Abstract
The emergence of complex and rapidly evolving pathogens necessitates innovative vaccine platforms that move beyond traditional methods. This review explores the transformative potential of next-generation vaccine technologies, focusing on the combined use of synthetic biology, nanotechnology, and systems immunology. Synthetic biology provides modular tools for designing antigenic components with improved immunogenicity, as seen in mRNA, DNA, and peptide-based platforms featuring codon optimization and self-amplifying constructs. At the same time, nanotechnology enables precise antigen delivery and controlled immune activation through engineered nanoparticles such as lipid-based carriers, virus-like particles, and polymeric systems to improve stability, targeting, and dose efficiency. Systems immunology aids these advancements by analyzing immune responses through multi-omics data and computational modeling, which assists in antigen selection, immune profiling, and adjuvant optimization. This approach enhances both humoral and cellular immunity, solving challenges like antigen presentation, response durability, and vaccine personalization. Case studies on SARS-CoV-2, Epstein–Barr virus, and Mycobacterium tuberculosis highlight the practical application of these platforms. Despite promising progress, challenges include scalability, safety evaluation, and ethical concerns with data-driven vaccine designs. Ongoing interdisciplinary collaboration is crucial to fully develop these technologies for strong, adaptable, globally accessible vaccines. This review emphasizes next-generation vaccines as foundational for future immunoprophylaxis, especially against emerging infectious diseases and cancer immunotherapy.
1. The Evolution of Vaccines: From Traditional to Next-Generation Platforms
Vaccines are biological agents designed to stimulate the immune system, promoting the production of pathogen-specific antibodies and facilitating the development of active immunity [1]. There is a long history of vaccine development, representing one of the most important aspects of modern medicine. In this context, there is an observable path in vaccine development, initiated from live-attenuated forms and advancing to genetically engineered ones [2]. From another viewpoint, all vaccine history can be subdivided into only three generations [3].
Live attenuated vaccines were the first form produced; these were designed based on the ideas proposed by Jenner. He suggested that incubation with cowpox virus in healthy individuals may protect them against smallpox [4]. After that, Pasteur developed Jenner’s ideas about attenuation and designed three vaccines based on this idea, comprising the Pasteurella multocida vaccine (from a pathogen in chickens), the sheep anthrax vaccine, and a rabies vaccine. In the following years and because of the development of cell culture techniques, other types of live attenuated vaccines were also designed, including those for tuberculosis, yellow fever, polio, measles, mumps, rubella, and varicella [5]. While live-attenuated vaccines elicit robust, long-lasting humoral and cellular immune responses, their use is contraindicated in immunocompromised individuals and pregnant women, due to the potential risk of reversion to a virulent form, especially in immunocompromised individuals [6].
Frederick Griffith’s discovery in the 19th century paved the way for vaccine technology. Based on Griffith’s work and subsequent scientific studies, preserving immunogenicity is possible when bacteria are inactivated using controlled heat or chemical methods [7]. This was the key to the discovery of transforming processes, in which pathogen-like bacteria transform whole or partial parts (plasmid) of their genome into that of another. In this context, the inactivation of bacteria and the formulation of their components represent a new perspective in vaccine design. The first vaccine of this type was the typhoid vaccine, which led to other bacterial and viral vaccines encompassing those for cholera, pertussis, influenza, rabies, and hepatitis A [8].
The identification of polysaccharide capsules around certain pathogens led to the formulation of a novel type of vaccine that was not traditionally an inactivated form; these were known as conjugated vaccines because of the presence of a carrier molecule in association with the main antigen (which was the polysaccharide) [9]. This cluster comprises vaccines against Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae. Likewise, proteins could be purified and utilized for vaccine formulation. In this context, the protein was an antigen, stimulating the host’s immunity response [10,11]. The first vaccine of this type employed diphtheria toxoid [2]. Others, including anti-anthrax secreted proteins and anti-hepatitis B [12] vaccines, were developed later.
Advancements in genetic and molecular biology technologies led to the development of recombinant vaccines. Unlike subunit vaccines, which employ purified proteins, these types employ genes that produce antigens [13]. Following antigen selection, an expression system (such as yeast, mammalian cells, and bacteria) is used to produce the antigen in huge amounts. The hepatitis B vaccine is the best example of this family, which has exhibited remarkable success. The vaccine comprises a sequence of viruses that encode HbsAg, which is responsible for huge levels of antibody production. Other types of subunit vaccines are anti-HPV [14], anti-herpes, and anti-rotavirus vaccines [15].
Lessons from emerging infectious outbreaks such as influenza, Ebola, SARS-CoV-1, MERS, and SARS-CoV-2, alongside the likelihood of future pandemics arising from RNA viruses, underscore the growing need for innovative vaccine platforms. The rapid evolution of pathogens, their high transmission potency, and the adaptability of virulent strains are key concerns in the face of new outbreaks [16,17]. These challenges have driven the development of next-generation vaccine platforms, incorporating advancements like nanotechnology to enable rapid adaptation, strengthen immune responses, and optimize dosing regimens. Recent advancements in nanotechnology have introduced novel vaccine delivery systems, demonstrating the potential to enhance immune responses more effectively, even with lower or potentially single-dose regimens, a concept supported by experimental results that will be discussed in later sections [18]. As next-generation vaccine platforms evolve, integrating AI-driven antigen prediction and personalized immunoprofiling may further revolutionize preventive medicine, fostering rapid responses to future pandemics.
2. Synthetic Biology in Vaccine Design
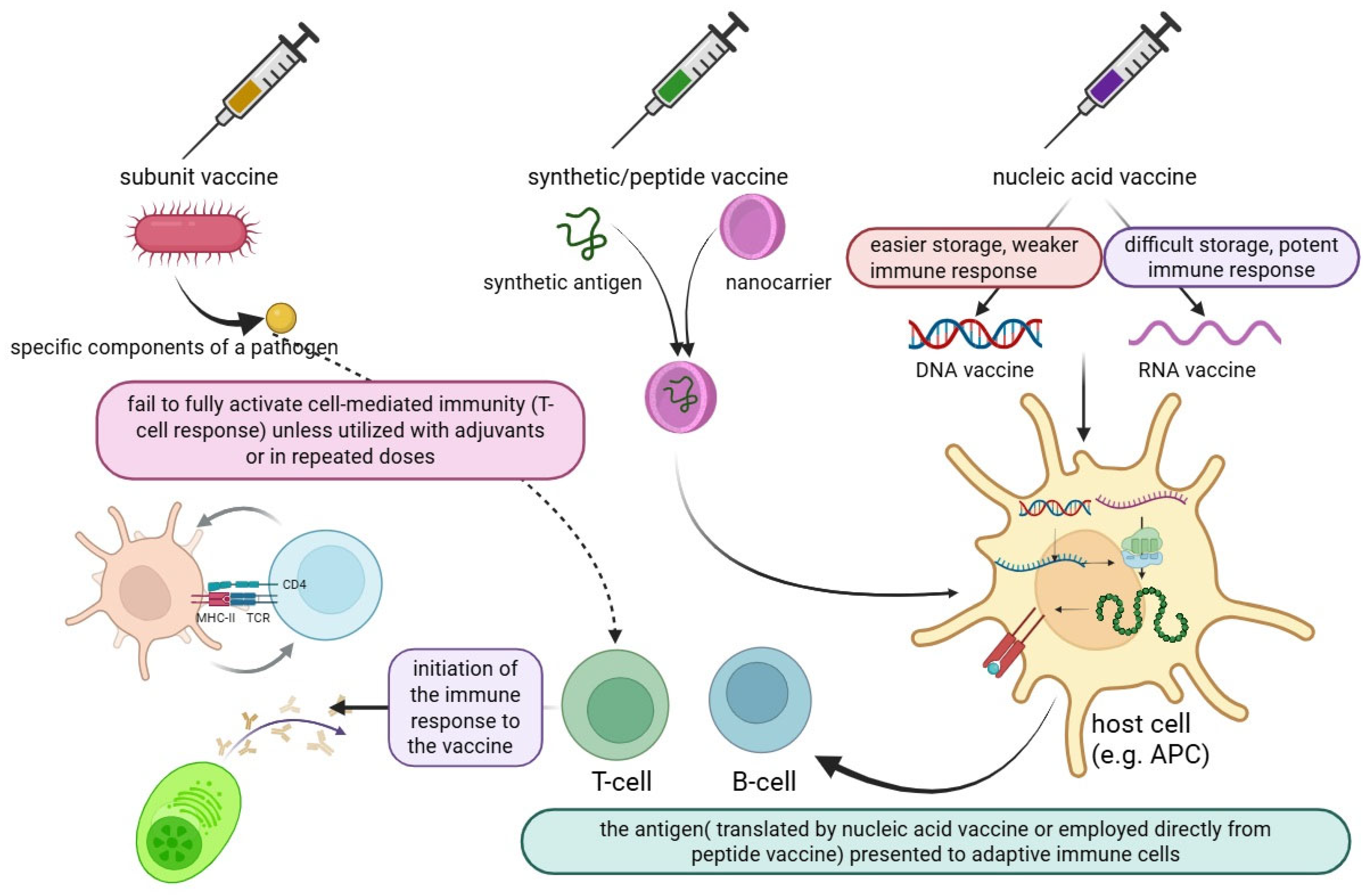
Vaccines are one of the main components of public health, and they can reduce mortality and morbidity without causing severe illness, a process that requires two steps: (1) selecting the antigen, and (2) introducing it into the body [19]. Subunit vaccines, which deliver essential pathogen components, are a new trend in the vaccination industry. They are more common and safer than traditional whole-pathogen vaccines, but subunit vaccines are typically weak, they need multiple doses to be effective and must be combined with an adjuvant to enhance the immune response, while live attenuated viruses frequently elicit strong immune responses. In recent decades, subunit vaccines have been processed and/or chemically modified to develop synthetic vaccines with improved immunogenicity. Specifically, synthetic vaccine systems such as polymeric nanoparticles, inorganic nanoparticles, liposomes, and micelles have shown significant effects in enhancing vaccine efficacy [20].
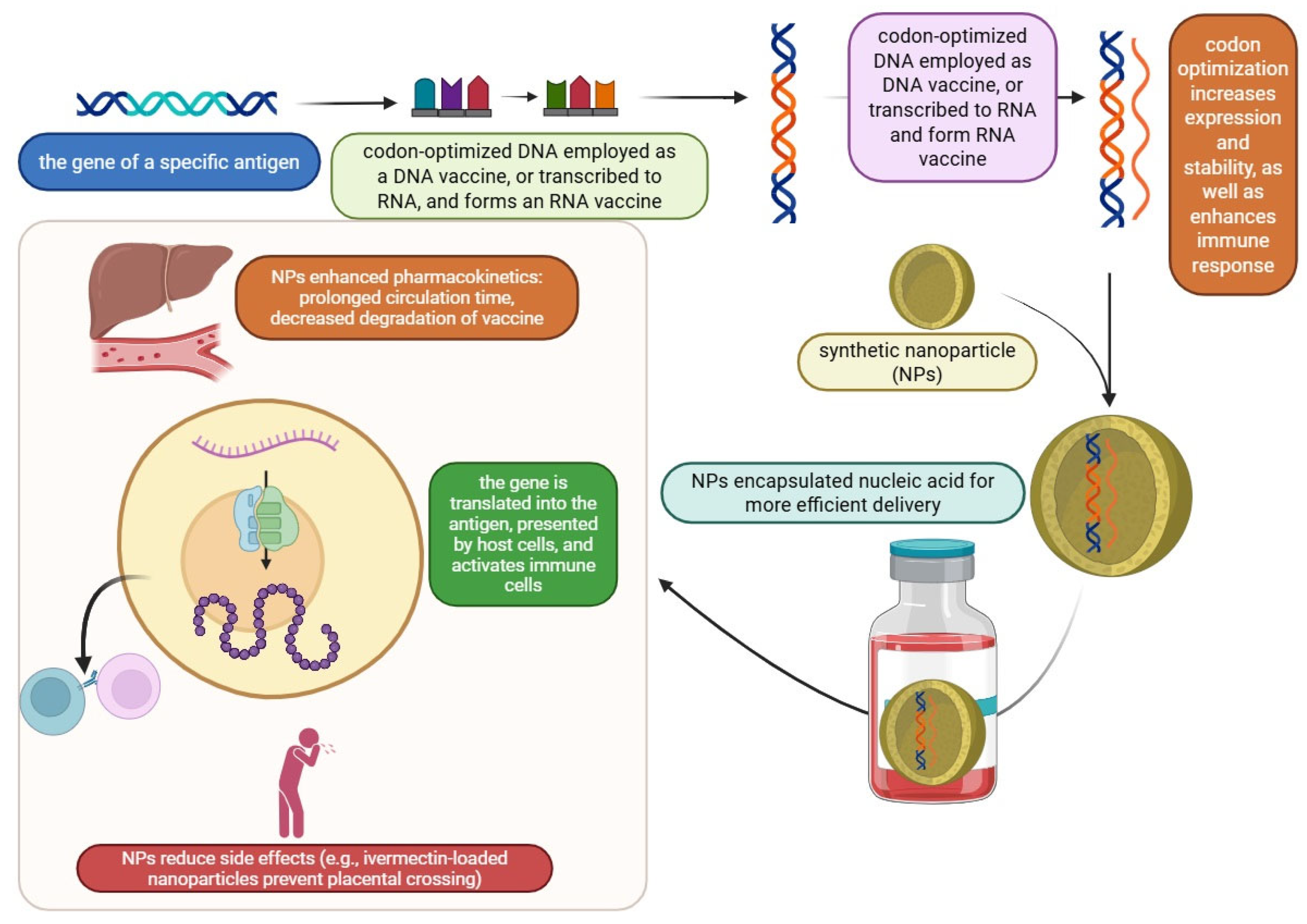
Synthetic vaccines consist of molecular antigens linked to a carrier protein. This approach can reduce the issues associated with traditional vaccines, such as pathogenicity and the risk of infection caused by attenuated bacteria or viruses, or inactivated pathogens that are killed by heat or formaldehyde [21]. Nucleic acid vaccines based on DNA work either through cloned plasmids or directly via mRNA to express the antigen in host cells, and this novel therapeutic approach uses the cell’s machinery for protein synthesis to create the desired proteins that the given mRNA encodes. Protein synthesis in this context mimics natural infection, activating both the humoral and cell-mediated immune responses. This approach also enhances immunity and enables the rapid production of vaccines and customizable vaccines. Although the mRNA platform made rapid vaccine development possible, the need for very cold storage still limits its widespread use. Most resource-limited countries lack the cold-chain storage necessary to carry out mass vaccinations [22,23,24]. During the SARS-CoV-2 pandemic, some mRNA vaccines, such as Moderna’s mRNA-1273 and BNT 162b2, were improved by Pfizer-BioNTech in 2020. COVID-19 mRNA vaccines have become the first FDA-approved mRNA vaccination for human use [25].
The production of proteins without the requirement for cell culture systems significantly reduces manufacturing costs. mRNA vaccines are generated through in vitro transcription using enzymatic reactions, although DNA serves as the initial template. The plasmid DNA required for this process is replicated in Escherichia coli. DNA-based vaccines exhibit greater stability compared to mRNA vaccines, thereby eliminating the need for ultra-low temperature storage and specialized cold-chain logistics. However, a recurring theme in human DNA vaccine trials has been their suboptimal immunogenicity when compared to traditional protein-based vaccine approaches. If they are to be used in humans, methods to improve the immunogenicity of DNA vaccines must be developed. These methods include electroporation, the co-expression of plasmids encoding adjuvants like cytokine-encoding genes, the co-formulation of DNA vaccines with conventional adjuvant compounds, codon optimization to maximize protein expression, the boosting of DNA vaccines with live viral vectors, or adjuvanted protein vaccines [26,27].
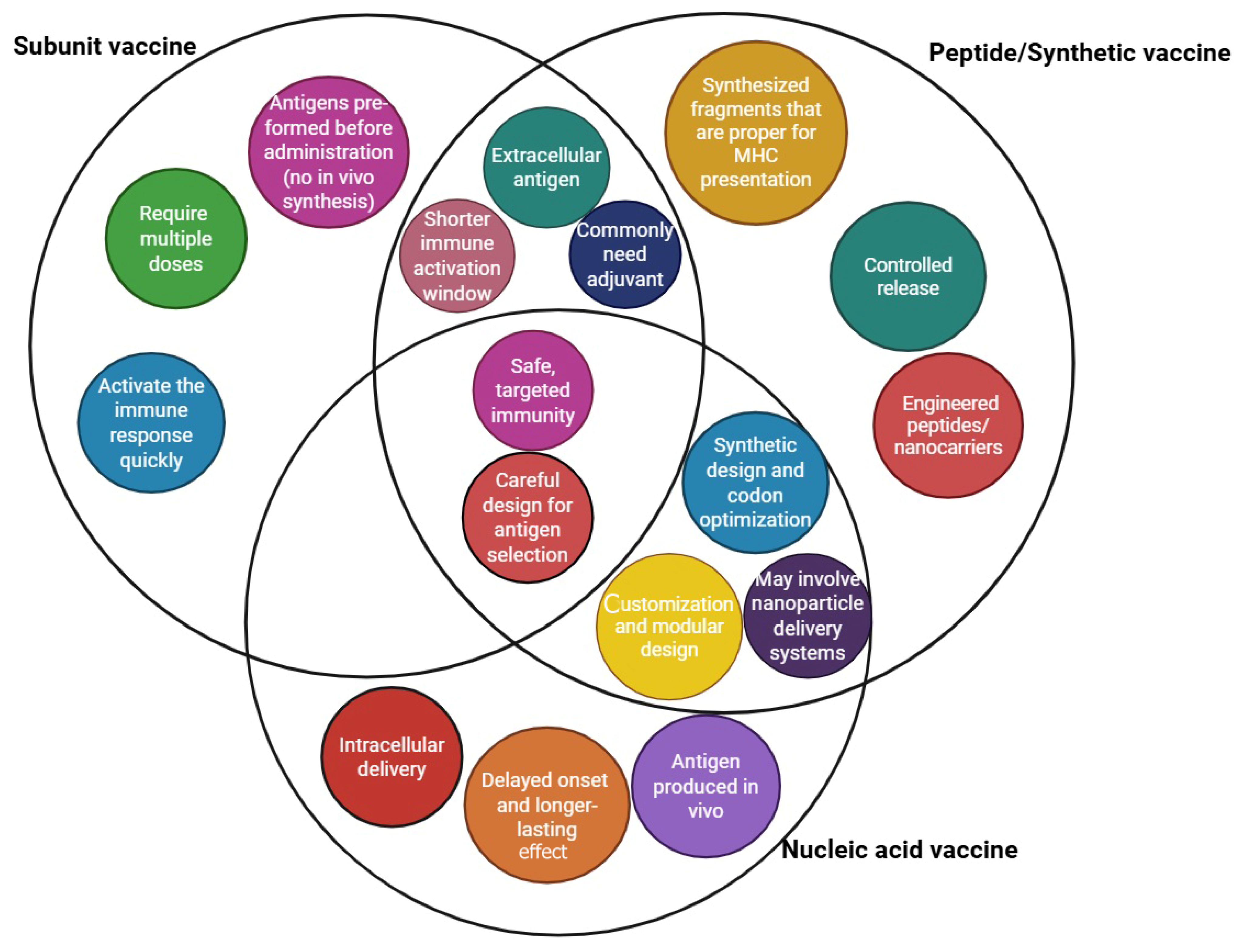
Peptide vaccines are synthesized using fragment condensation methods, and these vaccines are typically administered with an adjuvant to enhance the immune response. Additionally, these vaccines are considered safer than live or attenuated vaccines [28]. As biotechnology has advanced quickly, recombinant proteins for application in vaccines and medications have been produced by heterologous expression. As the fundamental unit of correspondence between information-carrying proteins and nucleic acids, the codon serves as the fundamental conduit for information transfer in vivo. Known as synonymous codons, they encode the same amino acid. Codon usage bias refers to the tendency of a species or a gene to favor the use of one or more specific synonymous codons, known as optimum codons, even when the usage probability of synonymous codons is not the same during protein synthesis. Additionally, the bias governing codon use by genes varies greatly, depending on the function. This phenomenon is called codon usage bias. Heterologous expression has been used to produce recombinant proteins for application in medications and vaccines, as biotechnology has advanced quickly, even though the usage probability of synonymous codons is not the same during protein synthesis [29,30]. There are also other advantages to employing the attenuation technique of codon deoptimization. A successful response to infectious epidemics requires speed, and this method has the advantage of not requiring an in-depth knowledge of viral function. The three novel aspects of vaccine technology are illustrated in Figure 1. Additionally, a summary of these three technologies is provided in a diagram highlighting their exclusive and shared features, as shown in Figure 2.
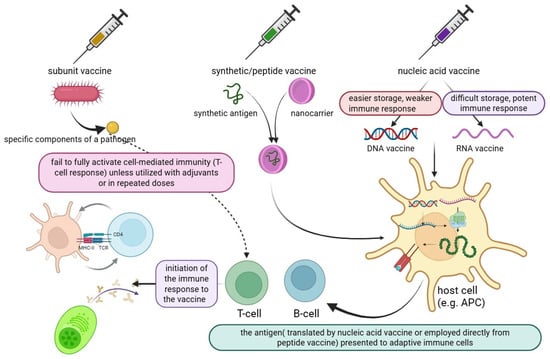
Figure 1.
An overview of the three main novel vaccine technologies, synthetic biology, nanotechnology, and systems immunology, along with a depiction of their underlying molecular mechanisms and points of interaction.
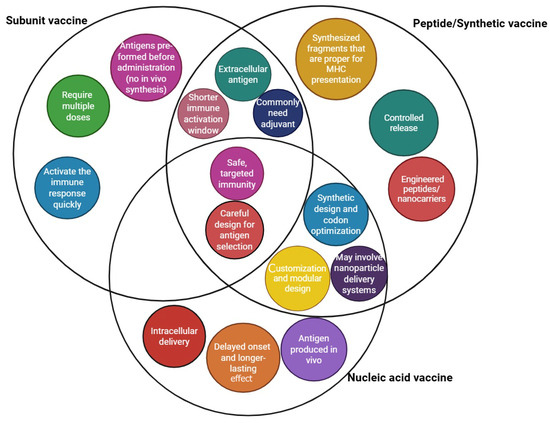
Figure 2.
Summary of peptide, nucleic acid, and synthetic vaccine technologies in the context of their unique and common characteristics.
Computational methods make it possible to characterize codon biases and forecast protein-coding areas using genomic data [31,32]. Because of the hundreds of mutations, virulent reversion is extremely uncommon, and a single dose may be enough to produce long-lasting protective immunity, making deployment easier. Furthermore, the genes’ codon use bias varies greatly, depending on their function. Codon deoptimization vaccines have been evaluated in several phase I clinical trials, including CodaVax-H1N1 (for influenza A H1N1), CodaVax-RSV (for respiratory syncytial virus), and CDX-005 (for SARS-CoV-2), with clinical trials for the latter planned for early 2021 [33].
2.1. Integrated Perspective on Vaccine Adjuvants: Common Mechanisms and Strategic Insights
Adjuvants are defined as different substances that, when given with vaccination antigens, increase the immunogenicity of vaccines [34]. Adjuvants can be anything from complicated natural extracts and particulate matter to tiny artificial molecules. Alexander Glenny discovered, in 1926, that injecting aluminum salts with antigens into guinea pigs produced more antibodies than injecting antigens alone, providing the first proof of adjuvants. Freund and his associates subsequently created water-in-oil emulsions in the 1940s, which resulted in the development of Freund’s adjuvants [35]. However, due to its toxicity to people, Freund’s adjuvant is not authorized for use in human vaccinations. Like Freund’s adjuvants, bacterial lipopolysaccharide (LPS) adjuvants have had limited usage in human vaccines because of both their systemic and local adverse effects. Aluminum adjuvants are the most common mineral salts. For more than 60 years, many nations have used aluminum adjuvants in their regular childhood vaccination regimens, mainly in tetanus, diphtheria, pertussis, and poliomyelitis vaccines. Aluminum adjuvants were then added to vaccines for use against the hepatitis A and hepatitis B viruses, the human papillomavirus (which causes cervical cancer and genital warts), Lyme disease/Borreliose, and Japanese encephalitis. For specific risk populations, additional aluminum-adsorbed vaccines are available to prevent diseases like anthrax. Emulsions are another type of adjuvant. Both AS03 and MF59 are traditional adjuvants for oil-in-water emulsions [35].
The first non-aluminum adjuvant authorized for use in human vaccinations was MF59, which was licensed in 1997 as an adjuvant for the influenza vaccine. The two functions of MF59 emulsions are immune activation and antigen delivery. By gradually releasing antigens into the lymph nodes, MF59 can be employed as an emulsion delivery method in conjunction with antigens to enhance antigen presentation and prolong antigen engagement with the immune system [36,37]. Despite attempts to create new adjuvants for human vaccinations, only aluminum adjuvants were licensed between the 1920s and the 1990s. The oil-in-water emulsion MF59 was not approved for use as an adjuvant in influenza vaccines in Europe until 1997. The monotony of adjuvants for human vaccinations was broken in the next two decades with the licensing of four additional adjuvants (AS04, AS03, AS01, and CpG ODN 1018) for use in vaccines [38]. During this period, numerous other kinds of chemicals, such as mineral salts, microbial products, emulsions, saponins, synthetic small molecule agonists, polymers, nanoparticles, and liposomes, were also assessed as adjuvants [39]. Preclinical and clinical research has demonstrated that they improve the robustness, range, and durability of immune responses [40]. Adjuvants function through two mechanisms: delivery systems and immunostimulants. By targeting particular pattern-recognition receptors (PRRs), immunostimulants stimulate the antigen-presenting cells (APCs), resulting in improved antigen-presenting and co-stimulatory signaling and an improvement in the adaptive immune system. By extending the bioavailability of antigens, the delivery systems direct the antigens to APCs or lymph nodes, improving the absorption and presentation of antigens by APCs and boosting the adaptive immune response. In order to help with the selection of sensible adjuvants during the development of a particular vaccine, this review will go into detail on both traditional adjuvant platforms and the adjuvant platforms that are currently being studied. We believe that the following issues might need to be addressed in order to encourage the more widespread development of adjuvants [35]. Clinically approved adjuvants collectively exhibit common mechanisms that are capable of increasing immunogenicity. Most adjuvants achieve this either by activating innate immunity via PRRs, as seen with CpG 1018 and AS01, or by establishing an antigen depot effect, as exemplified by alum and MF59, which extends antigen availability to bolster adaptive immune responses. Additionally, emulsions such as AS03 and MF59 promote antigen uptake and the recruitment of APCs, while Toll-like receptor (TLR) agonists like CpG actively drive dendritic cell maturation. Notably, Th1/Th2 polarization varies significantly among adjuvants: alum primarily induces a Th2-biased response, while AS01 and CpG favor Th1 responses, which are especially essential for addressing intracellular pathogens and cancer. This comparative analysis highlights the importance of strategically selecting adjuvants that align with the pathogen type, desired immune response, and characteristics of the target patient population (Table 1).

Table 1.
Clinically approved adjuvants and their mechanisms of action.
2.2. Key Functional Characteristics of Vaccine Adjuvants
Self-amplifying RNA (saRNA) and standard mRNA are the two forms of synthetic RNA vaccines that are currently available (see Figure 1). Numerous preclinical and clinical studies have examined the use of conventional mRNA strategies—also known as nonreplicating or non-amplifying mRNA—against cancer and infectious disorders. While these encoding therapeutic proteins, including antibodies or immune modulators, have been examined for immunotherapy, in vitro transcribed mRNAs encoding viral antigens have been investigated as vaccines [41,42]. Nonetheless, the quantity of conventional mRNA transcripts that are successfully administered during immunization is directly correlated with antigen expression. Therefore, high dosages or repeated administrations may be necessary to achieve sufficient expression for immunomodulation or protection. Genetically modified replicons generated from self-replicating single-stranded RNA viruses are known as saRNA vaccines. They can be administered as fully synthetic saRNA, generated following in vitro transcription, or delivered as viral replication particles (VRPs) with the saRNA enclosed within the viral particle. During manufacturing, envelope proteins are supplied in the form of faulty assistance constructions to produce replication-defective VRPs. After an initial infection, the resulting VRPs are unable to produce infectious viral particles, and only the RNA can be amplified further. Both positive-sense and negative-sense RNA viruses can produce VRPs, although the latter are more complicated and need reverse genetics to be saved (Table 2).

Table 2.
Synthetic biology-based vaccine platforms and their mechanisms.
3. Nanoparticle Properties and Their Immunological Impacts
3.1. Size
The size of nanoparticles plays a crucial role in their uptake by APCs and the subsequent activation of the immune system. Particles within the 20–200 nm range are efficiently internalized by dendritic cells and macrophages, enabling effective antigen processing and presentation. Smaller nanoparticles, which are typically less than 50 nm, can migrate into the lymph nodes, enhancing interactions with resident immune cells. In contrast, larger particles, those exceeding 500 nm, are more likely to be involved in phagocytosis by macrophages at the injection site.
3.2. Shape
Nanoparticle shape significantly influences cellular uptake and distribution throughout the body. Spherical nanoparticles are generally internalized more easily compared to rod-shaped or irregular forms. However, rod-shaped nanoparticles benefit from prolonged circulation times and offer improved tumor penetration, making them particularly valuable in cancer immunotherapy.
3.3. Surface Charge
The surface charge of nanoparticles impacts their interaction with cell membranes and proteins. Positively charged nanoparticles show increased cellular uptake due to electrostatic attraction to negatively charged cell membranes, although this can also lead to higher cytotoxicity and nonspecific binding. In contrast, negatively charged or neutral nanoparticles tend to reduce nonspecific interactions and exhibit longer circulation times.
3.4. Composition
The composition of nanoparticles determines factors such as biodegradability, antigen release dynamics, and intrinsic immunostimulatory properties. For example, lipid-based nanoparticles excel at facilitating endosomal escape, promoting cytosolic antigen delivery, while polymer-based nanoparticles can be tailored for controlled release and targeted delivery [49].
3.5. Molecular and Cellular Mechanisms
Nanoparticles boost immune responses through an interconnected network of molecular and cellular pathways.
Enhanced antigen presentation: Nanoparticles improve the precise delivery of antigens to APCs, such as dendritic cells and macrophages. This facilitates efficient processing and presentation through major histocompatibility complex (MHC) class I and II pathways, triggering the strong activation of CD4+ and CD8+ T cells.
Activation of innate immunity: Certain nanoparticle formulations can interact with PRRs, including TLRs and NOD-like receptors (NLRs). This interaction induces the production of proinflammatory cytokines, such as IL-6 and TNF-α, along with the upregulation of co-stimulatory molecules. Such innate immune activation plays a fundamental role in shaping effective adaptive immune responses.
Modulation of immune cell trafficking: By engineering nanoparticles with specific surface ligands or optimizing their size, it becomes possible to target particular tissues or subsets of immune cells. These tailored particles can influence immune cell migration, retention, and activation, particularly within the lymphoid tissues or areas of inflammation [50,51].
4. Nanotechnology as a Vaccine Delivery System
Recent advancements in nanotechnology have introduced novel vaccine delivery systems, demonstrating the potential to enhance immune responses more effectively, even with lower or potentially single-dose regimens. Nanoparticles (NPs) encompass a diverse class of materials and are characterized by a diameter of less than 100 nm; their unique physicochemical properties render them highly suitable for biomedical applications. Beyond their established potential for drug delivery and controlled release, nanoparticles can also exhibit intrinsic antigenic properties, sparking considerable scientific interest in harnessing this technology as an innovative vaccine platform [18]. Nanovaccines offer a targeted tissue delivery system, which represents one of their key advantages. Their structural composition protects vaccine antigens from enzymatic degradation and enhances antigen transport to the lymphatic system, improving immune activation. Conventional peptide-based vaccines often fail to induce cross-presentation by dendritic cells (DCs), limiting CD8+ T cell activation. Nanovaccines overcome this limitation by facilitating efficient antigen presentation through both the MHC I and MHC II pathways, promoting CD8+ T cell activation and enhancing CD4+ T cell responses, ultimately supporting B cell activation and antibody production. Moreover, nanovaccine adjuvants can engage pattern-recognition receptors on DCs, driving DC maturation, type I interferon expression, and antiviral cytokine production. Their enhanced delivery efficiency, superior DC activation, sustainable antigen release, and ability to present high antigen concentrations for optimal B cell receptor binding make nanovaccines promising candidates for inducing robust, long-lasting antibody responses [35,52]. LNPs, polymer-based nanoparticles, protein-based nanoparticles, metal-based nanoparticles, virus-like particles (VLPs), and liposomes are examples of nano-based vaccine platforms [18,53]. In a study evaluating an Epstein–Barr virus (EBV) vaccine, protein-based polymeric NPs were co-formulated with EBV glycoprotein B (gB) to assess immunogenicity in BALB/c mice. Control groups received either EBV gB or nanoparticles alone. To investigate the adjuvant effects, oil-in-water emulsion MF59 and Imject Alum were incorporated. Mice received three doses at weeks 0, 3, and 8. The results demonstrated that the gB-NP formulation elicited higher neutralizing antibody titers in epithelial cells compared to B cells [54].
Notably, anti-gB IgG titers were highest in the gB-NP group adjuvanted with MF59, maintaining elevated levels through week 20 that were comparable to the peak titers observed at week 10. The candidate vaccine was further evaluated in Macaca fascicularis as a non-human primate (NHP) model. Immunogenicity analysis revealed that total anti-gB IgG (p < 0.01) and anti-gB IgA titers (p < 0.01), along with B cell and epithelial cell neutralization titers (p < 0.0001), were significantly higher in the gB-NP vaccine group compared to the gB-alone formulation, with marked superiority in terms of epithelial cell neutralization. Moreover, the passive transfer of serum from vaccinated NHPs to humanized mice conferred protection against an EBV challenge, demonstrating the functional efficacy of the induced immune response [54].
A recent study introduced the vaccine delivery system X (VADEX), an innovative self-adjuvanted protein nanoparticle platform designed to simultaneously enhance antigen presentation and immune stimulation within a single molecular framework. The VADEX system is built on a fusion protein comprising an amphipathic helical peptide and a superfolder green fluorescent protein (sfGFP) that self-assembles into stable nanoparticles. This structural configuration not only supports multivalent antigen display but also provides intrinsic immunostimulatory properties, eliminating the requirement for external adjuvants. A key finding of the study emphasized the significance of the amphipathic helical peptide for maintaining the nanoparticle’s thermal stability and structural integrity, both of which are essential for ensuring vaccine stability during storage and distribution. By employing split-GFP technology, the researchers analyzed the impact of peptide sequence integrity and thermal resilience. Their results revealed that the VADEX platform exhibited superior self-adjuvanticity when compared to a clinical-stage adjuvant, as demonstrated by improved antibody binding affinity and a higher-quality immune response. Generally, VADEX marks a significant advancement in protein-based vaccine platforms, delivering a streamlined, stable, and highly effective method for inducing strong humoral and cellular immune responses without the need for additional adjuvants. The principles underlying its design could also be applied to therapeutic antibody development and other fields where robust immune activation is essential [55].
Furthermore, H1ssF recipients exhibited a fivefold rise in H5 stem-specific antibody titers by week 2, which persisted through week 16. Following the booster dose, the titers increased to eightfold by week 18 and remained elevated through week 40. Neutralizing antibodies against H5N1 similarly increased after both the prime and booster doses, sustaining significant elevation at week 40. A comparable trend was observed for H2N2 stem-binding and neutralizing antibodies, indicating broad cross-reactivity [56]. The first nano-based vaccine platform targeting Coxiella burnetii, the causative agent of Q fever, utilized an outer membrane protein antigen (CBU1910) conjugated to an E2 protein-based nanoparticle through three distinct methods: (1) the direct fusion of CBU1910 to E2, (2) Maleimide-tNTA-Ni linker chemistry using cysteine-rich E2 with His-tagged CBU1910, and (3) the SpyTag/SpyCatcher system for conjugating ST-E2 and SC-CBU1910. Among these, the third approach demonstrated superior physical stability and antigen-loading capacity. Notably, this formulation induced over ninefold higher IgG antibody levels compared to using the soluble antigen alone—even without an adjuvant. However, the response was skewed predominantly toward IgG1 (a Th2 response marker), with an imbalance in IgG2 (a Th1 response marker). This imbalance was corrected by incorporating CpG1826 as an adjuvant, leading to a more balanced Th1/Th2 response profile. Despite robust humoral immunity, antigen-specific IFN-γ production—a key marker of cellular immune activation—remained insufficient. This limitation was resolved by adding IVAX, which enhanced the cellular immune response. Collectively, these findings highlight the potential of nano-based vaccine platforms for C. burnetii and emphasize the need for further experimental optimization [57]. A recent study has developed a bionic nanovaccine that incorporates self-adjuvant properties using macrophage-derived vesicles co-loaded with antigens from the monkeypox virus. This innovative approach demonstrated strong antigen presentation capabilities and improved immune protection in preclinical models [58].
In another study, Mycobacterium tuberculosis (Mtb) antigens were first engineered as protein fusions with an N-terminal histidine tag and heparin-binding hemagglutinin adhesin, then they were loaded onto yellow carnauba palm wax nanoparticles with sodium myristate (YC-NaMA NPs). Two intranasal booster doses of Nano-FP1, administered at weeks 2 and 11, significantly enhanced short-term lung protection against Mtb infection compared to BCG alone. Additionally, the first booster dose increased the numbers of CD4+ resident memory T cells and T cells producing IFN-γ, TNF-α, IL-17, or combinations of IFN-γ+ TNF-α+ and IFN-γ+ TNF-α+ IL-2+, alongside an expansion in the CD8+ T cell population—although this response waned after 7 weeks [59]. In another study, the CV0501 vaccine, which is composed of SARS-CoV-2 Omicron BA.1 variant mRNA encapsulated in LNPs, was evaluated across five cohorts of up to 30 participants receiving 12, 25, 50, 100, or 200 μg doses. Participants had previously been vaccinated and were monitored for 6 months. Lower doses (3 and 6 μg, each administered to two groups of 15 participants) were subsequently evaluated after safety and immunogenicity assessments on day 29. Injection-site pain was the most frequently reported adverse event (57.5%, n = 180), followed by myalgia (36.9%) and fatigue (33%) [60].
Three serious adverse events (SAEs), namely, cholecystitis, diarrhea, and syncope, were reported but were determined to be unrelated to the vaccine. The neutralizing antibody titers peaked on day 15 and remained significantly elevated compared to pre-boost levels on day 181. Geometric mean titers (GMTs) were highest against the Omicron BA.1 subvariant, with cross-reactive responses observed against BA.2, BA.5, Delta, and wild-type strains. A dose-response relationship was evident, with higher doses generating correspondingly higher antibody titers. Cellular immunity analysis revealed a rise in polypositive CD4+ T cell frequencies by day 8, plateauing on day 15 against the wild-type, BA.1, and BA.5 subvariants. Notably, CD4+ T cells exhibited increased IFN-γ expression, which was indicative of a Th1-skewed immune response, while IL-13 and IL-17 expression remained unchanged. In contrast, no significant changes from baseline were observed in polypositive CD8+ T cell responses [60]. Another study on Listeria monocytogenes identified LMON_0149, a periplasmic oligopeptide-binding protein (OppA), as a promising vaccine target. Following a proteomics-based analysis, LMON_0149 was selected for an mRNA vaccine that was formulated using a lipid nanoparticle (LNP) platform. The vaccine demonstrated a significant induction of antigen-specific IFN-γ expression in mice, marking a strong CD8+ T cell response [61]. In another study on influenza and SARS-CoV-2, researchers encoded the Chemokine (C-X-C motif) ligand 13 (CXCL13) within antigen-encoding circular RNA (circRNA) strands—hemagglutinin (HA) for influenza and trimeric receptor-binding domain (RBD) for SARS-CoV-2—which were delivered via LNPs. The results demonstrated that HA-CXCL13–circRNA induced a threefold increase in germinal center B cell numbers compared to HA–circRNA in mice. Notably, while germinal center B cell numbers declined in the HA–circRNA group after day 14, they remained stable in the HA-CXCL13–circRNA group. Additionally, HA-CXCL13–circRNA elicited a stronger CD4+ and CD8+ T cell response, characterized by elevated IL-2 and TNF-α expression rather than IFN-γ. Moreover, cross-reactive antibody responses were significantly enhanced in the HA-CXCL13–circRNA group. Mice primed with an HA sequence from H3N2 and later challenged with PR8 (H1N1) exhibited 100% survival in the HA-CXCL13–circRNA group, whereas 4 out of 10 mice succumbed in the HA–circRNA group. Similarly, when comparing RBD-CXCL13–circRNA with RBD–circRNA, the former induced significantly higher antibody titers against the Wuhan-Hu-1 S protein, S1 protein, RBD protein, and multiple RBD mutants, while the latter showed markedly lower responses. These findings suggest that CXCL13 may enhance cross-reactivity in COVID-19 vaccines as well [62]. Metal-based nanoparticles, primarily of gold and iron oxides, are commonly employed as adjuvants within vaccine platforms [63]. However, a meta-analysis of animal studies indicated that these nanoparticles did not demonstrate superior efficacy compared to conventional adjuvants or antigen-alone formulations. Notably, 75% of the reviewed studies utilized gold nanoparticles as the adjuvant of choice [64].
Liposomes are bilayer structures that are primarily composed of phospholipids surrounding an aqueous core, making them highly biocompatible and, thus, an attractive candidate for vaccine delivery systems. While liposome-based vaccines have demonstrated promising results, their susceptibility to enzymatic degradation, pH fluctuations, and immune system interference limits their stability and effectiveness compared to LNPs [18]. Another study evaluated a Plasmodium falciparum full-length recombinant Circumsporozoite (rCSP) vaccine that was combined with glucopyranosyl lipid A–liposome Quillaja saponaria 21 formulation (GLA-LSQ), a nanoliposomal adjuvant, reporting that it demonstrated a favorable safety profile with no SAEs. However, the vaccine failed to induce protective immunity. Notably, higher doses, with 60 µg as the highest dose, elicited increased IgG titers, indicating the potential for dose optimization and the need for further investigation to enhance immunogenicity [65]. Another study investigated a Chlamydia trachomatis vaccine combining a recombinant major outer membrane protein (CTH522) with cationic liposomal adjuvants, CAF01 and CAF09b, comparing these formulations against each other and a placebo group. Intramuscular (IM), intradermal, and topical delivery methods were also evaluated. No SAEs were reported. IgG anti-CTH522 titers were highest in the 85 µg group, compared to the 15 µg group. However, no significant differences were observed between the 85 µg IM group, which received booster doses on days 28 and 112 with mucosal recall boosters at day 140, with either a placebo or topical vaccine using CAF01 or CAF09b. Intradermal CTH522 delivery induced robust neutralizing antibody responses against serovars B (trachoma) and D (urogenital infections) [66].
Additionally, topical ocular administration of the vaccine resulted in elevated IgA titers compared to baseline. Cell-mediated immune responses were detected across all active treatment groups [66]. VLPs are self-assembling structures composed of one or more viral proteins, organized symmetrically within a diameter range of 20 to 120 nm, mimicking the morphology of native virus particles. Their intrinsic adaptability to genetic and chemical modifications positions them as highly versatile antigen delivery platforms that are capable of incorporating multiple antigenic epitopes. This multi-epitope presentation enhances their potential for the broad-spectrum detection of diverse viral agents within a single assay format, offering a promising approach for multiplex vaccine applications [67]. In a recent study, the full-length SARS-CoV-2 spike protein was successfully expressed in Nicotiana benthamiana, purified, and subsequently formulated with the AS03 adjuvant. Healthy participants were randomized into nine groups, receiving CoVPL at doses of 3.75, 7.5, or 15 µg, combined with CpG 1018 or AS03, or administered without an adjuvant. Immunogenicity assessments from day 1 to day 42 demonstrated that AS03-adjuvanted formulations across all CoVPL doses elicited significantly higher and more durable anti-spike IgG and live virus-neutralizing antibody (nAb) responses, which are key indicators of humoral immunity [68]. Additionally, CoVPL used alone induced sustained IFN-γ and IL-4 responses after the second dose, whereas CoVPL with AS03 produced more robust cellular immunogenicity [68]. Long-term data collected on days 201 and 386 revealed that participants receiving CoVPL 3.75 µg + AS03 exhibited a significant decline in anti-spike IgG and live virus-neutralizing antibody titers at day 201 compared to day 42; however, these titers remained notably higher than those observed at baseline (day 21) [69].
Similarly, spike-specific IFN-γ and IL-4 responses decreased by day 201 relative to day 42 but remained elevated compared to day 21 levels. Importantly, no severe adverse events (SAEs), withdrawals, or fatalities were reported during the study, with only mild to moderate adverse events observed, alongside a single case of grade-three fatigue [69]. These findings suggest that a two-dose regimen of CoVPL + AS03, administered three weeks apart, effectively induces both cellular and humoral immunity against ancestral SARS-CoV-2 strains, supporting its potential as a viable vaccine candidate. In another study, a nanovaccine incorporating the pan-epitope peptide TBT, combined with CpG, was tested in mice to evaluate its immunogenicity against flaviviruses. The results demonstrated that bone marrow-derived DCs exhibited a higher uptake rate of TBT − CpG compared to TBT alone or TBT + CpG, leading to an increased expression of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α. Moreover, antigen-specific IgG production was observed, along with significantly elevated IL-4 (p < 0.001) and IFN-γ (p < 0.01) expression in spleen cells. Survival analysis revealed that 20% (p < 0.01) of three-day-old mice receiving TBT-CpG and challenged with DENV-2 or DENV-4 survived beyond 15 days, while a higher survival rate of 35% (p < 0.001) was recorded in one-day-old, immunized mice challenged with ZIKV (19). Notably, levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) remained within the normal range in immunized mice, suggesting the vaccine’s protective potential against Dengue virus (DENV) and Zika virus (ZIKV) infections [70].
In a first-in-human study, an enveloped virus-like particle (eVLP) vaccine targeting cytomegalovirus (CMV) was evaluated across a three-dose schedule (days 0, 56, and 168), with immunogenicity assessments being conducted on fibroblast and epithelial cell models. Participants received 0.5, 1, or 2 µg of glycoprotein B (gB) formulated with alum, 1 µg without alum, or a placebo. The most commonly reported local and systemic adverse events (AEs) were mild pain (without any associated swelling or erythema) and headache, both of which remained consistent in frequency and severity across the dosing groups. The serological analysis demonstrated that anti-CMV gB antibody titers and avidity were highest in the cohort receiving 2 µg with alum. Although antibody titers declined by 20–30% across all groups over time, the levels remained notably above baseline. Neutralizing antibody (nAb) responses demonstrated robust activity in fibroblasts, while responses in the epithelial cells were more modest, indicating the need for further optimization to enhance cross-cellular immunity [71]. Recent advancements in nanotechnology have significantly transformed vaccine development, offering innovative strategies to enhance immunogenicity, antigen stability, and targeted delivery. The studies reviewed highlight the diverse applications of nanovaccine platforms, including lipid-based nanoparticles, polymeric nanoparticles, virus-like particles, and metal-based nanoparticles, each demonstrating unique advantages in terms of antigen presentation, immune system activation, and cross-reactivity enhancement. Notably, nanovaccines facilitate both MHC I and MHC II antigen presentation, enhancing CD8+ T cell activation and supporting robust B cell-mediated antibody production. This property is particularly valuable for addressing the challenges associated with conventional peptide-based vaccines that fail to elicit strong cytotoxic responses. Furthermore, the use of nano-based adjuvants, such as CpG and CXCL13-conjugated platforms, underscores their potential to modulate immune responses toward a more balanced Th1/Th2 profile, a critical factor in effective and durable immunity. Preclinical and clinical evaluations have demonstrated promising immunogenicity and safety profiles across various infectious disease models, including Epstein–Barr virus, influenza, Mycobacterium tuberculosis, SARS-CoV-2, and Coxiella burnetii. Additionally, studies incorporating novel delivery mechanisms, such as circular RNA encoding immunomodulatory factors, suggest new avenues for the further optimization of vaccine efficacy. Despite these promising findings, several challenges remain, including the need for standardized formulations, long-term safety assessments, and large-scale production capabilities. Additionally, the immunogenic variability among different nanovaccine platforms underscores the necessity for further comparative studies to identify the most effective approaches for specific pathogens. Future research should focus on refining antigen delivery mechanisms, optimizing dose regimens, and leveraging artificial intelligence-driven models to predict immune responses and enhance vaccine design. Collectively, the evidence presented herein reinforces the transformative potential of nanovaccine platforms in advancing next-generation immunization strategies. With continued innovation and rigorous clinical validation, nano-based vaccines may redefine prophylactic and therapeutic approaches to infectious diseases, addressing global health challenges with greater precision and efficacy. Nanoparticle platforms exhibit several common functional advantages. Nanovaccines reliably boost antigen stability and ensure precise delivery to lymphoid tissues, addressing the shortcomings of traditional peptide vaccines. They support dual MHC class I and II presentation, increasing CD8+ cytotoxic and CD4+ helper T cell responses. Moreover, their pathogen-like structures and customizable surfaces facilitate multivalent antigen presentation and the direct activation of B cells. These attributes position nanoparticles as optimal frameworks for designing advanced vaccines that promote robust, durable, and broad-ranging immunity (Table 3).

Table 3.
Nanomaterials used in vaccine platforms: their properties and applications.
5. Systems Immunology: Decoding Immune Responses
The integration of computational modeling with multi-omics data, including transcriptomics and proteomics, to analyze and interpret immune system functions is referred to as systems immunology [72]. Transcriptomics is the study of all biological aspects of ribonucleic acids (RNA), including their structure, transcription, translation, and cellular functions [73]. Proteomics, on the other hand, focuses on investigating protein structures and functions, with mass spectrometry serving as a cornerstone technology [74]. Together, these disciplines form a powerful, integrated approach that enables holistic and quantitative insights into molecular interactions, facilitating the prediction of biological responses and enhancing therapeutic performance [75]. The immune response to threats involves various subsets of leukocytes, categorized into innate and adaptive immune responses. The primary distinction lies in the adaptive response’s reliance on antigen-specific receptors and direct antigen interactions with T and B cells. While immune cell dynamics and cytokine signaling are essential, this discussion focuses specifically on antigen recognition and receptor interactions, given their critical role in vaccine function. Monocytes and macrophages act as phagocytes, processing microbial antigens through proteolysis to generate peptide fragments. These fragments are then presented to T cells via their T cell receptors (TCRs), contributing to T cell activation [76].
Among APCs, dendritic cells (DCs) are the most potent. They are widely distributed across tissues, with a high concentration in secondary lymphoid organs, where they facilitate antigen recognition through MHC class I and II molecules interacting with TCRs a key step in initiating adaptive immunity. B cells, alongside atypical APCs such as mast cells, eosinophils, basophils, epithelial cells, endothelial cells, and innate lymphoid cells (ILCs), have also demonstrated antigen-presenting capabilities [76,77,78]. MHC class I interactions arise from endogenous antigen processing, where intracellular proteins undergo proteasomal degradation, triggered by IFN-γ, yielding peptide fragments that bind to MHC class I molecules. This process is essential for activating CD8+ cytotoxic T cells, enabling the targeted destruction of infected or malignant cells [76]. Interestingly, cross-presentation, where exogenous antigens are presented on MHC class I molecules, plays a pivotal role in antiviral immunity. This mechanism can bypass the traditional endogenous antigen pathway, offering a strategic advantage in terms of immune response modulation. In contrast, MHC class II interactions stem from the exogenous antigen presentation pathway. Antigens are internalized via endocytosis or phagocytosis and processed into peptide fragments. This pathway, which can also be induced by IFN-γ in the epithelial and endothelial cells of an inflammation site, leads to the activation of CD4+ T helper cells, orchestrating broader immune responses, including B cell activation and cytokine signaling. Co-stimulatory signaling, through CD28 on T cells binding to CD80 and CD86 on APCs, plays a crucial role in achieving full T cell activation. Without this interaction, T cells may enter an anergic state, rendering them unresponsive [76].
Regulatory T cells (Tregs) are essential for modulating immune responses and maintaining tolerance. They can express both CD4 and CD8 markers and are classified into two subsets: natural Tregs (nTregs) and adaptive (induced) Tregs (iTregs). nTregs develop in the thymus and are characterized by the expression of CD4, CD25, and forkhead box protein 3 (Foxp3), a transcription factor that is critical for their development and function. In contrast, iTregs differentiate from naive CD4+ T cells in the peripheral tissues upon encountering specific antigens, with their formation influenced by IL-10 concentration during the initial phase. Both subsets secrete IL-10 and TGF-β to regulate immune activity. Resting naive CD4+ T cells, or T helper (Th) cells—those that have not yet differentiated into functional subtypes—produce IL-2 and can polarize into distinct effector subsets, based on the cytokine environment at the activation site [76].
Key differentiation pathways include Th1 cells, which are driven by IL-12, express the transcription factor T-box expressed in T cells (T-bet), and produce IL-2, IFN-γ, and lymphotoxin. They are primarily involved in cellular immunity, promoting macrophage activation and cytotoxic responses. Th2 cells, induced by IL-4, express the transcription factor GATA3 and produce IL-4, IL-5, IL-6, IL-9, IL-13, and GM-CSF. They support humoral immunity, driving B cell differentiation and antibody production [76,79]. Th17 cells, promoted by IL-6 (synergistically with IL-1β [80]) and TGF-β, express retinoic acid-related orphan receptor C isoform 2 (RORC2) and produce IL-6, IL-17, and other proinflammatory cytokines. They are crucial for defense against extracellular pathogens and play a role in autoimmune responses. The Th1 and Th2 subsets represent distinct immune pathways, with Th1 driving cellular immunity and Th2 supporting humoral immunity. B cells can be activated through both T cell-dependent and T cell-independent pathways. The T cell-dependent pathway, which generates a stronger and longer-lasting memory, involves B cells acting as APCs. This leads to MHC class II, CD80, and CD86 expression. Co-stimulation between the CD40 ligand on T cells and CD40 on B cells then drives antibody class switching and somatic mutation. In the T cell-independent pathway, B cells are activated by polymeric antigens, likely through cross-linking and the clustering of immunoglobulins on the B cell surface [76].
Some studies suggest that nanoparticle delivery systems may also directly activate B cells, contributing to optimal antibody responses. Numerous studies have shown that vaccine adjuvants stimulate IL-1 family, IL-6, IL-10, IL-12, and TNF-α cytokines upon reaching the injection site by activating APCs [35,80]. IL-1α, produced by endothelial cells, epithelial cells, monocytes, macrophages, DCs, and B lymphocytes [81], and IL-1β, produced by monocytes, macrophages, and DCs [81], are potent activators of conventional DCs and Langerhans cells (LCs), which are specialized phagocytes in the epidermis. LCs can promote naïve CD4+ T cell polarization into Th2 and Th17 subsets, alongside the priming and cross-priming of CD8+ cytotoxic T cells. Additionally, IL-1α enhances monocyte-derived DCs, boosting IFN-γ and IL-13 production, while IL-1β supports CD40L-mediated DC activation, leading to increased IFN-γ production in T cells and IL-12 secretion. Moreover, IL-1β directly boosts antigen-specific CD8+ T cell function, enhancing granzyme B and IFN-γ expression, as well as improving their migration and survival. IL-18, produced by blood monocytes, intestinal epithelial cells, and human LCs, promotes both conventional and plasmacytoid DC, which are specialized producers of type I interferons (IFN-I) and key mediators of antiviral immunity, in terms of migration to the lymph nodes. In synergy with IL-12 and IL-1β, IL-18 plays a key role in Th1 polarization and further upregulates IFN-γ expression [80]. IL-6 influences monocyte differentiation, promoting macrophage development in the presence of macrophage colony-stimulating factor (M-CSF) at high concentrations, while encouraging DC generation under high granulocyte-macrophage colony-stimulating factor (GM-CSF) and TNF-α conditions. It plays a pivotal role in DC maturation, indirectly supporting maturation by synergizing with IFN-γ or by counteracting TGF-β’s inhibitory effects, while also directly inhibiting maturation [82].
Furthermore, IL-6 supports DC migration to the lymph nodes or skin, suppresses regulatory T cell (Treg) activity, and promotes CD4+ T cell expansion in non-tumor environments [82]. It also stimulates B cells, enhancing IgG production. IL-4 and IL-21 are key factors influencing memory B cell formation, with IL-21, produced by T follicular helper cells, specifically promoting germinal center memory. Meanwhile, IL-15 and IL-7 play pivotal roles in memory T cell development, with IL-7 making a lesser but notable contribution to central memory T cell formation. Although these cytokines play major roles in memory formation, they are not the only factors involved in this complex process. IL-7 is produced by fibroblastic reticular cells, thymic epithelial cells, bone marrow stromal cells, and lymphatic endothelial cells within both the primary and secondary lymphoid organs. IL-15 is produced by APCs, with its production significantly upregulated in response to IFN-I [83]. Building on the foundation of systems immunology and multi-omics analysis, recent advances in synthetic biology and nanotechnology are reshaping vaccine design and delivery. Nanoparticles such as LNPs, VLPs, poly (lactide-co-glycolide) (PLGA), and caged protein nanoparticles facilitate MHC class antigen presentation by mimicking antigen size and spatial conformation. This structural mimicry, coupled with their ability to target specific dendritic cell (DC) receptors, optimizes antigen uptake, processing, and subsequent T cell activation [35].
Functionalizing NP surfaces with specific ligands enables targeted delivery to distinct APC subsets. For example, coupling NPs with anti-CD169 antibodies facilitates macrophage targeting, while CLEC9A antibodies direct NPs to conventional type I DCs. Coupling CLEC9A antibodies with NPs targets conventional type I DCs. Additionally, NPs sized between 50 and 100 nm preferentially accumulate in follicular dendritic cells, promoting durable, high-affinity humoral responses—a process further enhanced by increasing NP antigenic valence and glycosylation. Multivalent NP designs can also engage and activate B cells directly, supporting robust antibody production. Moreover, NPs can be engineered to facilitate cross-presentation, such as through membrane fusion mechanisms that enable antigen escape from endosomes, enhancing CD8+ T cell responses [35]. Integrating machine learning (ML) predictive models with multi-omics data enhances our understanding of the role of NPs in antigen presentation and APC targeting, supporting vaccine optimization before in vivo trials. In a 2023 study, Shuaib and colleagues analyzed nasopharyngeal swab samples from COVID-19 patients, dividing them into two cohorts based on the presence or absence of the R203K and G204R (KR) mutations in the SARS-CoV-2 nucleocapsid, with healthy individuals as controls [84].
The goal was to determine whether these mutations drive a stronger inflammatory response. The impact of the KR mutation on immune responses was further examined through transcriptomic and proteomic analyses of cells incubated with VLPs expressing KR nucleocapsids. Results showed that KR nucleocapsids alone triggered a significantly higher expression of immune and inflammatory response genes, including cytokines and interferon-stimulated genes (ISGs), and upregulated immune response proteins like ISG15/20, IFIT1/2/3/5/M3, IFI16/44, OAS3, and MX1 from the ISG family, alongside STAT1/2, IRF6, and IRF7 from the interferon-regulated group [84]. Additionally, KR mutations induced elevated cytokine levels (IFN-γ, IL-8, IL-6, and IL-1) and increased the neutrophil-to-lymphocyte ratio—a marker linked to COVID-19 severity [84,85]. These findings provide insight into COVID-19 pathogenesis and could inform future vaccine design. In 2022, Wang and colleagues accelerated mRNA vaccine optimization using an ML-driven approach for lipid LNP delivery systems. They analyzed 325 mRNA vaccine LNP formulations alongside the corresponding IgG titers. Remarkably, the algorithm identified key ionizable lipid substructures. Animal experiments confirmed that LNPs incorporating DLin-MC3-DMA (MC3) as the ionizable lipid induced higher efficiency in mice, aligning with the model predictions. This marked the first experimentally validated predictive model, later refined through integration with molecular modeling [86,87,88].
In 2024, Maharjan and colleagues conducted a study focusing on optimizing microfluidic conditions and lipid mix ratios for messenger RNA-lipid nanoparticles (mRNA-LNPs), although immunogenicity was not assessed. They evaluated key parameters, including particle size (PS), polydispersity index (PDI), zeta potential, pKa, heat trend cycle, encapsulation efficiency (EE), recovery ratio, and encapsulated mRNA. The results demonstrated that a self-validated ensemble model outperformed XGBoost and Bayesian optimization, achieving prediction accuracies of over 97% and 94%, respectively, while also aligning more closely with the actual experimental data [89]. In a study in 2024 by Suyash and colleagues on designing a multi-epitope vaccine for Marburg virus (MARV), they first identified immunogenic proteins from seven key MARV proteins that were capable of triggering B and T cell responses. Computational analysis confirmed the non-allergenic nature and strong antigenicity of the selected proteins. VLPs were then optimized using machine learning, although no experimental validation was performed. The in silico results provided promising leads for future in vitro and in vivo studies [90].
Similarly, in 2024, Prosper and colleagues conducted an in silico study focusing on optimizing VLP vaccine protein components (chimVLP) for SARS-CoV-2. Molecular dynamics analysis (proteomics) compared mutated and wild-type VLP proteins. Their findings indicated that while the mutant virus proteins showed slight allergenicity (except for VP2), they remained non-toxic, with an antigenicity predictive value of 0.55, according to the VaxiJen v.2.0 server [91]. An in silico study conducted in 2024 by Garmeh Motlagh and colleagues evaluated the receptor binding domain (RBD) of a SARS-CoV-2 monomeric spike protein combined with a ferritin-based nanoparticle, using molecular docking, molecular dynamics simulations, and immune simulations [92]. The study employed the root mean squared deviation (RMSD), root mean squared fluctuations (RMSF), principal component analysis (PCA), the cross-correlation matrices of the Cα atoms, solvent-accessible surface area (SASA), and the C-IMMSIM server to comprehensively assess structural stability, flexibility, and immunogenicity. The results demonstrated the robust stability and structural integrity of the nanoparticle complex, alongside a notable rise in B cell, CD4+, and CD8+ T cell populations following each dose. Moreover, elevated levels of IgG, IgM, IFN-γ, and IL-2 were observed after each immunization. Despite these promising immunogenic responses, the durability of the immune response remained limited when tested in a three-dose regimen with 30-day intervals over a two-month period. This suggests that further optimization—including structural refinement and in vivo experimentation—is essential to validate and enhance the platform’s performance [92].
As mentioned earlier, Mayer and colleagues conducted a study in 2022, developing the first mRNA vaccine targeting an intracellular bacterium, Listeria monocytogenes. They employed proteomics, specifically immunopeptidomics, to identify the surface antigens presenting on infected cells, guiding the design of an mRNA vaccine delivered via a lipid nanoparticle platform. The results highlighted LMON_0149 as a promising vaccine target. Mice that were vaccinated with this formulation demonstrated detectable antigen-specific IFN-γ responses—a key indicator of CD8+ T cell activation—alongside significant protection in both the liver and spleen [61]. These computational approaches pave the way for further model development, potentially correlating with the immunogenicity of pathogens such as influenza, CMV, Plasmodium falciparum, and Chlamydia trachomatis (Table 4).

Table 4.
Systems immunology tools and their contributions to vaccine design.
The reviewed studies demonstrate a consistent shift toward using computational tools to enhance predictive and personalized vaccine design. Although the methodologies differ, ranging from transcriptomics to machine learning-guided lipid nanoparticle optimization and in silico epitope mapping, they share a unified aim to streamline and improve antigen selection, delivery mechanisms, and immune profiling. These advancements cover the way for rationally designed platforms that can be customized for specific pathogens, immune environments, and even individual patient genotypes, signaling a transformative move from traditional experimental approaches to systems-driven vaccine engineering.
6. Synergistic Integration of the Three Pillars
Based on different research studies, synthetic nanoparticles in immunotherapy and vaccinations will greatly affect public health. Antibiotic resistance is one of the main global issues nowadays, which has promoted new diagnostic techniques, as well as alternative therapeutic and preventative strategies. The successful treatment of infectious diseases depends on early detection, and recently, developed applications based on nanotechnology have provided a more sensitive and effective diagnostic model [93]. In the past three years, nucleoside-modified mRNA-lipid nanoparticle (mRNA-LNP) vaccines have been developed against the SARS-CoV-2 Wuhan-Hu-1 strain to stimulate humoral and cellular immunity. Although rare, there are reports of adverse effects from COVID-19 mRNA vaccines, such as acute myocardial infarction, Bell’s palsy, cerebral venous sinus thrombosis, stroke, and thrombosis with thrombocytopenia syndrome. Codon optimization plays a crucial role in the antigen design of mRNA therapeutics and vaccines through multiple aspects of the target protein expression: mRNA abundance, mRNA stability, translational efficiency, and correct protein folding [94].
Synonymous codons and their cognate tRNAs occur at different frequencies across proteins, their domains, and the organisms from which they originate [95,96]. A previous study by Chih-Jen Lai et al. showed that spike protein codon optimization using Herpes Simplex Virus-1 (HSV) codons, instead of human codons, increased immunity. This optimization resulted in higher levels of spike-specific antibodies, neutralizing antibodies (NAbs), and better protective immunity against lethal SARS-CoV-2 infection. Additionally, the HSV codon-optimized mRNA vaccines for the Delta and Omicron variants showed improved efficacy compared to human codon-optimized vaccines. The results suggest that using viral codon optimization platforms could lead to the development of mRNA therapeutics with lower dosages and fewer side effects [97]. A study by Bapurao Surnar et al. showed that IVM (ivermectin) can accumulate in the circulation at a greater concentration when administered via synthetic NPs, and the initial findings indicated that NP-delivered IVM can specifically target the Zika virus (ZIKV) nonstructural 1 protein. The long-term storable formulation of IVM-nanoparticles in a dry powder state for inclusion in capsule form and as a cryoprotectant, including frozen forms, showed encouraging results with possible clinical significance. Additionally, the initial in vitro research showed that ivermectin crosses the placental barrier, making it dangerous for pregnant ZIKV patients. In contrast, the ivermectin-loaded nanoparticle showed no discernible placental barrier-crossing behavior, suggesting that it might be appropriate for this population [98].
Based on a case study by Peter G. Kremsner et al., conducted on 245 adults aged 18 to 60 years old who were divided into vaccine injection and placebo injection groups, the research showed that two doses of the CVnCoV vaccine had acceptable reactogenicity and were safe. Immune responses at levels similar to those seen in recovered antibodies from COVID-19 patients were induced by a dosage of 12 μg [99]. In recent years, nanotechnology has developed quickly in the field of healthcare research, and nanovaccines hold great promise for resolving the issues seen with traditional peptide-based vaccines, as follows. First, APC phagocytosis and lymph node (LN) retention can affect nanoparticles. Second, the administration of antigens and adjuvants by structured nanovaccines promotes strong antigen presentation activation. Third, the pharmacokinetic characteristics of encapsulated antigens and adjuvants, such as prolonging drug circulation time and delaying drug breakdown, may be markedly enhanced by nanotechnology [96]. With basic chemical synthesis techniques, a study created a novel self-assembly vehicle-free multi-component antitumor nano-vaccine (SVMAV) that stimulated strong antitumor immune responses with fewer harmful side effects. The SVMAV is made up of antigen peptides, R848, and static. Following subcutaneous injection, the SVMAV homed in on LNs and was caught by the LN-residing DCs, which, in turn, stimulated the CD8+ T cells, facilitated DC maturation and antigen cross-presentation, and ultimately began the targeted destruction of tumor cells. Additionally, this method could be used to administer customized cancer vaccinations. Considering all these factors, our research presents a compelling and feasible strategy for enhancing the therapeutic efficacy of vaccine-based cancer immunotherapy through the application of nanotechnology. A self-assembly nanovaccine, containing the TLR7/8 agonist and STAT3 inhibitor, enhances tumor immunotherapy by augmenting the tumor-specific immune response. Tumor cells, however, have detrimental impacts on immune activities in general, including the release of metabolic products, immunosuppressive cytokines, and ligands for immunological checkpoint receptors. Furthermore, neo-antigen-specific cytotoxic T lymphocytes (CTLs) are inherently uncommon in a variety of cancer types. Neoantigen-specific CTLs, for instance, make up less than 0.001% of the peripheral T cell population in individuals with colorectal cancer [100,101].
The vaccine-induced activation of tumor-specific immune responses is a highly desirable approach to address these issues. Personalized tumor neoantigens can now be quickly identified, thanks to recent developments in next-generation sequencing (NGS) and analytics, especially for tumors with modest mutation burdens or with no shared neoantigens [102]. For the time being, there are two widely used COVID-19 vaccines in the world, which are mRNA-based and encapsulated in lipid nanoparticles: mRNA-12734 [103] and BNT162b25 [104], from Moderna and Pfizer-BioNTech’s lipid-based nanoparticle vaccine. Compared to conventional vaccines, these showed high efficiency (above 94%), which established their combat potential for future SARS-CoV-2 variants [105,106]. In contrast, there are nanoparticle-based DNA vaccines, which, compared to mRNA vaccines, are more cost-effective, easy to generate, and perform with greater stability. Human anti-tumor immunity efficiency is related to T cells, which attack cancer-specific neoantigens. Tumor cells produce these specific protein markers, which do not exist in normal tissues. Therefore, they trigger a strong immune response through the avoidance of natural body tolerance mechanisms [107,108]. According to a study by Patrick A. Ott et al., even in the absence of previous or current treatments, a personalized neoantigen vaccination is effective, safe, and capable of eliciting robust T cell responses in cancer patients. This can move beyond the two main challenges in cancer treatment: tumor incompatibility and targeting tumor tissue versus healthy tissue. It has been demonstrated that the vaccine causes the production of new T cell clones that are able to identify processed antigens along with the patient’s own tumor cells, as well as several patient-specific neoantigens. This indicates that a range of malignant clones may be targeted by the vaccination, addressing tumor diversity and reducing the possibility of tumor escape as a result of antigen loss [109].
The development of vaccines is being entirely altered by the use of artificial intelligence (AI), which is bringing about notable breakthroughs at every level from discovery to dissemination. Through antigen identification, immune response prediction, and vaccine design optimization, AI speeds up the vaccine discovery process. By facilitating real-time data monitoring and enhancing participant enrollment, AI also improves clinical trials. AI enhances process efficiency, equipment maintenance, and quality control in vaccine manufacturing. It also streamlines logistics and cold-chain management for distribution, guaranteeing a quicker and more equitable vaccine supply. Notwithstanding these developments, issues like data integration, model transparency, and moral and legal dilemmas still exist. Personalized vaccines and enhancing global health equity will be the main topics of future research. Working together, AI specialists, vaccine creators, and medical institutions can improve global health outcomes and expedite illness response [110]. A comprehensive review of next-generation vaccine innovations is shown in Figure 3 and Table 5.
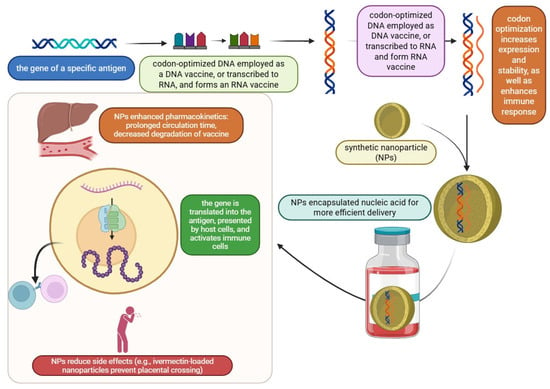
Figure 3.
Three-pillar strategy for next-generation vaccines: nanoparticles, codon optimization, and mRNA/DNA technologies.

Table 5.
Selected clinical trials and approved vaccines based on next-generation platforms.

Table 7.
Comparative analysis of the various vaccine platform strategies.

Table 6.
Synergistic integration of synthetic biology, nanotechnology, and systems immunology in next-generation vaccines.
Table 5.
Selected clinical trials and approved vaccines based on next-generation platforms.
| Platform Type | Product/Trial Name | Technology Used | Target Disease | Clinical Phase/Status |
|---|---|---|---|---|
| mRNA-based (LNP) | BNT162b2 (Pfizer–BioNTech) | Synthetic mRNA + lipid nanoparticles | COVID-19 | Approved (EMA, FDA) |
| saRNA | ARCT-154 (Arcturus) | Self-amplifying RNA + LNP | COVID-19 | Phase 3 |
| DNA Vaccine | INO-4800 | Synthetic plasmid DNA | COVID-19 | Phase 3 |
| Nanoparticle Protein-based | NVX-CoV2373 (Novavax) | Recombinant protein + Matrix-M (saponin NP) | COVID-19 | Approved (e.g., WHO, EU) |
| VLP-based | Mosquirix (RTS,S/AS01) | Hepatitis B-based VLP + AS01 adjuvant | Malaria | Approved (WHO, 2021) |
| Personalized Neoantigen | mRNA-4157/V940 + Keytruda | mRNA encoding patient-specific tumor neoantigens | Melanoma (Cancer) | Phase 2 (Positive results) |
7. Integrated Conceptual Framework: Synergistic Interaction of Synthetic Biology, Nanotechnology, and Systems Immunology
Synthetic biology, nanotechnology, and systems immunology each bring distinctive strengths to the development of next-generation vaccines. When integrated, they create a synergistic framework that amplifies immunogenicity through interconnected molecular and cellular processes. On the molecular front, synthetic biology facilitates the programmable design of antigens, whether mRNA, DNA, or peptides, utilizing advancements like codon optimization, epitope mapping, and self-amplifying RNA systems. These engineered antigens are delivered using nanocarriers such as LNPs, VLPs, or polymer-based systems. These carriers stabilize the antigens, protect them from degradation, and ensure efficient delivery to APCs. Many nanocarriers also function as adjuvants by supporting depot formation, promoting membrane fusion, or directly activating PRRs. At the cellular level, this delivery system ensures efficient antigen processing and presentation through both MHC class I and II pathways, strengthening the CD4+ and CD8+ T cell responses. Systems immunology provides an additional layer by decoding the host immune response through multi-omics approaches (transcriptomics, proteomics, and epigenetics). This enables iterative vaccine optimization by identifying the ideal antigens, modifying immune responses (e.g., Th1 vs. Th2), and personalizing regimens according to individual immune profiles. Together, these disciplines form a dynamic feedback loop: synthetic biology can design optimized antigens, nanotechnology facilitates precise delivery, and systems immunology analyzes the immune outcomes to guide further modifications. This integrated approach supports precision immunoprophylaxis, resulting in vaccines that are safer, more effective, and adaptable to emerging pathogens or unique immune landscapes [114,115] (Table 8).

Table 8.
Case studies of next-generation vaccine platforms across diverse pathogen classes and disease contexts.
8. Challenges and Ethical Considerations
The broad field of nanomedicine makes use of nanotechnology for therapeutic purposes. It entails the creation, advancement, and use of nanoscale tools and materials for illness detection, management, and prevention. Additionally, it covers a broad spectrum of uses, such as medicinal substances, diagnostic instruments, and drug delivery systems [116]. The safety assessment of nanomaterials and nanodevices is one of the main regulatory concerns in nanomedicine. Because of their small size, nanomaterials have unique physicochemical properties that can lead to different biological interactions from those with conventional materials. Regulatory agencies, including the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA), have been working to establish guidelines for evaluating the safety of and conducting risk assessments of nanomedicine products. These guidelines center on the characterization, toxicity, and environmental impact of nanomaterials, as well as the requirement for appropriate preclinical and clinical studies to evaluate their safety and efficacy [117].
Public health plays a critical role in the implementation of nanotechnology, particularly in the context of medicine. Nanomedicine, due to its direct application in healthcare, faces heightened public scrutiny. Avoiding negative public perception influences the acceptance of nanomedicine significantly [118]. People’s fear of death and their concern over medical conditions result in less difficulty regarding the acceptance of novelty in this area. However, any accident related to nanomedicine may cause widespread distrust in the field [119].
A major regulatory challenge in nanomaterials is the lack of standardized characterization techniques, due to their diverse properties. Developing and adopting standardized protocols for assessing key attributes like physicochemical properties, their stability, and potential aggregation or transformation is essential. These methods will improve the accuracy of any safety and efficacy evaluations and streamline regulatory approval for nanomedicine products [120]. The absence of proper classification and regulatory processes specific to these products is another significant regulatory hurdle in nanomedicine. The special qualities and complexity of nanomedicines are frequently not adequately addressed by conventional frameworks. Regulatory bodies are creating certain guidelines in response. The FDA, for instance, has released guideline documents that list crucial factors to take into account when assessing the efficacy and safety of applications that include nanomedicine [121]. Although VLP-based vaccines have the potential to protect against diseases such as the Zika virus, HCV, HBV, and HPV, their commercialization is hindered by a number of issues. Researchers are enhancing formulations to navigate these obstacles by refining adjuvants, stabilizers, administration routes, and delivery systems (such as liposomes and microparticles) in order to lower dosage and prolong shelf life without the need for cold chains. Additionally, efforts are being made to develop more reasonably priced vaccinations for LMICs, or low- and middle-income nations. However, prior experiences, like the delayed availability of HPV vaccines because of their high initial costs, make financial barriers a crucial problem that needs to be resolved in order to guarantee fair distribution around the world [122].
8.1. Regulatory Challenges
The intersection of synthetic biology, nanotechnology, and systems immunology has highlighted regulatory uncertainty, as existing frameworks were established primarily with traditional vaccines in mind. Agencies such as the FDA and EMA lack standardized pathways that are tailored to evaluating highly modular or self-amplifying constructs. For instance, the development of mRNA vaccines necessitated emergency use authorizations (EUAs), given the absence of prior precedent; their approval relied heavily on accelerated clinical trials, bridging data from similar platforms, and real-time audits of the production process. In addition, platforms integrating novel delivery methods such as LNPs or VLPs often encounter delays, due to the extensive testing required for toxicity, biodistribution, and adjuvant compatibility. Regulatory approaches must evolve to address the unique challenges posed by these technologies. Core questions include how synthetic components interact with host immune systems, the potential long-term risks associated with engineered vectors, and the implications of in silico-driven vaccine personalization on population-wide efficacy and equity [123].
8.2. Manufacturing Scalability and Complexity
Manufacturing next-generation vaccine platforms presents significant hurdles, due to intricate supply chains and scaling difficulties. Self-amplifying RNA (saRNA) vaccines, for example, demand precise enzymatic transcription and purification steps, while VLPs rely on expression in advanced systems like insect or mammalian cells, requiring stringent glycosylation control. Nanoparticle formulation, particularly for cold chain-reliant LNPs, adds another layer of complexity to production logistics. Globally scaling these technologies remains challenging because of the limited availability of standardized GMP-compatible microfluidic production systems and the high cost of essential materials such as ionizable lipids and synthetic polymers. Unlike conventional recombinant protein vaccines, these platforms also necessitate multi-step quality control processes to ensure consistency in batch production, antigen stability, and immune response performance [124].
8.3. Cost Implications and Global Access
Despite their remarkable potential, modular vaccine platforms come with substantial up-front research, development, and infrastructure costs. The cost-per-dose of mRNA- and nano-enabled vaccines is significantly higher than that of traditional inactivated or protein subunit vaccines, limiting their widespread adoption in low- and middle-income countries (LMICs). Furthermore, personalized or stratified vaccine approaches that are informed by systems immunology may not be feasible on a global scale without affordable and scalable diagnostic systems [125].
8.4. Case Studies on Regulatory Approval
The COVID-19 pandemic offered valuable insights into regulatory processes. The authorization of Pfizer-BioNTech’s and Moderna’s mRNA vaccines required unprecedented regulatory coordination through rolling reviews and post-marketing measures to monitor rare adverse events. Similarly, the RTS,S/AS01 malaria vaccine—the first to utilize the AS01 adjuvant system (a combination of liposomes, MPL, and QS-21)—required over a decade of data collection to satisfy the safety and efficacy benchmarks, primarily due to its novel adjuvant component. These case studies underscore the critical importance of engaging with regulators early in the development process, maintaining platform master files for efficient evaluation, and establishing robust pharmacovigilance systems. Moving forward, vaccine developers must strike a balance between innovation and adherence to the existing regulatory frameworks to enable timely, equitable access to these advanced technologies [126].
9. Conclusions
The landscape of vaccinology is being reshaped by the integration of synthetic biology, nanotechnology, and systems immunology. In contrast to traditional methods, next-generation platforms enable precise design, efficient delivery, and personalized immune engagement. mRNA and DNA vaccines, which have been improved through codon optimization and lipid nanoparticle delivery, have shown rapid development and high immunogenicity, as evidenced during the COVID-19 pandemic. Nanoparticle-based systems not only protect antigens but actively enhance antigen presentation via the MHC I and II pathways, leading to better T and B cell responses. Additionally, systems immunology uses multi-omics data and AI-driven modeling to unravel the intricacies of immune reactions, aiding in the rational design of vaccines. These technologies collectively offer solutions to challenges such as immunogenicity, delivery challenges, and antigen instability. Despite problems such as regulatory standards and equitable access, the potential of these platforms is clear. They indicate a significant change toward vaccines that are quicker to develop, safer, adaptable, and more efficient for combating infectious diseases and cancer.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis, R.N. and B.F.D.; the first draft of the manuscript, B.F.D. and R.N.; writing—original draft preparation, A.H., S.K., S.Y. and M.E.; software, S.Y.; data curation and writing—review and editing, M.E. and V.O.; supervision and project administration, M.E. and V.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The corresponding author will provide the datasets created during and/or analyzed during the current investigation upon reasonable request.
Acknowledgments
Cancer Research Center, Semnan University of Medical Sciences, Semnan, Iran.
Conflicts of Interest
The authors declare that they have no competing interests.
Glossary of Key Technical Terms
| Term | Definition |
| Codon Optimization | The modification of gene codons to improve the efficiency of protein expression in the target host organism. |
| Virus-like Particles (VLPs) | VLPs are structures that resemble viruses but lack genetic material, making them a safe and effective tool for triggering immune responses to vaccines. |
| Self-amplifying RNA (saRNA) | A synthetic RNA platform that replicates within host cells, producing higher antigen levels from lower vaccine doses. |
| Pattern Recognition Receptors (PRRs) | Cellular receptors that recognize pathogen-associated molecular patterns (PAMPs) to trigger immune responses. |
| Multi-omics | An integrative approach combining datasets from various “omics” fields, such as genomics, transcriptomics, and proteomics. |
| Codon Usage Bias | The preferential usage of certain synonymous codons over others in a gene, which can affect protein translation efficiency. |
| Nanovaccine | A vaccine platform that utilizes nanoparticles to deliver antigens directly and efficiently to immune cells. |
| Adjuvant | A substance added to vaccines to enhance the body’s immune response to the presented antigen. |
| Transcriptomics | The comprehensive analysis of all RNA transcripts in a cell or tissue used to study gene activity and regulation. |
| Immunopeptidomics | A proteomics subfield that is focused on identifying the peptides presented by MHC molecules to inform precise vaccine design. |
References
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Tahamtan, A.; Charostad, J.; Hoseini Shokouh, S.J.; Barati, M. An overview of history, evolution, and manufacturing of various generations of vaccines. J. Arch. Mil. Med. 2017, 5, e12315. [Google Scholar] [CrossRef]
- Levine, M.M.; Lagos, R. Vaccines and vaccination in historical perspective. In New Generation Vaccines; CRC Press: Boca Raton, FL, USA, 2016; pp. 29–39. [Google Scholar]
- Sandle, T. The Manufacture and Quality Control of Immunological Products. In Hugo and Russell’s Pharmaceutical Microbiology; John Wiley & Sons Ltd.: London, UK, 2023. [Google Scholar]
- Kozak, M.; Hu, J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef]
- Méthot, P.-O. Bacterial transformation and the origins of epidemics in the interwar period: The epidemiological significance of Fred Griffith’s “transforming experiment”. J. Hist. Biol. 2016, 49, 311–358. [Google Scholar] [CrossRef]
- Hicks, D.; Fooks, A.; Johnson, N. Developments in rabies vaccines. Clin. Exp. Immunol. 2012, 169, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Bröker, M.; Berti, F.; Schneider, J.; Vojtek, I. Polysaccharide conjugate vaccine protein carriers as a “neglected valency”–potential and limitations. Vaccine 2017, 35, 3286–3294. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef]
- Eslami, M.; Arjmand, N.; Mahmoudian, F.; Babaeizad, A.; Tahmasebi, H.; Fattahi, F.; Oksenych, V. Deciphering Host–Virus Interactions and Advancing Therapeutics for Chronic Viral Infection. Viruses 2025, 17, 390. [Google Scholar] [CrossRef]
- Romano’, L.; Zanetti, A.R. Hepatitis B vaccination: A historical overview with a focus on the Italian achievements. Viruses 2022, 14, 1515. [Google Scholar] [CrossRef]
- Hudu, S.A.; Shinkafi, S.H.; Umar, S. An overview of recombinant vaccine technology, adjuvants and vaccine delivery methods. Int. J. Pharm. Pharm. Sci. 2016, 8, 19–24. [Google Scholar] [CrossRef]
- Harper, D.M.; DeMars, L.R. HPV vaccines–a review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Anwari, P. Benefit-Risk and Cost-Utility of Rotavirus Vaccination in Afghanistan: A Modelling Study Informed by Post-Marketing Surveillance Data. Ph.D. Thesis, London School of Hygiene & Tropical Medicine, London, UK, 2025. [Google Scholar]
- Alizon, S.; Michalakis, Y. Adaptive virulence evolution: The good old fitness-based approach. Trends Ecol. Evol. 2015, 30, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.A.C.; Clemens, R. The need and challenges for development of vaccines against emerging infectious diseases. J. Pediatria 2023, 99, S37–S45. [Google Scholar] [CrossRef]
- Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials 2023, 13, 1828. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Ellis, D.; King, N.P. New Vaccine Design and Delivery Technologies. J. Infect. Dis. 2019, 219, S88–S96. [Google Scholar] [CrossRef]
- Zhang, R.; Ulery, B. Synthetic Vaccine Characterization and Design. J. Bionanosci. 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Jones, L.H. Recent advances in the molecular design of synthetic vaccines. Nat. Chem. 2015, 7, 952–960. [Google Scholar] [CrossRef]
- Gary, E.N.; Weiner, D.B. DNA vaccines: Prime time is now. Curr. Opin. Immunol. 2020, 65, 21–27. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kwon, M.; Im, S.; Lee, K.; Lee, H. mRNA vaccines: The most recent clinical applications of synthetic mRNA. Arch. Pharm. Res. 2022, 45, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Saade, F.; Petrovsky, N. Technologies for enhanced efficacy of DNA vaccines. Expert. Rev. Vaccines 2012, 11, 189–209. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Han, J.H.; Choi, Y.S.; Kim, W.J.; Jeon, Y.H.; Lee, S.K.; Lee, B.J.; Ryu, K.S. Codon optimization enhances protein expression of human peptide deformylase in E. coli. Protein Expr. Purif. 2010, 70, 224–230. [Google Scholar] [CrossRef]
- Liu, B.; Kong, Q.; Zhang, D.; Yan, L. Codon optimization significantly enhanced the expression of human 37-kDa iLRP in Escherichia coli. 3 Biotech 2018, 8, 210. [Google Scholar] [CrossRef]
- Hyatt, D.; LoCascio, P.F.; Hauser, L.J.; Uberbacher, E.C. Gene and translation initiation site prediction in metagenomic sequences. Bioinformatics 2012, 28, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Schlub, T.E.; Buchmann, J.P.; Holmes, E.C. A Simple Method to Detect Candidate Overlapping Genes in Viruses Using Single Genome Sequences. Mol. Biol. Evol. 2018, 35, 2572–2581. [Google Scholar] [CrossRef]
- Codagenix, Inc. Codagenix Announces the Synthesis and Preliminary Safety of Scalable Live-Attenuated Vaccine Candidate Against COVID-19. Available online: https://www.prnewswire.com/news-releases/codagenix-announces-the-synthesis-and-preliminary-safety-of-scalable-live-attenuated-vaccine-candidate-against-covid-19-301079306.html (accessed on 18 June 2020).
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, R.; Pezzicoli, A.; Cioncada, R.; Malzone, C.; De Gregorio, E.; D’Oro, U.; Piccioli, D. Vaccine adjuvant MF59 promotes retention of unprocessed antigen in lymph node macrophage compartments and follicular dendritic cells. J. Immunol. 2015, 194, 1717–1725. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Omer, S.B. Why and How Vaccines Work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2003, 2, 727–735. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Schmidt, C.; Schnierle, B.S. Self-Amplifying RNA Vaccine Candidates: Alternative Platforms for mRNA Vaccine Development. Pathogens 2023, 12, 138. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Yang, J.; Han, Z.; Chen, L.; Jiang, W.; Zhou, H.; Li, T.; Tang, Z.; Deng, J.; et al. Deep Generative Optimization of mRNA Codon Sequences for Enhanced Protein Production and Therapeutic Efficacy. bioRxiv 2024. [Google Scholar] [CrossRef]
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef]
- Andrade, V.M.; Christensen-Quick, A.; Agnes, J.; Tur, J.; Reed, C.; Kalia, R.; Marrero, I.; Elwood, D.; Schultheis, K.; Purwar, M.; et al. INO-4800 DNA vaccine induces neutralizing antibodies and T cell activity against global SARS-CoV-2 variants. NPJ Vaccines 2021, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, E.B.; Duroux, L. Mineral Adjuvants. In Immunopotentiators in Modern Vaccines; Academic Press: New York, NY, USA, 2017; pp. 347–375. [Google Scholar] [CrossRef]
- Hershberg, R.; Petrov, D.A. Selection on codon bias. Annu. Rev. Genet. 2008, 42, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Tews, B.A.; Meyers, G. Self-Replicating RNA. Methods Mol. Biol. 2017, 1499, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef]
- Farooq, M.A.; Johnston, A.P.; Trevaskis, N.L. Impact of Nanoparticle Properties on Immune Cell Interactions in the Lymph Node. Acta Biomater. 2024, 193, 65–82. [Google Scholar] [CrossRef]
- Perez-Potti, A.; Rodríguez-Pérez, M.; Polo, E.; Pelaz, B.; Del Pino, P. Nanoparticle-based immunotherapeutics: From the properties of nanocores to the differential effects of administration routes. Adv. Drug Deliv. Rev. 2023, 197, 114829. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Chen, Y.; Huang, K. Nano-based approaches in the development of antiviral agents and vaccines. Life Sci. 2021, 265, 118761. [Google Scholar] [CrossRef]
- Curley, S.M.; Putnam, D. Biological Nanoparticles in Vaccine Development. Front. Bioeng. Biotechnol. 2022, 10, 867119. [Google Scholar] [CrossRef]
- Sun, C.; Kang, Y.-F.; Fang, X.-Y.; Liu, Y.-N.; Bu, G.-L.; Wang, A.-J.; Li, Y.; Zhu, Q.-Y.; Zhang, H.; Xie, C.; et al. A gB nanoparticle vaccine elicits a protective neutralizing antibody response against EBV. Cell Host Microbe 2023, 31, 1882–1897.E10. [Google Scholar] [CrossRef]
- Lyu, J.H.; Liou, G.G.; Wang, M.; Kan, M.C. The genetic, biophysical and immunological studies of a self-adjuvanted protein nanoparticle. Vaccine 2025, 56, 127087. [Google Scholar] [CrossRef]
- Widge, A.T.; Hofstetter, A.R.; Houser, K.V.; Awan, S.F.; Chen, G.L.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Holman, L.A.; et al. An influenza hemagglutinin stem nanoparticle vaccine induces cross-group 1 neutralizing antibodies in healthy adults. Sci. Transl. Med. 2023, 15, eade4790. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Felgner, J.; Jain, A.; Jan, S.; Albin, T.J.; Badten, A.J.; Gregory, A.E.; Nakajima, R.; Jasinskas, A.; Felgner, P.L.; et al. Engineering Protein Nanoparticles Functionalized with an Immunodominant Coxiella burnetii Antigen to Generate a Q Fever Vaccine. Bioconjug. Chem. 2023, 34, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Shen, C.; Li, M.; Ma, S.; Liu, C.; Huang, J.; Ren, Z.; Yang, Y.; Zhao, M.; Xie, Q. Programmable Macrophage Vesicle Based Bionic Self-Adjuvanting Vaccine for Immunization against Monkeypox Virus. Adv. Sci. 2025, 12, 2408608. [Google Scholar] [CrossRef]
- Martínez-Pérez, A.; Igea, A.; Estévez, O.; Ferreira, C.M.; Torrado, E.; Castro, A.G.; Fernández, C.; Spetz, A.L.; Adam, L.; López González, M.; et al. Changes in the Immune Phenotype and Gene Expression Profile Driven by a Novel Tuberculosis Nanovaccine: Short and Long-Term Post-immunization. Front. Immunol. 2020, 11, 589863. [Google Scholar] [CrossRef] [PubMed]
- Essink, B.J.; Shapiro, C.; Isidro, M.G.D.; Bradley, P.; Pragalos, A.; Bloch, M.; Santiaguel, J.; Frias, M.V.; Miyakis, S.; Alves de Mesquita, M.; et al. Safety and immunogenicity of a modified mRNA-lipid nanoparticle vaccine candidate against COVID-19: Results from a phase 1, dose-escalation study. Hum. Vaccin. Immunother. 2024, 20, 2408863. [Google Scholar] [CrossRef]
- Mayer, R.L.; Verbeke, R.; Asselman, C.; Aernout, I.; Gul, A.; Eggermont, D.; Boucher, K.; Thery, F.; Maia, T.M.; Demol, H.; et al. Immunopeptidomics-based design of mRNA vaccine formulations against Listeria monocytogenes. Nat. Commun. 2022, 13, 6075. [Google Scholar] [CrossRef]
- Wan, J.; Wang, C.; Wang, Z.; Wang, L.; Wang, H.; Zhou, M.; Fu, Z.F.; Zhao, L. CXCL13 promotes broad immune responses induced by circular RNA vaccines. Proc. Natl. Acad. Sci. USA 2024, 121, e2406434121. [Google Scholar] [CrossRef]
- Marques Neto, L.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development. Front. Immunol. 2017, 8, 239. [Google Scholar] [CrossRef]
- Hoseini, Z.S.; Hajizade, A.; Easton, A.J.; Ahmadian, G.; Ramezani, F. A meta-analysis of the efficiency of metal nanoparticles in vaccine delivery against infectious disease. Nanomedicine 2021, 16, 481–495. [Google Scholar] [CrossRef]
- Friedman-Klabanoff, D.J.; Berry, A.A.; Travassos, M.A.; Shriver, M.; Cox, C.; Butts, J.; Lundeen, J.S.; Strauss, K.A.; Joshi, S.; Shrestha, B.; et al. Recombinant Full-length Plasmodium falciparum Circumsporozoite Protein-Based Vaccine Adjuvanted With Glucopyranosyl Lipid A-Liposome Quillaja saponaria 21: Results of Phase 1 Testing with Malaria Challenge. J. Infect. Dis. 2024, 229, 1883–1893. [Google Scholar] [CrossRef]
- Pollock, K.M.; Borges, Á.H.; Cheeseman, H.M.; Rosenkrands, I.; Schmidt, K.L.; Søndergaard, R.E.; Day, S.; Evans, A.; McFarlane, L.R.; Joypooranachandran, J.; et al. An investigation of trachoma vaccine regimens by the chlamydia vaccine CTH522 administered with cationic liposomes in healthy adults (CHLM-02): A phase 1, double-blind trial. Lancet Infect. Dis. 2024, 24, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Rastogi, A.; Ayyavoo, V.; Srivastava, S. Nanotechnology-Based Approaches for the Development of Diagnostics, Therapeutics, and Vaccines. Monoclon. Antibodies Immunodiagn. Immunother. 2014, 33, 186–191. [Google Scholar] [CrossRef]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y.; Couture, M.; D’Aoust, M.A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef]
- Gobeil, P.; Pillet, S.; Boulay, I.; Charland, N.; Lorin, A.; Cheng, M.P.; Vinh, D.C.; Boutet, P.; Van Der Most, R.; Roman, F.; et al. Durability and cross-reactivity of immune responses induced by a plant-based virus-like particle vaccine for COVID-19. Nat. Commun. 2022, 13, 6905. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ding, X.; Zhao, J.; Zeng, J.; Zhou, Y.; Xiao, W.; Hua, D.; Liu, M.; Guo, H.; Zhang, Y.; et al. A novel pan-epitope based nanovaccine self-assembled with CpG enhances immune responses against flavivirus. J. Nanobiotechnol. 2024, 22, 738. [Google Scholar] [CrossRef]
- Langley, J.M.; Gantt, S.; Halperin, S.A.; Ward, B.; McNeil, S.; Ye, L.; Cai, Y.; Smith, B.; Anderson, D.E.; Mitoma, F.D. An enveloped virus-like particle alum-adjuvanted cytomegalovirus vaccine is safe and immunogenic: A first-in-humans Canadian Immunization Research Network (CIRN) study. Vaccine 2024, 42, 713–722. [Google Scholar] [CrossRef]
- Sokouti, B.; Amjad, E. Chapter 16—Systems immunology. In Systems Biology and In-Depth Applications for Unlocking Diseases; Sokouti, B., Ed.; Academic Press: New York, NY, USA, 2025; pp. 207–217. [Google Scholar] [CrossRef]
- Skerrett-Byrne Anthony, D.; Jiang Chen, C.; Nixon, B.; Hondermarck, H. Transcriptomics. In Encyclopedia of Cell Biology, 2nd ed.; Bradshaw, R.A., Hart, G.W., Stahl, P.D., Eds.; Academic Press: Oxford, UK, 2023; pp. 363–371. [Google Scholar] [CrossRef]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 2021, 12, 57–69. [Google Scholar] [CrossRef]
- Yue, R.; Dutta, A. Computational systems biology in disease modeling and control, review and perspectives. Npj Syst. Biol. Appl. 2022, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Rao, A.; Strauss, O.; Kokkinou, E.; Bruchard, M.; Tripathi, K.P.; Schlums, H.; Carrasco, A.; Mazzurana, L.; Konya, V.; Villablanca, E.J.; et al. Cytokines regulate the antigen-presenting characteristics of human circulating and tissue-resident intestinal ILCs. Nat. Commun. 2020, 11, 2049. [Google Scholar] [CrossRef]
- Kambayashi, T.; Laufer, T.M. Atypical MHC class II-expressing antigen-presenting cells: Can anything replace a dendritic cell? Nat. Rev. Immunol. 2014, 14, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Kealy, L.; Good-Jacobson, K.L. Advances in understanding the formation and fate of B-cell memory in response to immunization or infection. Oxf. Open Immunol. 2021, 2, iqab018. [Google Scholar] [CrossRef]
- Muñoz-Wolf, N.; Lavelle, E.C. A Guide to IL-1 family cytokines in adjuvanticity. FEBS J. 2018, 285, 2377–2401. [Google Scholar] [CrossRef]
- Netea, M.G.; van de Veerdonk, F.L.; van der Meer, J.W.; Dinarello, C.A.; Joosten, L.A. Inflammasome-independent regulation of IL-1-family cytokines. Annu. Rev. Immunol. 2015, 33, 49–77. [Google Scholar] [CrossRef]
- Xu, Y.D.; Cheng, M.; Shang, P.P.; Yang, Y.Q. Role of IL-6 in dendritic cell functions. J. Leukoc. Biol. 2022, 111, 695–709. [Google Scholar] [CrossRef]
- Raeber, M.E.; Zurbuchen, Y.; Impellizzieri, D.; Boyman, O. The role of cytokines in T-cell memory in health and disease. Immunol. Rev. 2018, 283, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, M.; Adroub, S.; Mourier, T.; Mfarrej, S.; Zhang, H.; Esau, L.; Alsomali, A.; Alofi, F.S.; Ahmad, A.N.; Shamsan, A.; et al. Impact of the SARS-CoV-2 nucleocapsid 203K/204R mutations on the inflammatory immune response in COVID-19 severity. Genome Med. 2023, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, B.; Valizadeh, S.; Ghaffari, H.; Vahedi, A.; Karbalaei, M.; Eslami, M. A global treatments for coronaviruses including COVID-19. J. Cell. Physiol. 2020, 235, 9133–9142. [Google Scholar] [CrossRef]
- Wang, W.; Feng, S.; Ye, Z.; Gao, H.; Lin, J.; Ouyang, D. Prediction of lipid nanoparticles for mRNA vaccines by the machine learning algorithm. Acta Pharm. Sin. B 2022, 12, 2950–2962. [Google Scholar] [CrossRef]
- Haghmorad, D.; Eslami, M.; Orooji, N.; Halabitska, I.; Kamyshna, I.; Kamyshnyi, O.; Oksenych, V. mRNA vaccine platforms: Linking infectious disease prevention and cancer immunotherapy. Front. Bioeng. Biotechnol. 2025, 13, 1547025. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M. Genetic and structure of novel coronavirus COVID-19 and molecular mechanisms in the pathogenicity of coronaviruses. Rev. Res. Med. Microbiol. 2022, 33, e180–e188. [Google Scholar] [CrossRef]
- Maharjan, R.; Kim, K.H.; Lee, K.; Han, H.-K.; Jeong, S.H. Machine learning-driven optimization of mRNA-lipid nanoparticle vaccine quality with XGBoost/Bayesian method and ensemble model approaches. J. Pharm. Anal. 2024, 14, 100996. [Google Scholar] [CrossRef]
- Suyash, S.; Khan, W.H.; Maitra, P.; Jangid, V.; Punia, P.; Mishra, A. Machine Learning Optimization Approach to Design Multi-Epitope Marburg Vaccine Construct. Biotech. Res. Asia 2024, 21, 1463–1484. [Google Scholar] [CrossRef]
- Prosper, P.; Rodríguez Puertas, R.; Guérin, D.M.A.; Branda, M.M. Computational method for designing vaccines applied to virus-like particles (VLPs) as epitope carriers. Vaccine 2024, 42, 3916–3929. [Google Scholar] [CrossRef]
- Garmeh Motlagh, F.; Azimzadeh Irani, M.; Masoomi Nomandan, S.Z.; Assadizadeh, M. Computational design and investigation of the monomeric spike SARS-CoV-2-ferritin nanocage vaccine stability and interactions. Front. Mol. Biosci. 2024, 11, 1403635. [Google Scholar] [CrossRef]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced Nanobiomaterials: Vaccines, Diagnosis and Treatment of Infectious Diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef]
- Blank, F.; Stumbles, P.A.; Seydoux, E.; Holt, P.G.; Fink, A.; Rothen-Rutishauser, B.; Strickland, D.H.; von Garnier, C. Size-dependent uptake of particles by pulmonary antigen-presenting cell populations and trafficking to regional lymph nodes. Am. J. Respir. Cell Mol. Biol. 2013, 49, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.S.; Alonso, M.J.; de la Fuente, M. Nanoengineering of vaccines using natural polysaccharides. Biotechnol. Adv. 2015, 33, 1279–1293. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, F.; Liu, Y.; Wang, Z.; Yu, G.; Liang, B.; Niu, G.; Su, T.; Zhu, G.; Lu, G.; et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef]
- Lai, C.-J.; Kim, D.; Kang, S.; Li, K.; Cha, I.; Sasaki, A.; Porras, J.; Xia, T.; Jung, J.U. Viral codon optimization on SARS-CoV-2 Spike boosts immunity in the development of COVID-19 mRNA vaccines. J. Med. Virol. 2023, 95, e29183. [Google Scholar] [CrossRef]
- Surnar, B.; Kamran, M.Z.; Shah, A.S.; Basu, U.; Kolishetti, N.; Deo, S.; Jayaweera, D.T.; Daunert, S.; Dhar, S. Orally Administrable Therapeutic Synthetic Nanoparticle for Zika Virus. ACS Nano 2019, 13, 11034–11048. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.G.; Mann, P.; Kroidl, A.; Leroux-Roels, I.; Schindler, C.; Gabor, J.J.; Schunk, M.; Leroux-Roels, G.; Bosch, J.J.; Fendel, R.; et al. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2: A phase 1 randomized clinical trial. Wien. Klin. Wochenschr. 2021, 133, 931–941. [Google Scholar] [CrossRef]
- Cohen, C.J.; Gartner, J.J.; Horovitz-Fried, M.; Shamalov, K.; Trebska-McGowan, K.; Bliskovsky, V.V.; Parkhurst, M.R.; Ankri, C.; Prickett, T.D.; Crystal, J.S.; et al. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J. Clin. Invest. 2015, 125, 3981–3991. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Li, L.; Goedegebuure, P.; Mardis, E.R.; Ellis, M.J.; Zhang, X.; Herndon, J.M.; Fleming, T.P.; Carreno, B.M.; Hansen, T.H.; Gillanders, W.E. Cancer genome sequencing and its implications for personalized cancer vaccines. Cancers 2011, 3, 4191–4211. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Hacohen, N.; Fritsch, E.F.; Carter, T.A.; Lander, E.S.; Wu, C.J. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol. Res. 2013, 1, 11–15. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, A.; Nizamullah, F.N.U.; Shah, Z. Ai in healthcare: Using cutting-edge technologies to revolutionize vaccine development and distribution. JURIHUM J. Inov. Dan Hum. 2023, 1, 17–29. [Google Scholar]
- Zhang, L.; Huang, J.; Chen, X.; Pan, C.; He, Y.; Su, R.; Guo, D.; Yin, S.; Wang, S.; Zhou, L.; et al. Self-assembly nanovaccine containing TLR7/8 agonist and STAT3 inhibitor enhances tumor immunotherapy by augmenting tumor-specific immune response. J. Immunother. Cancer 2021, 9, e003132. [Google Scholar] [CrossRef]
- Guimaraes, L.C.; Costa, P.A.C.; Scalzo Júnior, S.R.A.; Ferreira, H.A.S.; Braga, A.C.S.; de Oliveira, L.C.; Figueiredo, M.M.; Shepherd, S.; Hamilton, A.; Queiroz-Junior, C.M.; et al. Nanoparticle-based DNA vaccine protects against SARS-CoV-2 variants in female preclinical models. Nat. Commun. 2024, 15, 590. [Google Scholar] [CrossRef] [PubMed]
- Hoces, D.; Miguens Blanco, J.; Hernández-López, R.A. A synthetic biology approach to engineering circuits in immune cells. Immunol. Rev. 2023, 320, 120–137. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, H.; Zhang, D.; Zhang, M.; Chao, X.; Scimeca, L.; Wu, M.-R. Synthetic biology approaches for improving the specificity and efficacy of cancer immunotherapy. Cell. Mol. Immunol. 2024, 21, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Holzwarth, U.; Roebben, G.; Bogni, A.; Bremer-Hoffmann, S. Mapping of the available standards against the regulatory needs for nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1531. [Google Scholar] [CrossRef]
- Pautler, M.; Brenner, S. Nanomedicine: Promises and challenges for the future of public health. Int. J. Nanomed. 2010, 5, 803–809. [Google Scholar]
- Berube, D.M. The public acceptance of nanomedicine: A personal perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 2–5. [Google Scholar] [CrossRef]
- Sharifi, S.; Mahmoud, N.N.; Voke, E.; Landry, M.P.; Mahmoudi, M. Importance of Standardizing Analytical Characterization Methodology for Improved Reliability of the Nanomedicine Literature. Nanomicro Lett. 2022, 14, 172. [Google Scholar] [CrossRef]
- De Jong, W.H.; Geertsma, R.E.; Borchard, G. Regulatory safety evaluation of nanomedical products: Key issues to refine. Drug Deliv. Transl. Res. 2022, 12, 2042–2047. [Google Scholar] [CrossRef]
- Gupta, R.; Arora, K.; Roy, S.S.; Joseph, A.; Rastogi, R.; Arora, N.M.; Kundu, P.K. Platforms, advances, and technical challenges in virus-like particles-based vaccines. Front. Immunol. 2023, 14, 1123805. [Google Scholar] [CrossRef] [PubMed]
- Vallet, T.; Vignuzzi, M. Self-Amplifying RNA: Advantages and Challenges of a Versatile Platform for Vaccine Development. Viruses 2025, 17, 566. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. Three decades of messenger RNA vaccine development. Nano Today 2019, 28, 100766. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).