossRef]
- Sirard, J.C.; Niedergang, F.; Kraehenbuhl, J.P. Live attenuated Salmonella: A paradigm of mucosal vaccines. Immunol. Rev. 1999, 171, 5–26. [Google Scholar] [CrossRef]
- Galen, J.E.; Buskirk, A.D.; Tennant, S.M.; Pasetti, M.F. Live Attenuated Human Salmonella Vaccine Candidates: Tracking the Pathogen in Natural Infection and Stimulation of Host Immunity. EcoSal Plus 2016, 7, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Moscoso, M.; Fuentes-Valverde, V.; Rodicio, M.R.; Herrera-León, S.; Bou, G. A highly-safe live auxotrophic vaccine protecting against disease caused by non-typhoidal Salmonella Typhimurium in mice. J. Microbiol. Immunol. Infect. 2023, 56, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Klasa, B.; Kędzierska, A.E.; Grzymajło, K. Pre-Growth Culture Conditions Affect Type 1 Fimbriae-Dependent Adhesion of Salmonella. Int. J. Mol. Sci. 2020, 21, 4206. [Google Scholar] [CrossRef] [PubMed]
- Hansmeier, N.; Miskiewicz, K.; Elpers, L.; Liss, V.; Hensel, M.; Sterzenbach, T. Functional expression of the entire adhesiome of Salmonella enterica serotype Typhimurium. Sci. Rep. 2017, 7, 10326. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Mirelman, D.; Altmann, G.; Eshdat, Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeasts. J. Clin. Microbiol. 1980, 11, 328–331. [Google Scholar] [CrossRef]
- Yu, S.; Lowe, A.W. The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli type 1 Fimbriae. BMC Gastroenterol. 2009, 9, 58. [Google Scholar] [CrossRef]
- Boddicker, J.D.; Ledeboer, N.A.; Jagnow, J.; Jones, B.D.; Clegg, S. Differential binding to and biofilm formation on, HEp-2 cells by Salmonella enterica serovar Typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol. Microbiol. 2002, 45, 1255–1265. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J., 2nd; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Angelakopoulos, H.; Hohmann, E.L. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 2000, 68, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Hindle, Z.; Chatfield, S.N.; Phillimore, J.; Bentley, M.; Johnson, J.; Cosgrove, C.A.; Ghaem-Maghami, M.; Sexton, A.; Khan, M.; Brennan, F.R.; et al. Characterization of Salmonella Enterica Derivatives Harboring Defined aroC and Salmonella Pathogenicity Island 2 Type III Secretion System (Ssav) Mutations by Immunization of Healthy Volunteers. Infect. Immun. 2002, 70, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Galen, J.E.; Curtiss, R., 3rd. The delicate balance in genetically engineering live vaccines. Vaccine 2014, 32, 4376–4385. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.P.; García, P.; Beceiro, A.; Rumbo, C.; Pérez, A.; Moscoso, M.; Bou, G. Design of live attenuated bacterial vaccines based on D-glutamate auxotrophy. Nat. Commun. 2017, 8, 15480. [Google Scholar] [CrossRef]
- Sydenham, M.; Douce, G.; Bowe, F.; Ahmed, S.; Chatfield, S.; Dougan, G. Salmonella enterica serovar Typhimurium surA mutants are attenuated and effective live oral vaccines. Infect. Immun. 2000, 68, 1109–1115. [Google Scholar] [CrossRef]
- Abd El Ghany, M.; Jansen, A.; Clare, S.; Hall, L.; Pickard, D.; Kingsley, R.A.; Dougan, G. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect. Immun. 2007, 75, 1835–1842. [Google Scholar] [CrossRef]
- Tennant, S.M.; Wang, J.Y.; Galen, J.E.; Simon, R.; Pasetti, M.F.; Gat, O.; Levine, M.M. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect. Immun. 2011, 79, 4175–4185. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Pati, N.B.; Chandel, H.S.; Sahoo, S.S.; Saha, B.; Suar, M. Evaluation of Salmonella enterica serovar Typhimurium TTSS-2 deficient fur mutant as safe live-attenuated vaccine candidate for immunocompromised mice. PLoS ONE 2012, 7, e52043. [Google Scholar] [CrossRef]
- Zhi, Y.; Lin, S.M.; Jang, A.Y.; Ahn, K.B.; Ji, H.J.; Guo, H.C.; Lim, S.; Seo, H.S. Effective mucosal live attenuated Salmonella vaccine by deleting phosphotransferase system component genes ptsI and crr. J. Microbiol. 2019, 57, 64–73. [Google Scholar] [CrossRef]
- Park, S.; Jung, B.; Kim, E.; Hong, S.T.; Yoon, H.; Hahn, T.W. Salmonella Typhimurium lacking YjeK as a candidate live attenuated vaccine against invasive Salmonella infection. Front. Immunol. 2020, 11, 1277. [Google Scholar] [CrossRef]
- Troxell, B.; Mendoza, M.; Ali, R.; Koci, M.; Hassan, H. Attenuated Salmonella enterica serovar Typhimurium, strain NC983, is immunogenic and protective against virulent Typhimurium challenges in mice. Vaccines 2020, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Thi, T.H.; Huynh, K.Q.; Dinh-Thi, P.L.; Tran, L.T.; Jang, Y.S.; Tran-Van, H. Expression, Purification, and in vivo Evaluation of GFP-Fused M Cell Targeting Receptor Binding Domain of Protein FimH. Protein Pept. Lett. 2019, 26, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Moriel, D.G.; Beatson, S.A.; Wurpel, D.J.; Lipman, J.; Nimmo, G.R.; Paterson, D.L.; Schembri, M.A. Identification of novel vaccine candidates against multidrug-resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e77631. [Google Scholar] [CrossRef] [PubMed]
- Zargaran, F.N.; Akya, A.; Rezaeian, S.; Ghadiri, K.; Lorestani, R.C.; Madanchi, H.; Safaei, S.; Rostamian, M. Cell Epitopes of Four Fimbriae Antigens of Klebsiella pneumoniae: A Comprehensive In Silico Study for Vaccine Development. Int. J. Pept. Res. Ther. 2021, 27, 875–886. [Google Scholar] [CrossRef]
- Rostamian, M.; Farasat, A.; Chegene Lorestani, R.; Nemati Zargaran, F.; Ghadiri, K.; Akya, A. Immunoinformatics and molecular dynamics studies to predict T-cell-specific epitopes of four Klebsiella pneumoniae fimbriae antigens. J. Biomol. Struct. Dyn. 2022, 40, 166–176. [Google Scholar] [CrossRef]
- Chen, J.; Dai, W.; Cui, S.; Lei, W.; Dai, D. Screening of antigenic epitopes related to the adhesion of the avian Escherichia coli Type 1 Fimbrial Agglutinin Domain. BMC Vet. Res. 2023, 19, 187. [Google Scholar] [CrossRef]
- Tajuelo, A.; Gato, E.; Oteo-Iglesias, J.; Pérez-Vázquez, M.; McConnell, M.J.; Martín-Galiano, A.J.; Pérez, A. Deep Intraclonal Analysis for the Development of Vaccines against Drug-Resistant Klebsiella pneumoniae Lineages. Int. J. Mol. Sci. 2024, 25, 9837. [Google Scholar] [CrossRef]
- Griffin, A.J.; McSorley, S.J. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 2011, 4, 371–382. [Google Scholar] [CrossRef]
- Wijburg, O.L.; Uren, T.K.; Simpfendorfer, K.; Johansen, F.E.; Brandtzaeg, P.; Strugnell, R.A. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 2006, 203, 21–26. [Google Scholar] [CrossRef]
- Mantis, N.J.; Forbes, S.J. Secretory IgA: Arresting microbial pathogens at epithelial borders. Immunol. Investig. 2010, 39, 383–406. [Google Scholar] [CrossRef]
- Bioley, G.; Monnerat, J.; Lötscher, M.; Vonarburg, C.; Zuercher, A.; Corthésy, B. Plasma-Derived Polyreactive Secretory-Like IgA and IgM Opsonizing Salmonella enterica Typhimurium Reduces Invasion and Gut Tissue Inflammation Through Agglutination. Front. Immunol. 2017, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, S.; Stoppato, M.; Sedic, M.; Ejemel, M.; Pondish, J.R.; Wisheart, D.; Schiller, Z.A.; Thomas, W.D., Jr.; Barry, E.M.; Cavacini, L.A.; et al. Identification and Characterization of Human Monoclonal Antibodies for Immunoprophylaxis against Enterotoxigenic Escherichia coli Infection. Infect. Immun. 2018, 86, e00355-18. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Dutta, P.; Pal, A.; Chakraborty, S.; Banik, G.; Halder, P.; Gope, A.; Miyoshi, S.I.; Das, S. Intranasal immunization of mice with chimera of Salmonella Typhi protein elicits protective intestinal immunity. NPJ Vaccines 2024, 9, 24. [Google Scholar] [CrossRef] [PubMed]
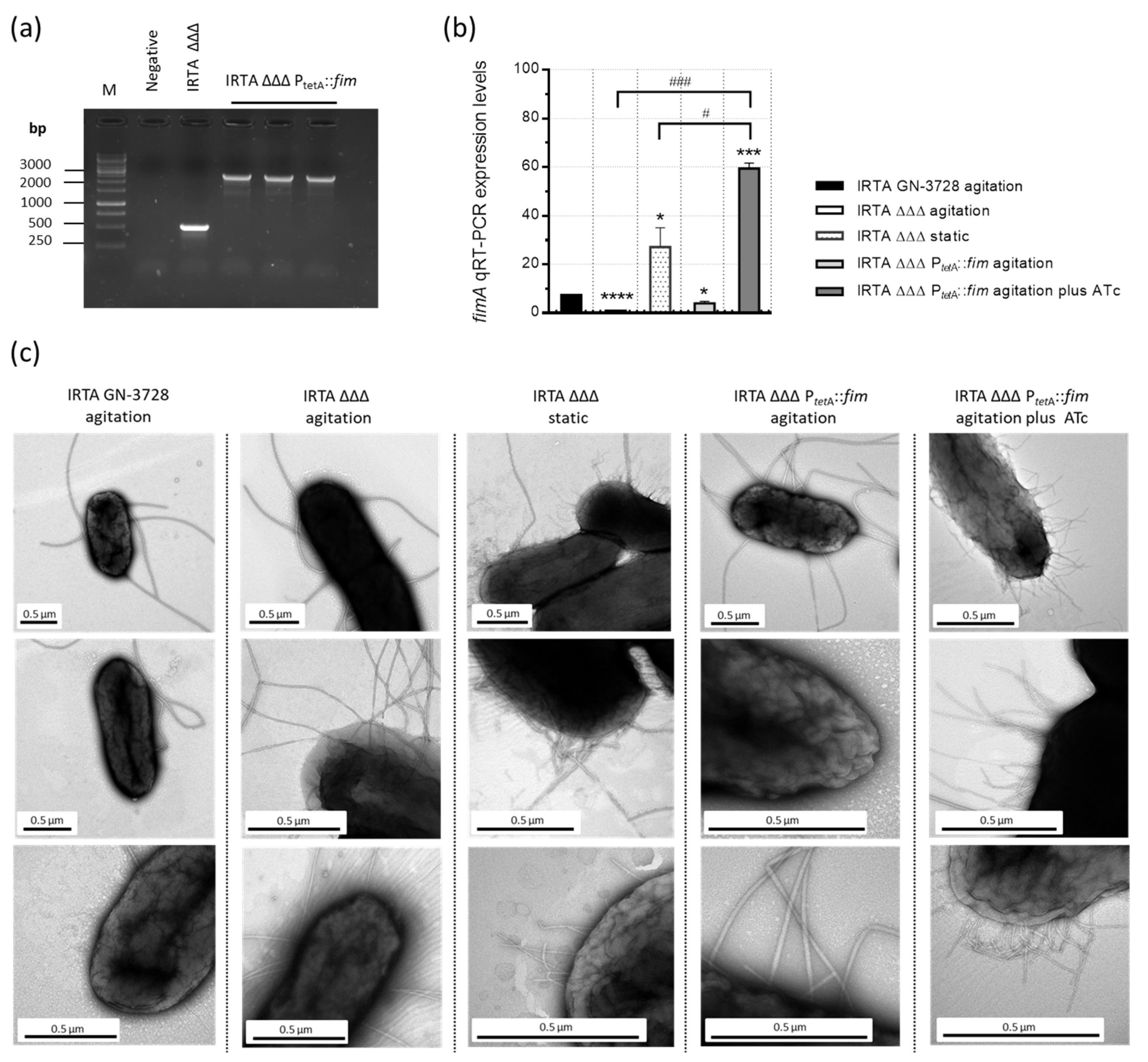

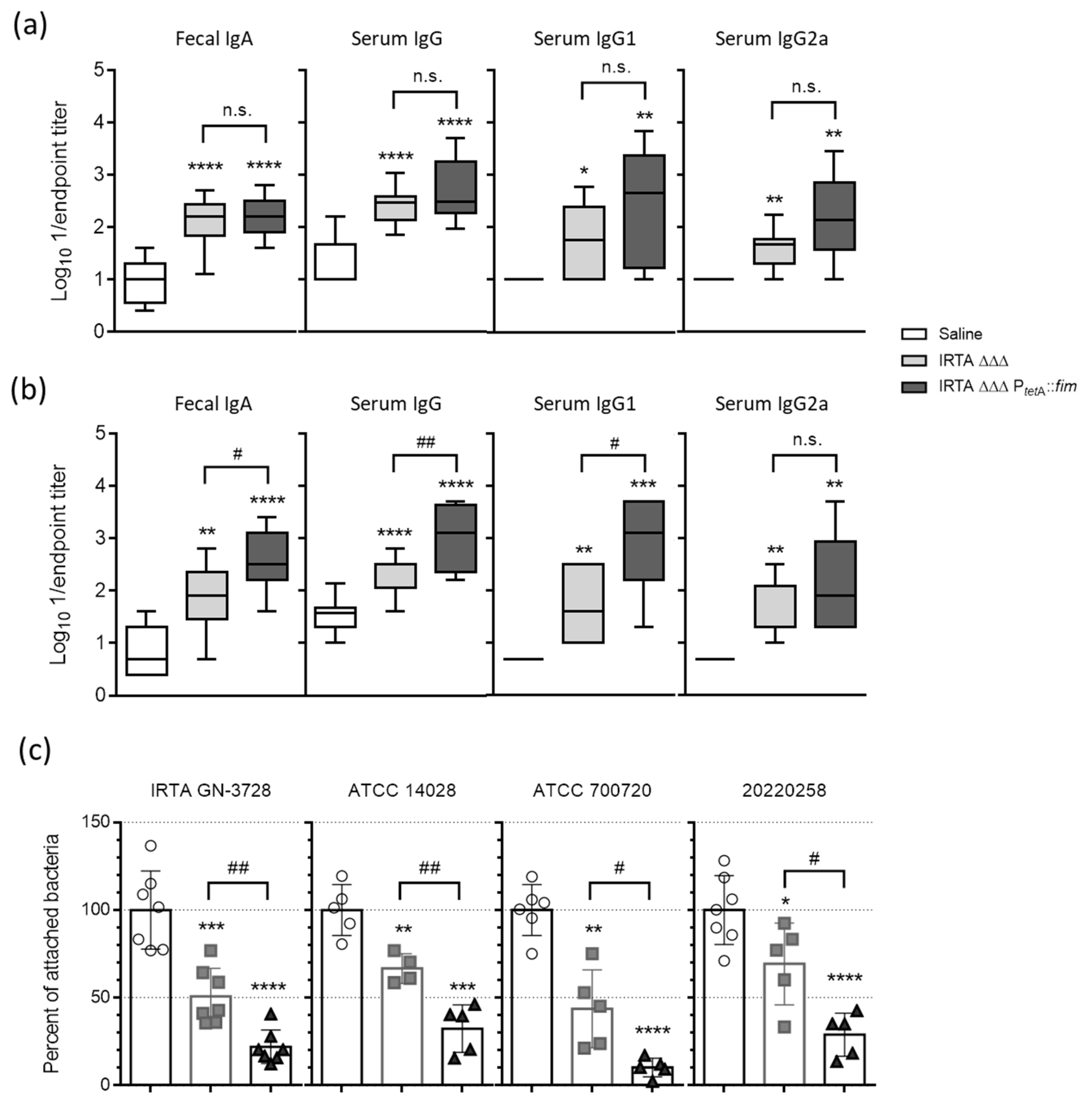
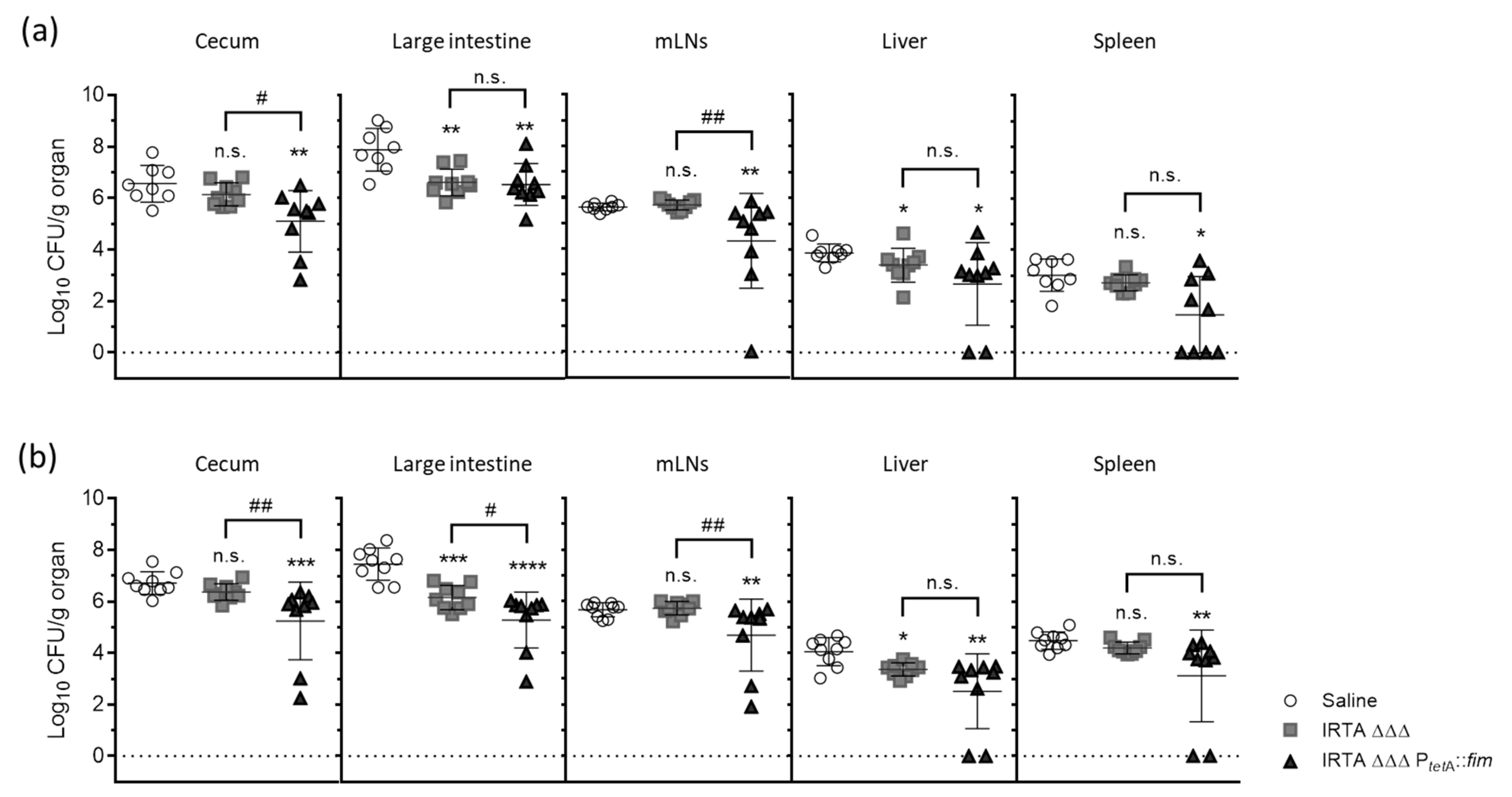
| Strain | Relevant Features | Experimental Use | Source |
|---|---|---|---|
| IRTA GN-3728 | Wild-type rif-resistant | PCR, qRT-PCR, TEM and HT-29 adherence FimH sequence analysis ELISA tests Blocking HT-29 adhesion assays Mouse challenge | [33] |
| IRTA ΔΔΔkanS | Double auxotroph (ΔmurI Δalr ΔdadX) | λ-Red Mutagenesis | [33] |
| IRTA ΔΔΔ (formerly IRTA ΔΔΔ::aph(3)-IIIa) | Double auxotroph kan-resistant (ΔmurI Δalr:: aph(3)-IIIa ΔdadX) | PCR, qRT-PCR, mannose-binding, TEM and HT-29 adherence Mouse immunization | [33] |
| IRTA ΔΔΔ PtetA::fim | Double auxotroph with inducible fim expression, kan-resistant (ΔmurI Δalr ΔdadX aph(3)-IIIa-tetR-PtetA::fim) | PCR, qRT-PCR, mannose-binding, TEM and HT-29 adherence ELISA tests Mouse immunization | This study |
| ATCC 700720 (LT2) | Wild-type isolated from a natural source | Blocking HT-29 adhesion assays | ATCC |
| ATCC 14028 | Reference strain, isolated from pools of heart and liver from 4-week-old-chickens | Blocking HT-29 adhesion assays | ATCC |
| 20220258 (monophasic) | Multi-country outbreak strain isolated from 38 year-old woman, cgMLST cluster 1, ST34 and amp-, chl-, str-, kan-, gen-, smx-, tet-and tmp-resistant | Blocking HT-29 adhesion assays Mouse challenge | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, P.; Rodríguez-Coello, A.; García-Pose, A.; Fernández-López, M.D.C.; Muras, A.; Moscoso, M.; Beceiro, A.; Bou, G. Engineering and Evaluation of a Live-Attenuated Vaccine Candidate with Enhanced Type 1 Fimbriae Expression to Optimize Protection Against Salmonella Typhimurium. Vaccines 2025, 13, 659. https://doi.org/10.3390/vaccines13060659
García P, Rodríguez-Coello A, García-Pose A, Fernández-López MDC, Muras A, Moscoso M, Beceiro A, Bou G. Engineering and Evaluation of a Live-Attenuated Vaccine Candidate with Enhanced Type 1 Fimbriae Expression to Optimize Protection Against Salmonella Typhimurium. Vaccines. 2025; 13(6):659. https://doi.org/10.3390/vaccines13060659
Chicago/Turabian StyleGarcía, Patricia, Arianna Rodríguez-Coello, Andrea García-Pose, María Del Carmen Fernández-López, Andrea Muras, Miriam Moscoso, Alejandro Beceiro, and Germán Bou. 2025. "Engineering and Evaluation of a Live-Attenuated Vaccine Candidate with Enhanced Type 1 Fimbriae Expression to Optimize Protection Against Salmonella Typhimurium" Vaccines 13, no. 6: 659. https://doi.org/10.3390/vaccines13060659
APA StyleGarcía, P., Rodríguez-Coello, A., García-Pose, A., Fernández-López, M. D. C., Muras, A., Moscoso, M., Beceiro, A., & Bou, G. (2025). Engineering and Evaluation of a Live-Attenuated Vaccine Candidate with Enhanced Type 1 Fimbriae Expression to Optimize Protection Against Salmonella Typhimurium. Vaccines, 13(6), 659. https://doi.org/10.3390/vaccines13060659





