A Comparison of Tests for Detecting Prior Exposure to Coxiella burnetii for Use with Q-VAX in Australian Human Q Fever Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Human Study Cohorts
2.2.1. Vaccine Clinic Attendees with Varied Exposure Histories
2.2.2. Enrolled Study Cohorts with Unknown Exposure Histories
2.2.3. Enrolled Study Cohort with Known Prior Q Fever Vaccination or Disease
2.3. Tests of Immune Responses to Coxiella burnetii
2.3.1. Q-VAX Intradermal Skin Test
2.3.2. Assessment of Serological Status
2.3.3. Whole Blood IFNγ Release Assay (Q-Detect 1.0 and Q-Detect 2.0)
2.4. Determination of Vaccine Eligibility
2.5. Assessment of Post-Vaccination Immune Responses in Veterinary Student Cohorts
2.6. Computational and Statistical Analyses
3. Results
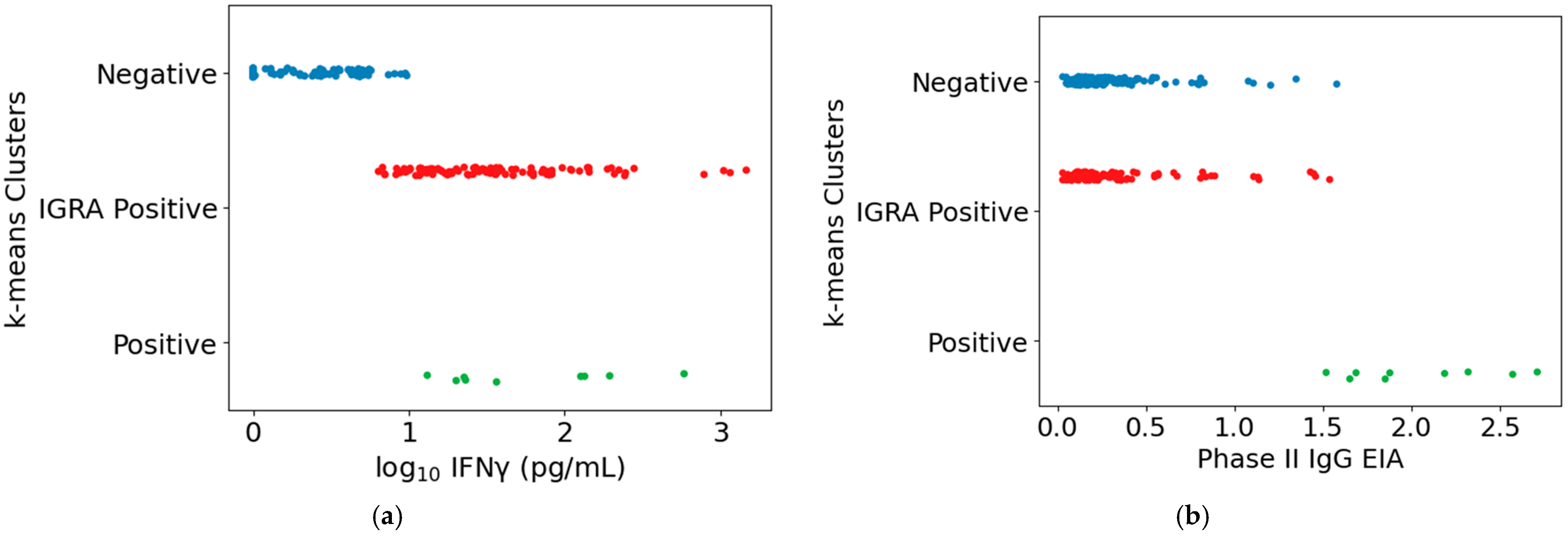
3.1. Pilot Comparison of Tests for Prior Exposure to Coxiella burnetii
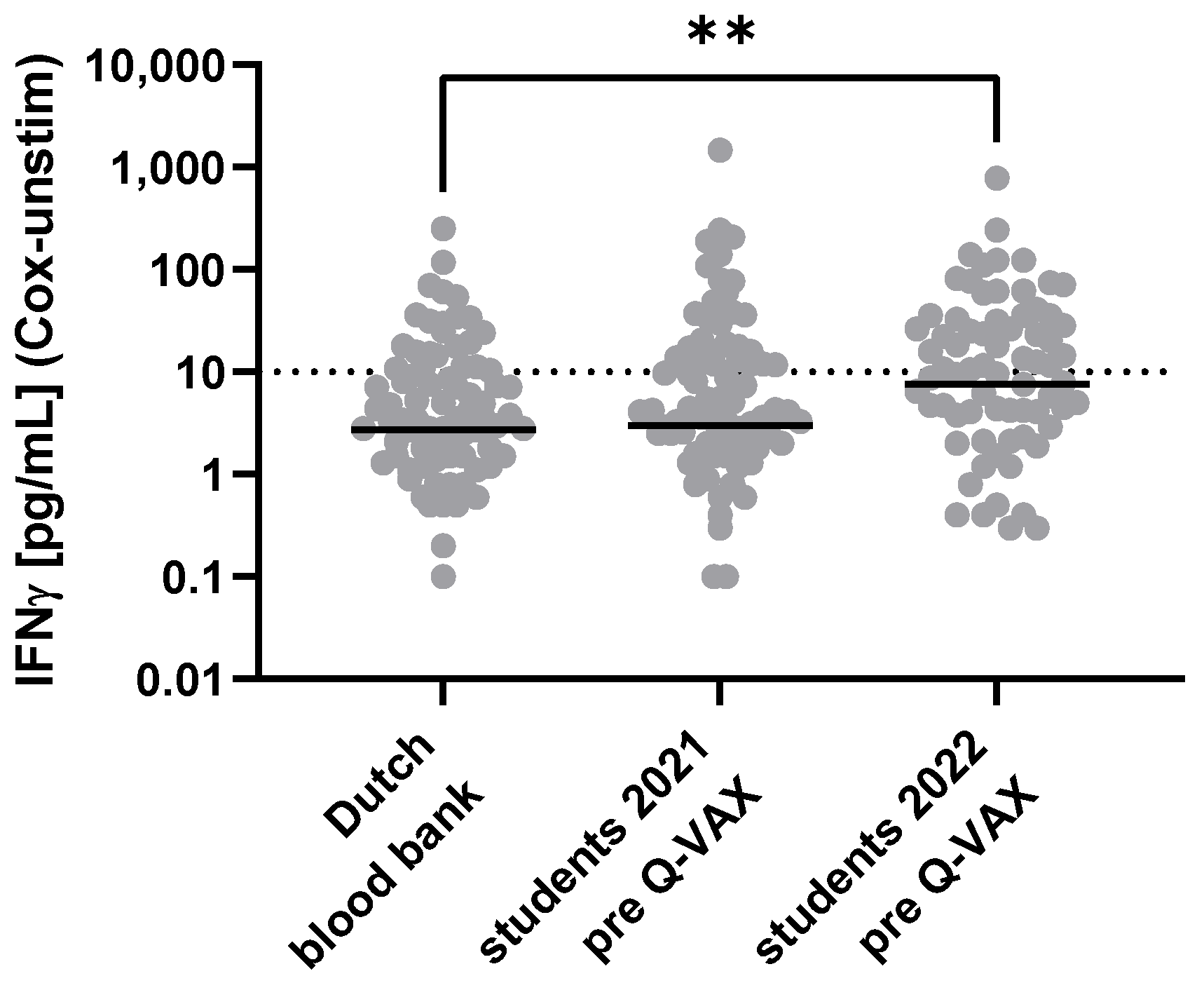
3.2. Pre-Vaccination Tests for Prior Exposure to Coxiella burnetii in Australian Veterinary Students
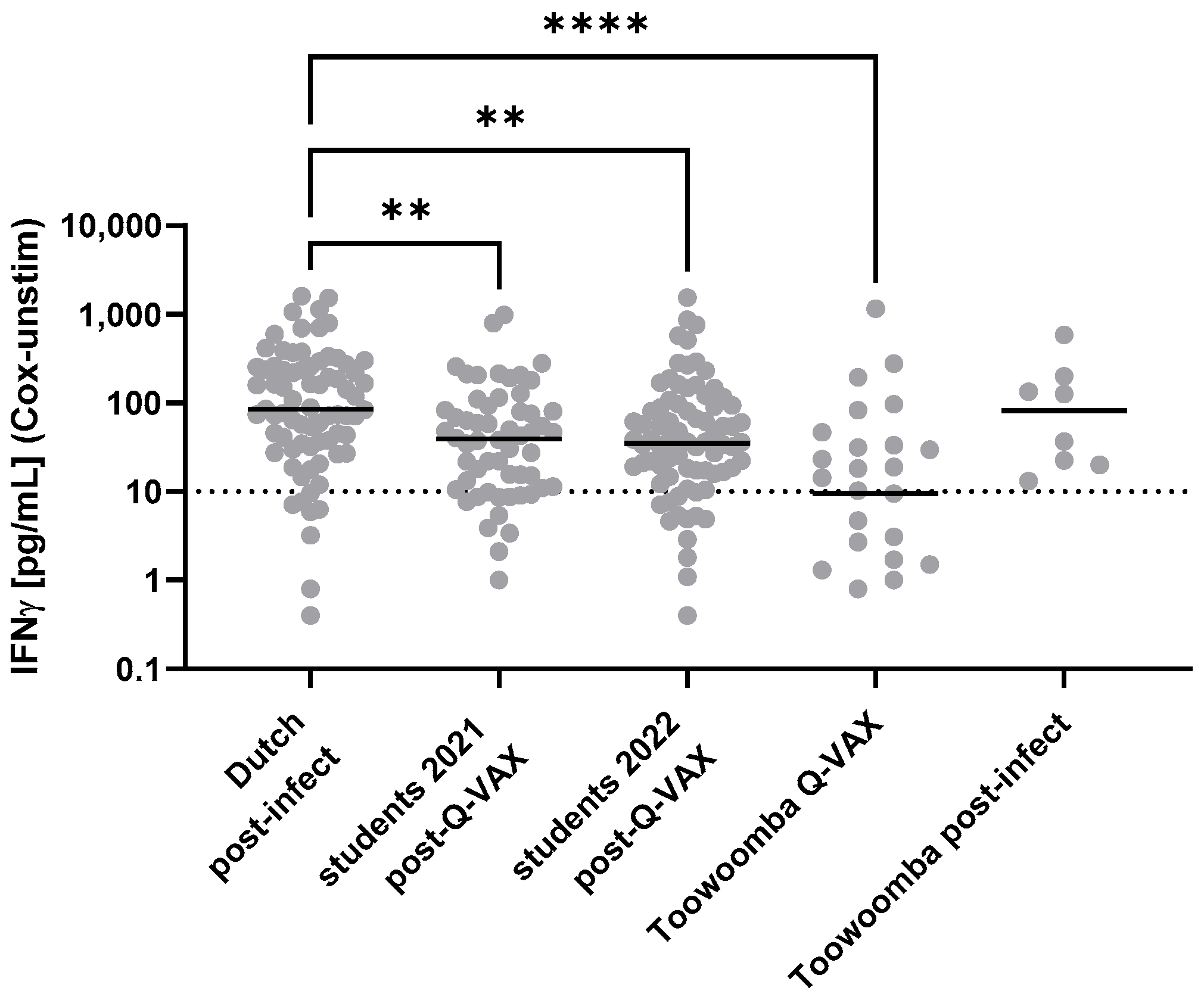
3.3. Evaluation of Markers of Immune Responses to Coxiella burnetii in Individuals with Known Prior Exposure
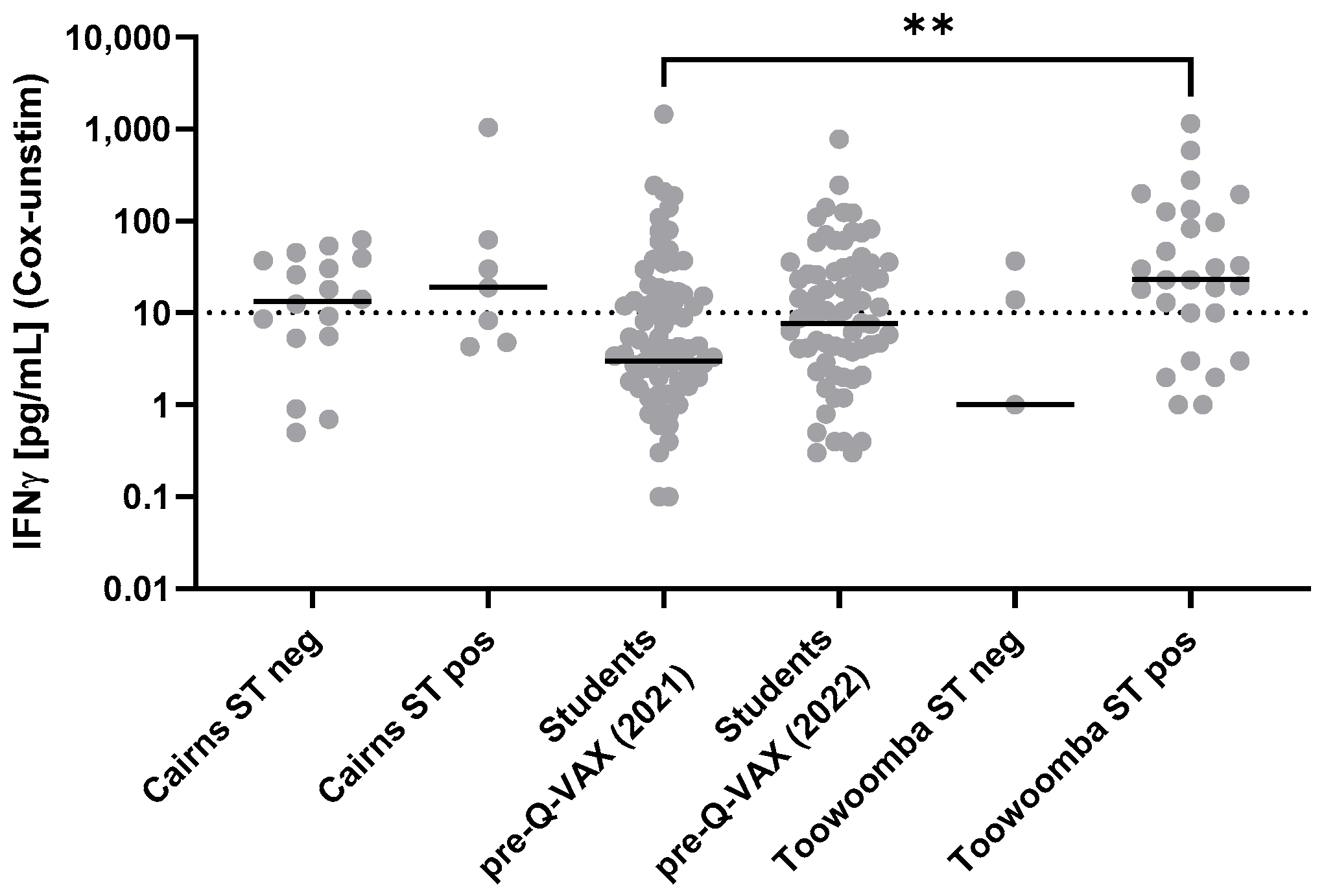
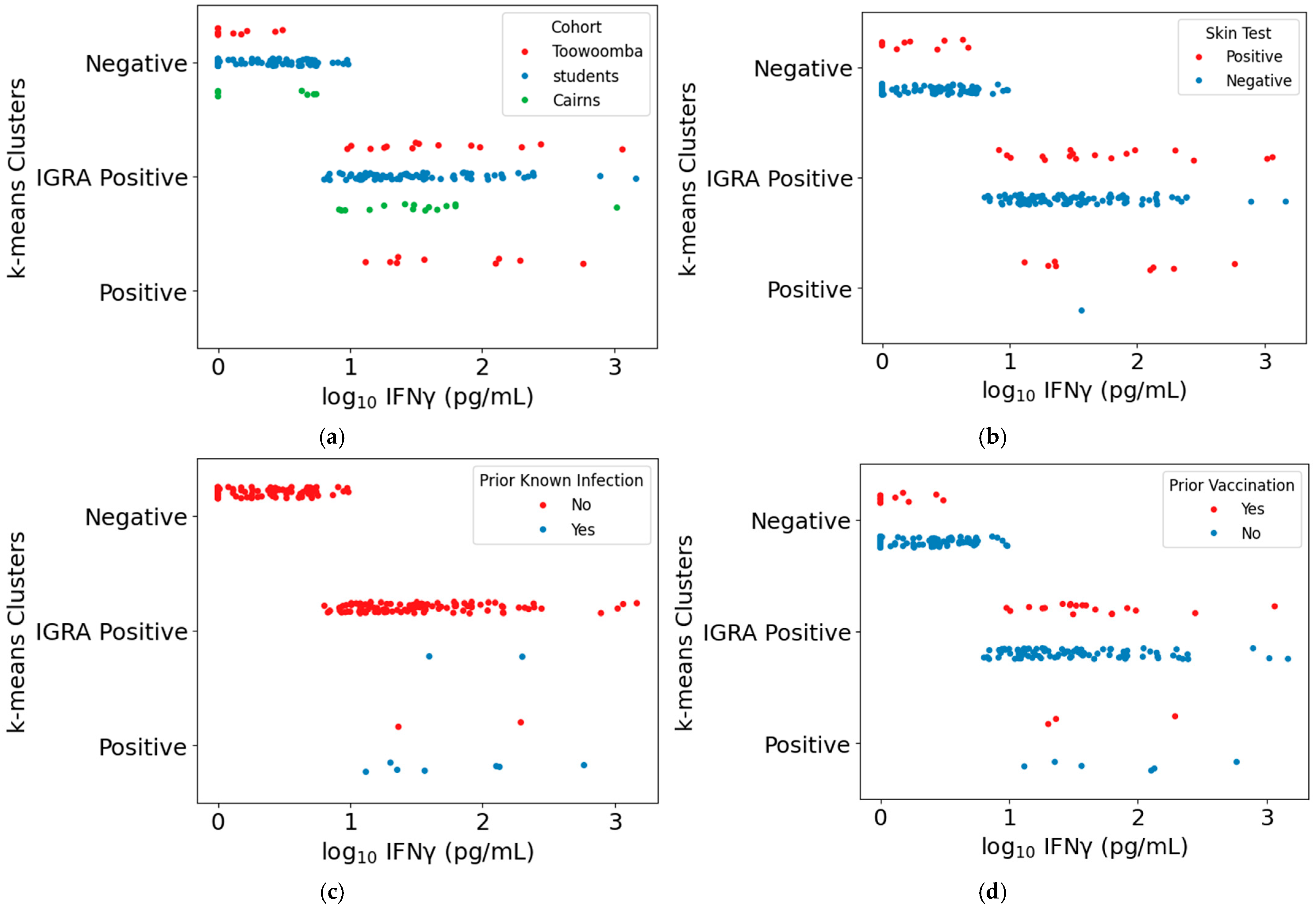
3.4. Comparison of the Intradermal Skin Test and Q-Detect IGRA as Indicators of Cellular Immune Memory of Prior Exposure to Coxiella burnetii Antigens
3.5. Comparison of the Intradermal Skin Test and Q-Detect IGRA for Determination of Q-VAX Vaccine Eligibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eldin, C.; Melenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Wolfe, D.N. Vaccination against Q fever for biodefense and public health indications. Front. Microbiol. 2014, 5, 726. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Bijlmer, H.; Fournier, P.E.; Graves, S.; Hartzell, J.; Kersh, G.J.; Limonard, G.; Marrie, T.J.; Massung, R.F.; McQuiston, J.H.; et al. Diagnosis and management of Q fever—United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR Recomm. Rep. 2013, 62, 1–30. [Google Scholar]
- Kampschreur, L.M.; Oosterheert, J.J.; Hoepelman, A.I.; Lestrade, P.J.; Renders, N.H.; Elsman, P.; Wever, P.C. Prevalence of chronic Q fever in patients with a history of cardiac valve surgery in an area where Coxiella burnetii is epidemic. Clin. Vaccine Immunol. 2012, 19, 1165–1169. [Google Scholar] [CrossRef]
- Morroy, G.; Keijmel, S.P.; Delsing, C.E.; Bleijenberg, G.; Langendam, M.; Timen, A.; Bleeker-Rovers, C.P. Fatigue following Acute Q-Fever: A Systematic Literature Review. PLoS ONE 2016, 11, e0155884. [Google Scholar] [CrossRef]
- Morroy, G.; Van Der Hoek, W.; Nanver, Z.D.; Schneeberger, P.M.; Bleeker-Rovers, C.P.; Van Der Velden, J.; Coutinho, R.A. The health status of a village population, 7 years after a major Q fever outbreak. Epidemiol. Infect. 2016, 144, 1153–1162. [Google Scholar] [CrossRef]
- Madariaga, M.G.; Rezai, K.; Trenholme, G.M.; Weinstein, R.A. Q fever: A biological weapon in your backyard. Lancet Infect. Dis. 2003, 3, 709–721. [Google Scholar] [CrossRef]
- Marmion, B.P. A Guide to Q Fever and Q Fever Vaccination; CSL Biotherapies: Parkville, Australia, 2009. [Google Scholar]
- Marmion, B.P.; Ormsbee, R.A.; Kyrkou, M.; Wright, J.; Worswick, D.A.; Izzo, A.A.; Esterman, A.; Feery, B.; Shapiro, R.A. Vaccine prophylaxis of abattoir-associated Q fever: Eight years’ experience in Australian abattoirs. Epidemiol. Infect. 1990, 104, 275–287. [Google Scholar] [CrossRef]
- Fries, L.F.; Waag, D.M.; Williams, J.C. Safety and immunogenicity in human volunteers of a chloroform-methanol residue vaccine for Q fever. Infect. Immun. 1993, 61, 1251–1258. [Google Scholar] [CrossRef]
- Kazar, J.; Brezina, R.; Palanova, A.; Tvrda, B.; Schramek, S. Immunogenicity and reactogenicity of a Q fever chemovaccine in persons professionally exposed to Q fever in Czechoslovakia. Bull. World Health Organ. 1982, 60, 389–394. [Google Scholar] [PubMed]
- Marmion, B.P. Development of Q-fever vaccines, 1937 to 1967. Med. J. Aust. 1967, 2, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, T.; Wong, A.; Rumke, H.C.; Netea, M.G.; Timen, A.; van Deuren, M.; Vermeer-de Bondt, P.E. Adverse events and association with age, sex and immunological parameters of Q fever vaccination in patients at risk for chronic Q fever in the Netherlands 2011. Vaccine 2014, 32, 6622–6630. [Google Scholar] [CrossRef] [PubMed]
- Sellens, E.; Bosward, K.L.; Willis, S.; Heller, J.; Cobbold, R.; Comeau, J.L.; Norris, J.M.; Dhand, N.K.; Wood, N. Frequency of Adverse Events Following Q Fever Immunisation in Young Adults. Vaccines 2018, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Ascher, M.S.; Berman, M.A.; Ruppanner, R. Initial clinical and immunologic evaluation of a new phase I Q fever vaccine and skin test in humans. J. Infect. Dis. 1983, 148, 214–222. [Google Scholar] [CrossRef]
- Ascher, M.S.; Williams, J.C.; Berman, M.A. Dermal granulomatous hypersensitivity in Q fever: Comparative studies of the granulomatous potential of whole cells of Coxiella burnetii phase I and subfractions. Infect. Immun. 1983, 42, 887–889. [Google Scholar] [CrossRef]
- Kazar, J.; Rehacek, J. Q fever vaccines: Present status and application in man. Zentralbl Bakteriol. Mikrobiol. Hyg. A 1987, 267, 74–78. [Google Scholar] [CrossRef]
- Long, C.M.; Beare, P.A.; Cockrell, D.C.; Fintzi, J.; Tesfamariam, M.; Shaia, C.I.; Heinzen, R.A. Contributions of lipopolysaccharide and the type IVB secretion system to Coxiella burnetii vaccine efficacy and reactogenicity. NPJ Vaccines 2021, 6, 38. [Google Scholar] [CrossRef]
- Greig, J.E.; Patel, M.S.; Clements, M.S.; Taylor, N.K. Control strategies for Q fever based on results of pre-vaccination screening in Victoria, 1988 to 2001. Aust. N. Z. J. Public Health 2005, 29, 53–57. [Google Scholar] [CrossRef]
- Healy, B.; van Woerden, H.; Raoult, D.; Graves, S.; Pitman, J.; Lloyd, G.; Brown, N.; Llewelyn, M. Chronic Q fever: Different serological results in three countries--results of a follow-up study 6 years after a point source outbreak. Clin. Infect. Dis. 2011, 52, 1013–1019. [Google Scholar] [CrossRef]
- Wegdam-Blans, M.C.; Tjhie, H.T.; Korbeeck, J.M.; Nabuurs-Franssen, M.N.; Kampschreur, L.M.; Sprong, T.; Teijink, J.A.; Koopmans, M.P. Serology in chronic Q fever is still surrounded by question marks. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Wegdam-Blans, M.C.; Wielders, C.C.; Meekelenkamp, J.; Korbeeck, J.M.; Herremans, T.; Tjhie, H.T.; Bijlmer, H.A.; Koopmans, M.P.; Schneeberger, P.M. Evaluation of commonly used serological tests for detection of Coxiella burnetii antibodies in well-defined acute and follow-up sera. Clin. Vaccine Immunol. 2012, 19, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Hussain-Yusuf, H.; Islam, A.; Healy, B.; Lockhart, M.; Nguyen, C.; Sukocheva, O.; Stenos, J.; Graves, S. An analysis of Q fever patients 6 years after an outbreak in Newport, Wales, UK. QJM 2012, 105, 1067–1073. [Google Scholar] [CrossRef]
- Teunis, P.F.; Schimmer, B.; Notermans, D.W.; Leenders, A.C.; Wever, P.C.; Kretzschmar, M.E.; Schneeberger, P.M. Time-course of antibody responses against Coxiella burnetii following acute Q fever. Epidemiol. Infect. 2013, 141, 62–73. [Google Scholar] [CrossRef]
- Hutson, B.; Deaker, R.A.; Newland, J. Vaccination of cattle workers at risk of Q fever on the north coast of New South Wales. Aust. Fam. Physician 2000, 29, 708–709. [Google Scholar] [PubMed]
- Slot, E.; Hogema, B.M.; Molier, M.; Zaaijer, H.L. Screening of blood donors for chronic Coxiella burnetii infection after large Q fever outbreaks. Transfusion 2014, 54, 2867–2870. [Google Scholar] [CrossRef]
- Kampschreur, L.M.; Hagenaars, J.C.; Wielders, C.C.; Elsman, P.; Lestrade, P.J.; Koning, O.H.; Oosterheert, J.J.; Renders, N.H.; Wever, P.C. Screening for Coxiella burnetii seroprevalence in chronic Q fever high-risk groups reveals the magnitude of the Dutch Q fever outbreak. Epidemiol. Infect. 2013, 141, 847–851. [Google Scholar] [CrossRef]
- Schneeberger, P.M.; Wintenberger, C.; van der Hoek, W.; Stahl, J.P. Q fever in the Netherlands—2007–2010: What we learned from the largest outbreak ever. Med. Mal. Infect. 2014, 44, 339–353. [Google Scholar] [CrossRef]
- Schoffelen, T.; Herremans, T.; Sprong, T.; Nabuurs-Franssen, M.; Wever, P.C.; Joosten, L.A.; Netea, M.G.; van der Meer, J.W.; Bijlmer, H.A.; van Deuren, M. Limited humoral and cellular responses to Q fever vaccination in older adults with risk factors for chronic Q fever. J. Infect. 2013, 67, 565–573. [Google Scholar] [CrossRef]
- Schoffelen, T.; Joosten, L.A.; Herremans, T.; de Haan, A.F.; Ammerdorffer, A.; Rumke, H.C.; Wijkmans, C.J.; Roest, H.I.; Netea, M.G.; van der Meer, J.W.; et al. Specific interferon gamma detection for the diagnosis of previous Q fever. Clin. Infect. Dis. 2013, 56, 1742–1751. [Google Scholar] [CrossRef]
- Morroy, G.; van der Hoek, W.; Albers, J.; Coutinho, R.A.; Bleeker-Rovers, C.P.; Schneeberger, P.M. Population Screening for Chronic Q-Fever Seven Years after a Major Outbreak. PLoS ONE 2015, 10, e0131777. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, A.; de Vries, M.; Duerr, H.P.; Roest, H.J.; Sluder, A.E.; Poznansky, M.C.; Kouwijzer, M.; Garritsen, A. Whole Blood Interferon gamma Release Is a More Sensitive Marker of Prior Exposure to Coxiella burnetii Than Are Antibody Responses. Front. Immunol. 2021, 12, 701811. [Google Scholar] [CrossRef] [PubMed]
- Raju Paul, S.; Scholzen, A.; Mukhtar, G.; Wilkinson, S.; Hobson, P.; Dzeng, R.K.; Evans, J.; Robson, J.; Cobbold, R.; Graves, S.; et al. Natural Exposure- and Vaccination-Induced Profiles of Ex Vivo Whole Blood Cytokine Responses to Coxiella burnetii. Front. Immunol. 2022, 13, 886698. [Google Scholar] [CrossRef] [PubMed]
- Beare, P.A.; Jeffrey, B.M.; Long, C.M.; Martens, C.M.; Heinzen, R.A. Genetic mechanisms of Coxiella burnetii lipopolysaccharide phase variation. PLoS Pathog. 2018, 14, e1006922. [Google Scholar] [CrossRef]
- Hackstadt, T. The role of lipopolysaccharides in the virulence of Coxiella burnetii. Ann. N. Y Acad. Sci. 1990, 590, 27–32. [Google Scholar] [CrossRef]
- Jager, M.M.; Weers-Pothoff, G.; Hermans, M.H.; Meekelenkamp, J.C.; Schellekens, J.J.; Renders, N.H.; Leenders, A.C.; Schneeberger, P.M.; Wever, P.C. Evaluation of a diagnostic algorithm for acute Q fever in an outbreak setting. Clin. Vaccine Immunol. 2011, 18, 963–968. [Google Scholar] [CrossRef]
- Worswick, D.; Marmion, B.P. Antibody responses in acute and chronic Q fever and in subjects vaccinated against Q fever. J. Med. Microbiol. 1985, 19, 281–296. [Google Scholar] [CrossRef]
- Australian Technical Advisory Group on Immunisation. Australian Immunisation Handbook; Australian Government Department of Health and Aged Care: Canberra, Australia, 2024.
- Bürkner, P.-C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Gabry, J.; Cesnovar, R.; Johnson, A.; Bronder, S. cmdstanr: R Interface to ’CmdStan’. R package version 0.8.1. 2024. Available online: https://discourse.mc-stan.org (accessed on 2 January 2024).
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval Estimation for a Binomial Population. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Scholzen, A.; Richard, G.; Moise, L.; Baeten, L.A.; Reeves, P.M.; Martin, W.D.; Brauns, T.A.; Boyle, C.M.; Raju Paul, S.; Bucala, R.; et al. Promiscuous Coxiella burnetii CD4 Epitope Clusters Associated With Human Recall Responses Are Candidates for a Novel T-Cell Targeted Multi-Epitope Q Fever Vaccine. Front. Immunol. 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Kersh, G.J.; Fitzpatrick, K.A.; Self, J.S.; Biggerstaff, B.J.; Massung, R.F. Long-Term immune responses to Coxiella burnetii after vaccination. Clin. Vaccine Immunol. 2013, 20, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Hamada, Y.; Gupta, R.K.; Quartagno, M.; Izzard, A.; Acuna-Villaorduna, C.; Altet, N.; Diel, R.; Dominguez, J.; Floyd, S.; Gupta, A.; et al. Predictive performance of interferon-gamma release assays and the tuberculin skin test for incident tuberculosis: An individual participant data meta-analysis. eClinicalMedicine 2023, 56, 101815. [Google Scholar] [CrossRef]
- Hilbink, F.; Penrose, M.; Kovacova, E.; Kazar, J. Q fever is absent from New Zealand. Int. J. Epidemiol. 1993, 22, 945–949. [Google Scholar] [CrossRef]
- Binette, P.; Tesfamariam, M.; Cockrell, D.; Heinzen, R.A.; Richards, C.; Shaia, C.; Long, C.M. Murine Q Fever Vaccination Model Reveals Sex Dimorphism in Early Phase Delayed-Type Hypersensitivity Responses. Front. Immunol. 2022, 13, 894536. [Google Scholar] [CrossRef]
- Fratzke, A.P.; Gregory, A.E.; van Schaik, E.J.; Samuel, J.E. Coxiella burnetii Whole Cell Vaccine Produces a Th1 Delayed-Type Hypersensitivity Response in a Novel Sensitized Mouse Model. Front. Immunol. 2021, 12, 754712. [Google Scholar] [CrossRef]
- Fratzke, A.P.; Szule, J.A.; Butler, S.M.; Schaik, E.; Samuel, J.E. Molecular mechanisms of Coxiella burnetii formalin-fixed cellular vaccine reactogenicity. Infect. Immun. 2024, 92, e0033524. [Google Scholar] [CrossRef]
- Bond, K.A.; Franklin, L.J.; Sutton, B.; Firestone, S.M. Q-Vax Q fever vaccine failures, Victoria, Australia 1994-2013. Vaccine 2017, 35, 7084–7087. [Google Scholar] [CrossRef]
- Woldeyohannes, S.M.; Perkins, N.R.; Baker, P.; Gilks, C.F.; Knibbs, L.D.; Reid, S.A. Q fever vaccine efficacy and occupational exposure risk in Queensland, Australia: A retrospective cohort study. Vaccine 2020, 38, 6578–6584. [Google Scholar] [CrossRef]


| Number | 25 |
| Gender | 10 F/15 M |
| Median Age (yr) | 49 |
| Age Range (yr) | 25–72 |
| Prior Q-VAX (n) | 7 |
| Prior Q fever (n) | 1 |
| 2021 | 2022 | |
|---|---|---|
| Number | 96 | 115 |
| Gender | 83 F/13 M | 83 F/32 M |
| Median Age (yr) | 19 | 21 |
| Age Range (yr) | 18–39 | 18–36 |
| Enrolled | Returned for Skin Test Reading | |||
|---|---|---|---|---|
| Abattoir | Community | Abattoir | Community | |
| Number | 23 | 14 | 20 a | 14 b |
| Gender | 7 F/16 M | 6 F/8 M | 7 F/13 M | 6 F/8 M |
| Median Age (yr) | 42 | 62 | 42 | 62 |
| Age Range (yr) | 18–63 | 26–78 | 18–63 | 26–78 |
| Prior Q-VAX (n) | 22 | 7 | 19 | 7 |
| Prior Q fever (n) | 1 | 7 | 1 | 7 |
| 2021 | 2022 | |
|---|---|---|
| Number | 58 | 82 |
| Gender | 51 F/7 M | 60 F/22 M |
| Median Age (yr) | 19 | 21 |
| Age Range (yr) | 18–32 | 18–29 |
| # Positive Tests/Total | |||||
|---|---|---|---|---|---|
| Screening Test | Prior Q-VAX a | Prior Q Fever a | No Exposure a | Sensitivity b (CI d) | Specificity c (CI d) |
| Q-VAX skin test | 2/7 | 1/1 | 4/17 | 38% (8.5; 76) | 76% (50; 93) |
| Q-Detect IGRA | 7/7 | 1/1 | 3/17 | 100% (63; 100) | 82% (57; 96) |
| Phase II IgM EIA | 0/7 | 0/1 | 0/17 | 0% (0; 37) | 100% (80; 100) |
| Phase II IgG EIA | 1/7 | 1/1 | 0/17 | 25% (3.2; 65) | 100% (80; 100) |
| Phase II CFT | 1/7 | 1/1 | 1/17 | 25% (3.2; 65) | 94% (71; 100) |
| Phase II IgG IFA | 4/7 | 1/1 | 0/17 | 62% (24; 91) | 100% (80; 100) |
| # Positive Tests/Total | |||||
|---|---|---|---|---|---|
| Abattoir | Community | ||||
| Screening Test | Prior Q-VAX a | Prior Q Fever a | Prior Q-VAX a | Prior Q Fever a | Sensitivity b (CI c) |
| Q-VAX skin test | 18/19 d | 1/1 | 4/7 | 6/7 | 85% (69; 95) |
| Q-Detect IGRA | 13/21 e | 1/1 | 1/7 | 7/7 | 61% (43; 77) |
| Phase II IgG EIA | 6/22 | 1/1 | 1/7 | 7/7 | 41% (25; 58) |
| Phase II CFT | 19/22 | 1/1 | 3/7 | 7/7 | 81% (65; 92) |
| Phase II IgG IFA | 10/22 | 1/1 | 2/7 | 7/7 | 54% (37; 71) |
| Cohorts | Both Positive n (%) | ST+ a IGRA− n (%) | ST− IGRA+ n (%) | Both Negative n (%) | Overall % Agreement | Cohen’s Kappa (95% CI) | Kappa Agreement Level |
|---|---|---|---|---|---|---|---|
| Student cohorts | 0 (0) | 0 (0) | 67 (33) | 135 (67) | 67% | 0.0 (−0.20; 0.20) | Poor |
| Cairns | 2 (8) | 5 (20) | 2 (8) | 16 (64) | 72% | 0.20 (−0.30; 0.70) | Poor |
| Toowoomba | 20 (59) | 9 (26) | 2 (8) | 3 (9) | 68% | 0.18 (−0.21; 0.58) | Poor |
| Combined cohorts | 22 (8) | 14 (5) | 71 (27) | 154 (59) | 67% | 0.18 (0.03; 0.32) | Poor |
| Cohorts | Both Positive n (%) | ST+ a IGRA− n (%) | ST− IGRA+ n (%) | Both Negative n (%) | Overall % Agreement | Cohen’s Kappa (95% CI) | Kappa Agreement Level |
|---|---|---|---|---|---|---|---|
| Student cohorts | 0 (0) | 0 (0) | 12 (6) | 190 (94) | 94% | 0.0 (−0.55; 0.55) | Poor |
| Cairns | 1 (4) | 6 (24) | 0 (0) | 18 (72) | 76% | 0.19 (−0.37; 0.75) | Poor |
| Toowoomba | 7 (20) | 22 (65) | 0 (0) | 5 (15) | 35% | 0.08 (−0.14; 0.31) | Poor |
| Combined cohorts | 8 (3) | 28 (11) | 12 (4) | 213 (82) | 85% | 0.21 (−0.04; 0.40) | Fair |
| Vaccine Eligibility Determinations a | |||||||
|---|---|---|---|---|---|---|---|
| IGRA + Serology Combinations | Both No n (%) | Clin Yes IGRA No n (%) | Clin No IGRA Yes n (%) | Both Yes n (%) | Overall % Agreement | Cohen’s Kappa (95% CI) | Kappa Agreement Level |
| IGRA + EIA | 28 (12) | 62 (27) | 13 (6) | 129 (56) | 68% | 0.24 (0.10; 0.38) | Fair |
| IGRA + CFT b | 34 (15) | 62 (27) | 5 (2) | 129 (56) | 71% | 0.34 (0.21; 0.48) | Fair |
| IGRA + IFA | 32 (14) | 62 (27) | 10 (4) | 128 (55) | 69% | 0.29 (0.16; 0.43) | Fair |
| Vaccine Eligibility Determinations a | |||||||
|---|---|---|---|---|---|---|---|
| IGRA + Serology Combinations | Both No n (%) | Clin Yes IGRA No n (%) | Clin No IGRA Yes n (%) | Both Yes n (%) | Overall % Agreement | Cohen’s Kappa (95% CI) | Kappa Agreement Level |
| IGRA + EIA | 18 (8) | 10 (4) | 23 (10) | 181 (78) | 86% | 0.44 (0.26; 0.62) | Moderate |
| IGRA + CFT b | 30 (13) | 10 (4) | 9 (4) | 181 (79) | 92% | 0.71 (0.58; 0.83) | Good |
| IGRA + IFA | 25 (11) | 10 (4) | 17 (7) | 180 (78) | 89% | 0.58 (0.43; 0.73) | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graves, S.; Robson, J.; Scholzen, A.; Dzeng, R.; Powell-Romero, F.; Evans, J.; Stenos, J.; Jeppesen, M.; Kouwijzer, M.L.C.E.; Lankhof, J.; et al. A Comparison of Tests for Detecting Prior Exposure to Coxiella burnetii for Use with Q-VAX in Australian Human Q Fever Vaccination. Vaccines 2025, 13, 615. https://doi.org/10.3390/vaccines13060615
Graves S, Robson J, Scholzen A, Dzeng R, Powell-Romero F, Evans J, Stenos J, Jeppesen M, Kouwijzer MLCE, Lankhof J, et al. A Comparison of Tests for Detecting Prior Exposure to Coxiella burnetii for Use with Q-VAX in Australian Human Q Fever Vaccination. Vaccines. 2025; 13(6):615. https://doi.org/10.3390/vaccines13060615
Chicago/Turabian StyleGraves, Stephen, Jennifer Robson, Anja Scholzen, Richard Dzeng, Francisca Powell-Romero, Jennifer Evans, John Stenos, Meg Jeppesen, Milou L. C. E. Kouwijzer, Jordi Lankhof, and et al. 2025. "A Comparison of Tests for Detecting Prior Exposure to Coxiella burnetii for Use with Q-VAX in Australian Human Q Fever Vaccination" Vaccines 13, no. 6: 615. https://doi.org/10.3390/vaccines13060615
APA StyleGraves, S., Robson, J., Scholzen, A., Dzeng, R., Powell-Romero, F., Evans, J., Stenos, J., Jeppesen, M., Kouwijzer, M. L. C. E., Lankhof, J., Raju Paul, S., Proboste Ibertti, T., Ball, L., Powell, H., Wilkinson, S., van Schuppen, E., Anker-Op den Brouw, W. J., Cobbold, R., Garritsen, A., ... Sluder, A. E. (2025). A Comparison of Tests for Detecting Prior Exposure to Coxiella burnetii for Use with Q-VAX in Australian Human Q Fever Vaccination. Vaccines, 13(6), 615. https://doi.org/10.3390/vaccines13060615





