The Promising Potential of Reverse Vaccinology-Based Next-Generation Vaccine Development over Conventional Vaccines against Antibiotic-Resistant Bacteria
Abstract
1. Introduction
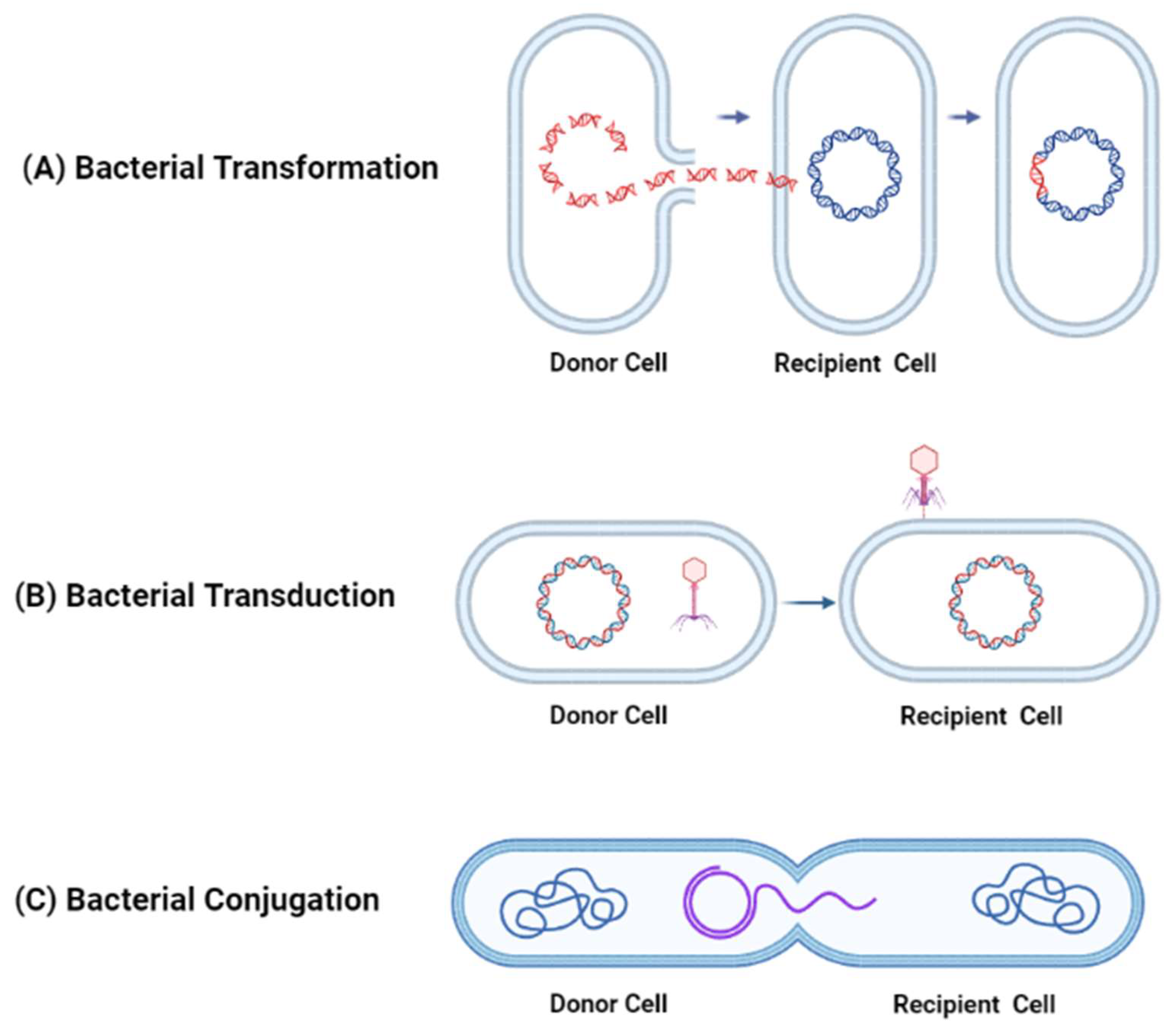
1.1. Mechanisms of Antibiotic Resistance Acquired through Horizontal Gene Transfer between Bacteria
1.2. Reverse Vaccinology: An Innovative Approach to Vaccine Development against Antibiotic-Resistant Bacteria
2. Vaccines against Antimicrobial Resistance Based on Conventional Vaccine Platforms
2.1. LAVs
2.2. IVs
2.3. Limitations of Conventional Vaccine Platforms
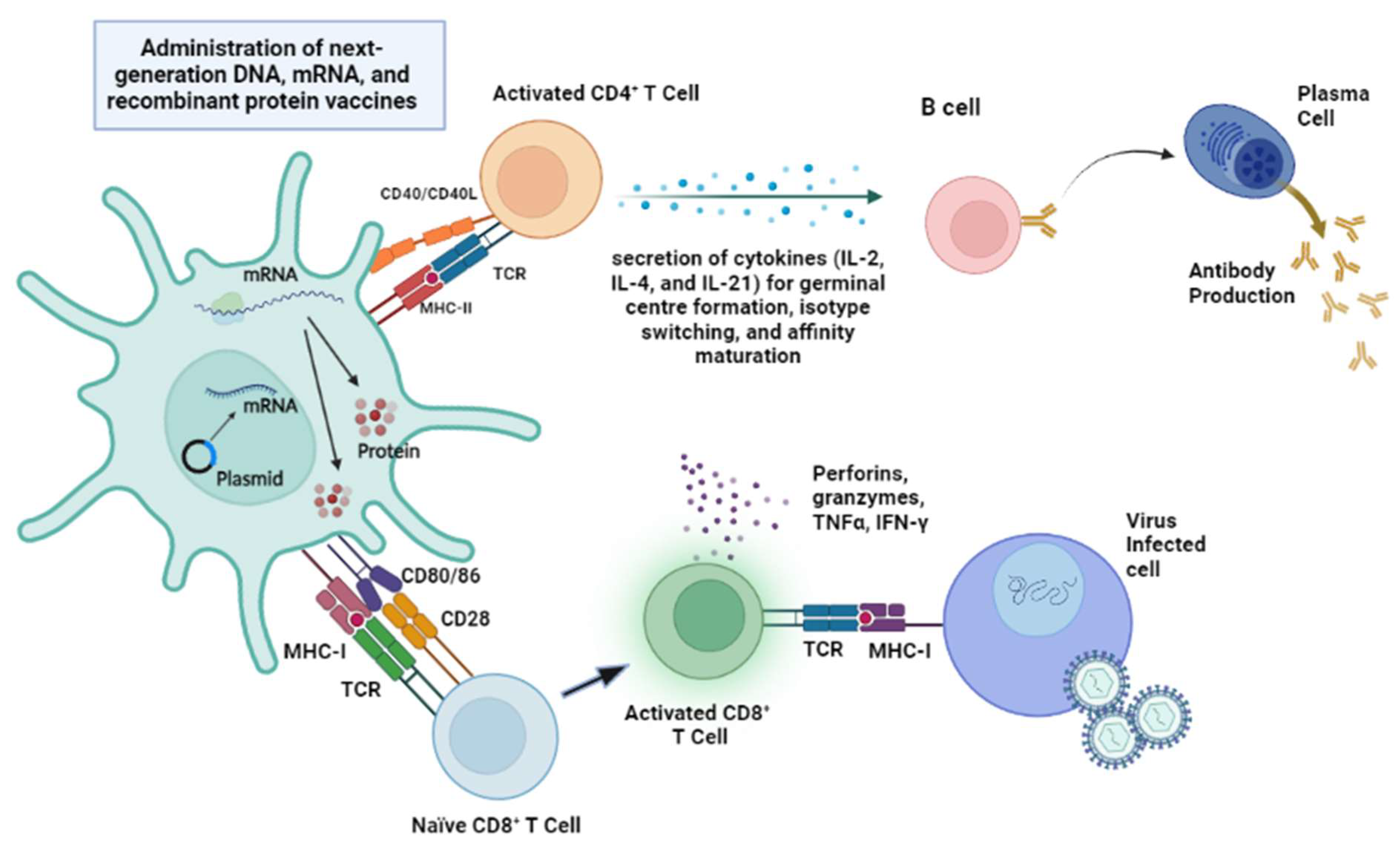
3. Current Preclinical and Clinical Development of Next-Generation Vaccines against AMR Bacteria
3.1. Recombinant Protein Vaccines
3.1.1. Advantages of Recombinant Protein Vaccines
3.1.2. Disadvantages of Recombinant Protein Vaccines
3.1.3. Recombinant Protein Vaccine Candidates
3.2. DNA Vaccines
3.2.1. Advantages of DNA Vaccines
3.2.2. Disadvantages of DNA Vaccines
3.2.3. DNA Vaccine Candidates
3.3. mRNA Vaccines
3.3.1. Advantages of mRNA Vaccines
3.3.2. Disadvantages of mRNA Vaccines
3.3.3. mRNA Vaccine Candidates
3.4. Comparison between Next-Generation mRNA, Recombinant Protein, and DNA Vaccines
3.4.1. Safety
3.4.2. Vaccine Protective Efficacy and Breadth of Immune Responses
4. Immunoinformatic Approaches for the Development of Multi-Epitope Vaccines
4.1. Immunoinformatics Tools for Epitope Prediction and Analysis
4.2. Literature Review: Immunoinformatics Approaches in Vaccine Development
4.3. Reverse Vaccinology: An Innovative Approach to Vaccine Development against Antibiotic-Resistant Bacteria
5. From Research to Real-World Experience: Currently Approved and Clinical Development of Experimental Vaccines against Antibiotic-Resistant Bacteria
5.1. S. enterica Serovar Typhi
5.2. H. influenzae Type b
5.3. S. pneumoniae
5.4. Extraintestinal Pathogenic E. coli (ExPEC)
5.5. S. enterica Serovar Paratyphi A
5.6. C. difficile
5.7. N. gonorrhoeae
5.8. N. meningitidis
5.9. ETEC
5.10. K. pneumoniae
5.11. P. aeruginosa
5.12. S. aureus
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paterson, I.K.; Hoyle, A.; Ochoa, G.; Baker-Austin, C.; Taylor, N.G. Optimising antibiotic usage to treat bacterial infections. Sci. Rep. 2016, 6, 37853. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public. Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef] [PubMed]
- Heaney, A.; Trenfield, S. Antibiotic usage in first time coronary artery surgery. J. Cardiothorac. Surg. 2015, 10, A294. [Google Scholar] [CrossRef]
- Kaviani, A.; Ince, D.; Axelrod, D.A. Management of antimicrobial agents in abdominal organ transplant patients in intensive care unit. Curr. Transplant. Rep. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Lee, L.Y.-H.; Ha, D.L.A.; Simmons, C.; de Jong, M.D.; Chau, N.V.V.; Schumacher, R.; Peng, Y.C.; McMichael, A.J.; Farrar, J.J.; Smith, G.L.; et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Investig. 2008, 118, 3478–3490. [Google Scholar] [CrossRef]
- Gaynes, R. The discovery of penicillin—New insights after more than 75 years of clinical use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- Komagamine, J. The efficacy of high-dose penicillin G for pneumococcal pneumonia diagnosed based on initial comprehensive assessment at admission: An observational study. BMC Res. Notes 2018, 11, 399. [Google Scholar] [CrossRef]
- Deshpande, D.; Srivastava, S.; Bendet, P.; Martin Katherine, R.; Cirrincione Kayle, N.; Lee Pooi, S.; Pasipanodya Jotam, G.; Dheda, K.; Gumbo, T. Antibacterial and Sterilizing Effect of Benzylpenicillin in Tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e02217–e02232. [Google Scholar] [CrossRef]
- Adedeji, W.A. The treasure called antibiotics. Ann. Ib. Postgrad. Med. 2016, 14, 56–57. [Google Scholar]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Molecules that inhibit bacterial resistance enzymes. Molecules 2018, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Schaenzer, A.J.; Wright, G.D. Antibiotic resistance by enzymatic modification of antibiotic targets. Trends Mol. Med. 2020, 26, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bbosa, G.; Mwebaza, N.; Odda, J.; Kyegombe, D.; Ntale, M. Antibiotics/antibacterial drug use, their marketing and promotion during the post-antibiotic golden age and their role in emergence of bacterial resistance. Health 2014, 6, 410–425. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations—2014 (December). Available online: http://amr-review.org (accessed on 14 July 2023).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef]
- Chen, W. Will the mRNA vaccine platform be the panacea for the development of vaccines against antimicrobial resistant (AMR) pathogens? Expert. Rev. Vaccines 2022, 21, 155–157. [Google Scholar] [CrossRef]
- Enayatkhani, M.; Hasaniazad, M.; Faezi, S.; Gouklani, H.; Davoodian, P.; Ahmadi, N.; Einakian, M.A.; Karmostaji, A.; Ahmadi, K. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: An in silico study. J. Biomol. Struct. Dyn. 2021, 39, 2857–2872. [Google Scholar] [CrossRef]
- Herrera, L.R.M. Reverse vaccinology approach in constructing a multi-epitope vaccine against cancer-testis antigens expressed in non-small cell lung cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1495–1506. [Google Scholar] [CrossRef]
- Tobuse, A.J.; Ang, C.W.; Yeong, K.Y. Modern vaccine development via reverse vaccinology to combat antimicrobial resistance. Life Sci. 2022, 302, 120660. [Google Scholar] [CrossRef]
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.; et al. Global tuberculosis report 2020—Reflections on the global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021, 113 (Suppl. 1), S7–S12. [Google Scholar] [CrossRef]
- Cabral, M.P.; García, P.; Beceiro, A.; Rumbo, C.; Pérez, A.; Moscoso, M.; Bou, G. Design of live attenuated bacterial vaccines based on D-glutamate auxotrophy. Nat. Commun. 2017, 8, 15480. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.P.; Sabourin, C.L.; Niemuth, N.A.; Li, H.; Semenova, V.A.; Rudge, T.L.; Mayfield, H.J.; Schiffer, J.; Mittler, R.S.; Ibegbu, C.C.; et al. A three-dose intramuscular injection schedule of anthrax vaccine adsorbed generates sustained humoral and cellular immune responses to protective antigen and provides long-term protection against inhalation anthrax in rhesus macaques. Clin. Vaccine. Immunol. 2012, 19, 1730–1745. [Google Scholar] [CrossRef] [PubMed]
- Little, S.F.; Ivins, B.E.; Webster, W.M.; Norris, S.L.; Andrews, G.P. Effect of aluminum hydroxide adjuvant and formaldehyde in the formulation of rPA anthrax vaccine. Vaccine 2007, 25, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.D.; Tenney, K.M.; Larsson, C.J.; O’Neill, J.P.; Ventrone, C.; Bentley, M.; Upton, A.; Hindle, Z.; Fidler, C.; Kutzko, D.; et al. The novel oral typhoid vaccine M01ZH09 Is well tolerated and highly immunogenic in 2 vaccine presentations. J. Infect. Dis. 2005, 192, 360–366. [Google Scholar] [CrossRef]
- Luca, S.; Mihaescu, T. History of BCG vaccine. Maedica 2013, 8, 53–58. [Google Scholar]
- Dijkman, K.; Sombroek, C.C.; Vervenne, R.A.W.; Hofman, S.O.; Boot, C.; Remarque, E.J.; Kocken, C.H.M.; Ottenhoff, T.H.M.; Kondova, I.; Khayum, M.A.; et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 2019, 25, 255–262. [Google Scholar] [CrossRef]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H.; Hughes, T.K.; Pokkali, S.; Swanson, P.A.; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef]
- Tsenova, L.; Harbacheuski, R.; Sung, N.; Ellison, E.; Fallows, D.; Kaplan, G. BCG vaccination confers poor protection against M. tuberculosis HN878-induced central nervous system disease. Vaccine 2007, 25, 5126–5132. [Google Scholar] [CrossRef]
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.-C.; Croda, J.; Hill, P.C.; et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet Glob. Health 2022, 10, e1307–e1316. [Google Scholar] [CrossRef]
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Sathkumara, H.D.; Pai, S.; Aceves-Sánchez, M.J.; Ketheesan, N.; Flores-Valdez, M.A.; Kupz, A. BCG vaccination prevents reactivation of latent lymphatic murine tuberculosis independently of CD4(+) T cells. Front. Immunol. 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, N.; Uranga, S.; Marinova, D.; Monzon, M.; Badiola, J.; Martin, C. MTBVAC vaccine is safe, immunogenic and confers protective efficacy against Mycobacterium tuberculosis in newborn mice. Tuberculosis 2016, 96, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Spertini, F.; Audran, R.; Chakour, R.; Karoui, O.; Steiner-Monard, V.; Thierry, A.-C.; Mayor, C.E.; Rettby, N.; Jaton, K.; Vallotton, L.; et al. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: A randomised, double-blind, controlled phase I trial. Lancet Respir. Med. 2015, 3, 953–962. [Google Scholar] [CrossRef]
- Arbues, A.; Aguilo, J.I.; Gonzalo-Asensio, J.; Marinova, D.; Uranga, S.; Puentes, E.; Fernandez, C.; Parra, A.; Cardona, P.J.; Vilaplana, C.; et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine 2013, 31, 4867–4873. [Google Scholar] [CrossRef]
- Verreck, F.A.; Vervenne, R.A.; Kondova, I.; van Kralingen, K.W.; Remarque, E.J.; Braskamp, G.; van der Werff, N.M.; Kersbergen, A.; Ottenhoff, T.H.; Heidt, P.J.; et al. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS ONE 2009, 4, e5264. [Google Scholar] [CrossRef]
- Martín, C.; Marinova, D.; Aguiló, N.; Gonzalo-Asensio, J. MTBVAC, a live TB vaccine poised to initiate efficacy trials 100 years after BCG. Vaccine 2021, 39, 7277–7285. [Google Scholar] [CrossRef]
- Moscoso, M.; García, P.; Cabral, M.P.; Rumbo, C.; Bou, G. A D-Alanine auxotrophic live vaccine is effective against lethal infection caused by Staphylococcus aureus. Virulence 2018, 9, 604–620. [Google Scholar] [CrossRef]
- Shu, M.H.; MatRahim, N.; NorAmdan, N.; Pang, S.P.; Hashim, S.H.; Phoon, W.H.; AbuBakar, S. An inactivated antibiotic-exposed whole-cell vaccine enhances bactericidal activities against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2016, 6, 22332. [Google Scholar] [CrossRef]
- Orenstein, W.A.; Ahmed, R. Simply put: Vaccination saves lives. Proc. Natl. Acad. Sci. USA 2017, 114, 4031–4033. [Google Scholar] [CrossRef]
- Vignuzzi, M.; Wendt, E.; Andino, R. Engineering attenuated virus vaccines by controlling replication fidelity. Nat. Med. 2008, 14, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Jones, J.O.; Andino, R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010, 28, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.K. Off-label abuse of antibiotics by bacteria. Gut Microbes 2014, 5, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Fertey, J.; Bayer, L.; Grunwald, T.; Pohl, A.; Beckmann, J.; Gotzmann, G.; Casado, J.P.; Schönfelder, J.; Rögner, F.H.; Wetzel, C.; et al. Pathogens inactivated by low-energy-electron irradiation maintain antigenic properties and induce protective immune responses. Viruses 2016, 8, 319. [Google Scholar] [CrossRef]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. (Eds.) Chapter 11—Vaccines and Vaccination. In Fenner and White’s Medical Virology (Fifth Edition); Academic Press: London, UK, 2017; pp. 155–167. [Google Scholar]
- Han, X.; Xu, P.; Ye, Q. Analysis of COVID-19 vaccines: Types, thoughts, and application. J. Clin. Lab. Anal. 2021, 35, e23937. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Gao, F.; Zhang, J.; He, Q.; Mao, Q.; Xu, M.; Liang, Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert. Rev. Vaccines 2021, 20, 365–373. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Liu, M.A. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef]
- Lai, C.-Y.; To, A.; Wong, T.A.S.; Lieberman, M.M.; Clements, D.E.; Senda, J.T.; Ball, A.H.; Pessaint, L.; Andersen, H.; Furuyama, W.; et al. Recombinant protein subunit SARS-CoV-2 vaccines formulated with CoVaccine HT™ adjuvant induce broad, Th1 biased, humoral and cellular immune responses in mice. Vaccine X 2021, 9, 100126. [Google Scholar] [CrossRef]
- Flood, A.; Estrada, M.; McAdams, D.; Ji, Y.; Chen, D. Development of a freeze-dried, heat-stable influenza subunit vaccine formulation. PLoS ONE 2016, 11, e0164692. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, e172. [Google Scholar] [CrossRef] [PubMed]
- Altmann, F.; Staudacher, E.; Wilson, I.B.; März, L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj. J. 1999, 16, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Thak, E.J.; Yoo, S.J.; Moon, H.Y.; Kang, H.A. Yeast synthetic biology for designed cell factories producing secretory recombinant proteins. FEMS Yeast Res. 2020, 20, foaa009. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Chen, W.H.; Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef]
- Fujita-Yamaguchi, Y. Affinity chromatography of native and recombinant proteins from receptors for insulin and IGF-I to recombinant single chain antibodies. Front. Endocrinol. 2015, 6, 166. [Google Scholar] [CrossRef]
- Schäfer, F.; Seip, N.; Maertens, B.; Block, H.; Kubicek, J. Purification of GST-tagged proteins. Methods Enzymol. 2015, 559, 127–139. [Google Scholar] [CrossRef]
- Liu, S.; Tobias, R.; McClure, S.; Styba, G.; Shi, Q.; Jackowski, G. Removal of endotoxin from recombinant protein preparations. Clin. Biochem. 1997, 30, 455–463. [Google Scholar] [CrossRef]
- Ghaemi, A.; Roshani Asl, P.; Zargaran, H.; Ahmadi, D.; Hashimi, A.A.; Abdolalipour, E.; Bathaeian, S.; Miri, S.M. Recombinant COVID-19 vaccine based on recombinant RBD/Nucleoprotein and saponin adjuvant induces long-lasting neutralizing antibodies and cellular immunity. Front. Immunol. 2022, 13, 974364. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Cotton, M.F.; Eisele, B.; Gengenbacher, M.; Grode, L.; Hesseling, A.C.; Walzl, G. The BCG replacement vaccine VPM1002: From drawing board to clinical trial. Expert. Rev. Vaccines 2014, 13, 619–630. [Google Scholar] [CrossRef]
- Loxton, A.G.; Knaul, J.K.; Grode, L.; Gutschmidt, A.; Meller, C.; Eisele, B.; Johnstone, H.; van der Spuy, G.; Maertzdorf, J.; Kaufmann, S.H.E.; et al. Safety and Immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin. Vaccine Immunol. 2017, 24, e00439-16. [Google Scholar] [CrossRef]
- Glynn, J.R.; Whiteley, J.; Bifani, P.J.; Kremer, K.; van Soolingen, D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: A systematic review. Emerg. Infect. Dis. 2002, 8, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Ordway, D.J.; Shang, S.; Henao-Tamayo, M.; Obregon-Henao, A.; Nold, L.; Caraway, M.; Shanley, C.A.; Basaraba, R.J.; Duncan, C.G.; Orme, I.M. Mycobacterium bovis BCG-mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clin. Vaccine Immunol. 2011, 18, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Grode, L.; Seiler, P.; Baumann, S.; Hess, J.; Brinkmann, V.; Nasser Eddine, A.; Mann, P.; Goosmann, C.; Bandermann, S.; Smith, D.; et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J. Clin. Investig. 2005, 115, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Dodd, P.J.; Sismanidis, C.; Seddon, J.A. Global burden of drug-resistant tuberculosis in children: A mathematical modelling study. Lancet Infect. Dis. 2016, 16, 1193–1201. [Google Scholar] [CrossRef]
- Grode, L.; Ganoza, C.A.; Brohm, C.; Weiner, J., 3rd; Eisele, B.; Kaufmann, S.H. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 2013, 31, 1340–1348. [Google Scholar] [CrossRef]
- Cotton, M.F.; Madhi, S.A.; Luabeya, A.K.; Tameris, M.; Hesseling, A.C.; Shenje, J.; Schoeman, E.; Hatherill, M.; Desai, S.; Kapse, D.; et al. Safety and immunogenicity of VPM1002 versus BCG in South African newborn babies: A randomised, phase 2 non-inferiority double-blind controlled trial. Lancet Infect. Dis. 2022, 22, 1472–1483. [Google Scholar] [CrossRef]
- Chiwala, G.; Liu, Z.; Mugweru, J.N.; Wang, B.; Khan, S.A.; Bate, P.N.N.; Yusuf, B.; Hameed, H.M.A.; Fang, C.; Tan, Y.; et al. A recombinant selective drug-resistant M. bovis BCG enhances the bactericidal activity of a second-line anti-tuberculosis regimen. Biomed. Pharmacother. 2021, 142, 112047. [Google Scholar] [CrossRef]
- Rodrigues, M.; Yang, Y.; Meira, E.; Silva, J.; Bicalho, R. Development and evaluation of a new recombinant protein vaccine (YidR) against Klebsiella pneumoniae infection. Vaccine 2020, 38, 4640–4648. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Khalid, K.; Poh, C.L. The development of DNA vaccines against SARS-CoV-2. Adv. Med. Sci. 2023, 68, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Hobernik, D.; Bros, M. DNA Vaccines-How Far from Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Aluminum vaccine adjuvants: Are they safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef]
- Al-Fattah Yahaya, A.A.; Khalid, K.; Lim, H.X.; Poh, C.L. Development of next generation vaccines against SARS-CoV-2 and variants of concern. Viruses 2023, 15, 624. [Google Scholar] [CrossRef]
- Ansari, H.; Tahmasebi-Birgani, M.; Bijanzadeh, M.; Doosti, A.; Kargar, M. Study of the immunogenicity of outer membrane protein A (ompA) gene from Acinetobacter baumannii as DNA vaccine candidate in vivo. Iran. J. Basic. Med. Sci. 2019, 22, 669–675. [Google Scholar] [CrossRef]
- Hashemzehi, R.; Doosti, A.; Kargar, M.; Jaafarinia, M. Cloning and expression of nlpA gene as DNA vaccine candidate against Acinetobacter baumannii. Mol. Biol. Rep. 2018, 45, 395–401. [Google Scholar] [CrossRef]
- Han, S. Clinical vaccine development. Clin. Exp. Vaccine Res. 2015, 4, 46–53. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, G.F. mRNA vaccines: A matter of delivery. eClinicalMedicine 2021, 32, 100746. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Mayer, R.L.; Verbeke, R.; Asselman, C.; Aernout, I.; Gul, A.; Eggermont, D.; Boucher, K.; Thery, F.; Maia, T.M.; Demol, H.; et al. Immunopeptidomics-based design of highly effective mRNA vaccine formulations against Listeria monocytogenes. bioRxiv 2022, 13, 6075. [Google Scholar] [CrossRef]
- Kon, E.; Levy, Y.; Elia, U.; Cohen, H.; Hazan-Halevy, I.; Aftalion, M.; Ezra, A.; Bar-Haim, E.; Naidu, G.S.; Diesendruck, Y.; et al. An effective mRNA-LNP vaccine against the lethal plague bacterium. bioRxiv 2022, 8, 503096. [Google Scholar] [CrossRef]
- Moghimi, S.M. Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines. Mol. Ther. 2021, 29, 898–900. [Google Scholar] [CrossRef]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 2021, 51, 861–863. [Google Scholar] [CrossRef]
- Krishna, M.; Nadler, S.G. Immunogenicity to biotherapeutics–the role of anti-drug immune complexes. Front. Immunol. 2016, 7, 21. [Google Scholar] [CrossRef]
- Medjitna, T.D.; Stadler, C.; Bruckner, L.; Griot, C.; Ottiger, H.P. DNA vaccines: Safety aspect assessment and regulation. Dev. Biol. 2006, 126, 261–270; discussion 327. [Google Scholar]
- Li, Y.; Xiao, J.; Chang, Y.F.; Zhang, H.; Teng, Y.; Lin, W.; Li, H.; Chen, W.; Zhang, X.; Xie, Q. Immunogenicity and protective efficacy of the recombinant Pasteurella multocida lipoproteins VacJ and PlpE, and outer membrane protein H from P. multocida A:1 in ducks. Front. Immunol. 2022, 13, 985993. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, H.; Meng, L.; Li, F.; Yu, C. Comparison of immune responses elicited by SARS-CoV-2 mRNA and recombinant protein vaccine candidates. Front. Immunol. 2022, 13, 906457. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.C.; Mahajan, S.; Peters, B.; Nielsen, M.; Marcatili, P. Antibody specific B-cell epitope predictions: Leveraging information from antibody-antigen protein complexes. Front. Immunol. 2019, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Rahman, M.K.; Saha, S.; Kaykobad, M.; Rahman, M.S. Antigenic: An improved prediction model of protective antigens. Artif. Intell. Med. 2019, 94, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, H.; Eslami, M.; Negahdaripour, M.; Ghoshoon, M.B.; Gholami, A.; Heidari, R.; Dehshahri, A.; Erfani, N.; Nezafat, N.; Ghasemi, Y. Vaccinomics approach for developing multi-epitope peptide pneumococcal vaccine. J. Biomol. Struct. Dyn. 2019, 37, 3524–3535. [Google Scholar] [CrossRef]
- Tan, C.; Zhu, F.; Xiao, Y.; Wu, Y.; Meng, X.; Liu, S.; Liu, T.; Chen, S.; Zhou, J.; Li, C.; et al. Immunoinformatics approach toward the introduction of a novel multi-epitope vaccine against Clostridium difficile. Front. Immunol. 2022, 13, 887061. [Google Scholar] [CrossRef]
- Alzarea, S.I. Identification and construction of a multi-epitopes vaccine design against Klebsiella aerogenes: Molecular modeling study. Sci. Rep. 2022, 12, 14402. [Google Scholar] [CrossRef]
- Stratmann, T. Cholera toxin subunit B as adjuvant--An accelerator in protective immunity and a break in autoimmunity. Vaccines 2015, 3, 579–596. [Google Scholar] [CrossRef]
- Albekairi, T.H.; Alshammari, A.; Alharbi, M.; Alshammary, A.F.; Tahir Ul Qamar, M.; Ullah, A.; Irfan, M.; Ahmad, S. Designing of a novel multi-antigenic epitope-based vaccine against E. Hormaechei: An intergraded reverse vaccinology and immunoinformatics approach. Vaccines 2022, 10, 665. [Google Scholar] [CrossRef]
- Alshabrmi, F.M.; Alrumaihi, F.; Alrasheedi, S.F.; Al-Megrin, W.A.I.; Almatroudi, A.; Allemailem, K.S. An in-silico investigation to design a multi-epitopes vaccine against multi-drug resistant Hafnia alvei. Vaccines 2022, 10, 1127. [Google Scholar] [CrossRef]
- Munia, M.; Mahmud, S.; Mohasin, M.; Kibria, K.M.K. In silico design of an epitope-based vaccine against choline binding protein A of Streptococcus pneumoniae. Inform. Med. Unlocked 2021, 23, 100546. [Google Scholar] [CrossRef]
- Kumar, A.; Harjai, K.; Chhibber, S. A multiepitopic theoretical fusion construct based on in-silico epitope screening of known vaccine candidates for protection against wide range of enterobacterial pathogens. Human Immunol. 2019, 80, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Buckner, M.M.C.; Croxen, M.; Arena, E.T.; Finlay, B.B. A comprehensive study of the contribution of Salmonella enterica serovar Typhimurium SPI2 effectors to bacterial colonization, survival, and replication in typhoid fever, macrophage, and epithelial cell infection models. Virulence 2011, 2, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Brockett, S.; Wolfe, M.K.; Hamot, A.; Appiah, G.D.; Mintz, E.D.; Lantagne, D. Associations among water, sanitation, and hygiene, and food exposures and typhoid fever in case-control studies: A systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 2020, 103, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.O.; Kim, Y.H.; Chung, S.J.; Lee, C.H.; Rhee, S.; Pratx, G.; Chung, J.K.; Youn, H. Identification of lymphatic and hematogenous routes of rapidly labeled radioactive and fluorescent exosomes through highly sensitive multimodal imaging. Int. J. Mol. Sci. 2020, 21, 7850. [Google Scholar] [CrossRef]
- Connor, B.A.; Schwartz, E. Typhoid and paratyphoid fever in travellers. Lancet Infect. Dis. 2005, 5, 623–628. [Google Scholar] [CrossRef]
- Ashurst, J.V.; Truong, J.; Woodbury, B. Salmonella typhi. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Hohmann, E.L.; Oletta, C.A.; Killeen, K.P.; Miller, S.I. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single dose typhoid fever vaccine in volunteers. J. Infect. Dis. 1996, 173, 1408–1414. [Google Scholar] [CrossRef]
- Crump, J.A.; Mintz, E.D. Global trends in typhoid and paratyphoid Fever. Clin. Infect. Dis. 2010, 50, 241–246. [Google Scholar] [CrossRef]
- Amicizia, D.; Arata, L.; Zangrillo, F.; Panatto, D.; Gasparini, R. Overview of the impact of typhoid and paratyphoid fever: Utility of Ty21a vaccine (Vivotif®). J. Prev. Med. Hyg. 2017, 58, E1–E8. [Google Scholar]
- Jackson, B.R.; Iqbal, S.; Mahon, B. Updated recommendations for the use of typhoid vaccine—Advisory committee on immunization practices, United States, 2015. Morb. Mortal. Wkly. Rep. 2015, 64, 305–308. [Google Scholar]
- Choi, S.K.; Baik, Y.O.; Lee, Y.; Lee, C.; Kim, S.K.; Park, J.; Sun, M.; Jung, D.; Jang, J.Y.; Yong, T.J. A phase ii/iii, multicenter, observer-blinded, randomized, non-inferiority and safety, study of typhoid conjugate vaccine (EuTCV) compared to Typbar-TCV® in healthy 6 months-45 years aged participants. Vaccine 2023, 41, 1753–1759. [Google Scholar] [CrossRef]
- Kumar Rai, G.; Saluja, T.; Chaudhary, S.; Tamrakar, D.; Kanodia, P.; Giri, B.R.; Shrestha, R.; Uranw, S.; Kim, D.R.; Yang, J.S.; et al. Safety and immunogenicity of the Vi-DT typhoid conjugate vaccine in healthy volunteers in Nepal: An observer-blind, active-controlled, randomised, non-inferiority, phase 3 trial. Lancet Infect. Dis. 2022, 22, 529–540. [Google Scholar] [CrossRef]
- Nampota-Nkomba, N.; Nyirenda, O.M.; Khonde, L.; Mapemba, V.; Mbewe, M.; Ndaferankhande, J.M.; Msuku, H.; Masesa, C.; Misiri, T.; Mwakiseghile, F.; et al. Safety and immunogenicity of a typhoid conjugate vaccine among children aged 9 months to 12 years in Malawi: A nested substudy of a double-blind, randomised controlled trial. Lancet Glob. Health 2022, 10, e1326–e1335. [Google Scholar] [CrossRef] [PubMed]
- Soulier, A.; Prevosto, C.; Chol, M.; Deban, L.; Cranenburgh, R.M. Engineering a novel bivalent oral vaccine against enteric fever. Int. J. Mol. Sci. 2021, 22, 3287. [Google Scholar] [CrossRef]
- Agrawal, A.; Murphy, T.F. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J. Clin. Microbiol. 2011, 49, 3728–3732. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Crane, R.J.; Karani, A.; Mutuku, A.; Morpeth, S.C.; Burbidge, P.; Goldblatt, D.; Kamau, T.; Sharif, S.; Mturi, N.; et al. Effect of Haemophilus influenza type b vaccination without a booster dose on invasive H. influenzae type b disease, nasopharyngeal carriage, and population immunity in Kilifi, Kenya: A 15-year regional surveillance study. Lancet Glob. Health 2016, 4, e185–e194. [Google Scholar] [CrossRef]
- World Health Organization. Bacterial Vaccines in Clinical and Preclinical Development 2021: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Tabarani, C.; Fletcher, S.A.; Heresi, G.P.; Wootton, S.H. Invasive Haemophilus influenza type b in an infant during the COVID-19 pandemic: The return of diseases we hoped never to see again. J. Pediatr. Infect. Dis. 2022, 41, e30–e31. [Google Scholar] [CrossRef]
- Jansen, K.U.; Anderson, A.S. The role of vaccines in fighting antimicrobial resistance (AMR). Hum. Vaccin. Immunother. 2018, 14, 2142–2149. [Google Scholar] [CrossRef]
- Jackson, C.; Mann, A.; Mangtani, P.; Fine, P. Effectiveness of Haemophilus influenzae type b vaccines administered according to various schedules: Systematic review and meta-analysis of observational data. Pediatr. Infect. Dis. J. 2013, 32, 1261–1269. [Google Scholar] [CrossRef]
- Obando-Pacheco, P.; Rivero-Calle, I.; Raguindin, P.F.; Martinón-Torres, F. DTaP5-HBV-IPV-Hib pediatric hexavalent combination vaccine for use in children from 6 weeks through to 4 years of age. Expert. Rev. Vaccines 2019, 18, 1115–1126. [Google Scholar] [CrossRef]
- Klein, N.P.; Abu-Elyazeed, R.; Cheuvart, B.; Janssens, W.; Mesaros, N. Immunogenicity and safety following primary and booster vaccination with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and Haemophilus influenzae type b vaccine: A randomized trial in the United States. Hum. Vaccin. Immunother. 2019, 15, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Vaez, H.; Sahebkar, A.; Pourfarzi, F.; Yousefi-Avarvand, A.; Khademi, F. Prevalence of Antibiotic Resistance of Haemophilus Influenzae in Iran—A Meta-Analysis. Iran. J. Otorhinolaryngol. 2019, 31, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.L.; Seon, S.H.; Rhee, D.K. Pneumonia and Streptococcus pneumoniae vaccine. Arch. Pharm. Res. 2017, 40, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Behrens, F.; Funk-Hilsdorf, T.C.; Kuebler, W.M.; Simmons, S. Bacterial membrane vesicles in pneumonia: From mediators of virulence to innovative vaccine candidates. Int. J. Mol. Sci. 2021, 22, 3858. [Google Scholar] [CrossRef]
- Masomian, M.; Ahmad, Z.; Gew, L.T.; Poh, C.L. Development of next generation Streptococcus pneumoniae vaccines conferring broad protection. Vaccines 2020, 8, 132. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.J.; Malley, R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 13564–13569. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E. Protein carriers of conjugate vaccines: Characteristics, development, and clinical trials. Hum. Vaccin. Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef]
- Brooks, W.A.; Chang, L.J.; Sheng, X.; Hopfer, R. Safety and immunogenicity of a trivalent recombinant PcpA, PhtD, and PlyD1 pneumococcal protein vaccine in adults, toddlers, and infants: A phase I randomized controlled study. Vaccine 2015, 33, 4610–4617. [Google Scholar] [CrossRef]
- NIH. A Study of Vaccination with 9-Valent Extraintestinal Pathogenic Escherichia coli Vaccine (ExPEC9V) in the Prevention of Invasive Extraintestinal Pathogenic Escherichia coli Disease in Adults Aged 60 Years and Older with a History of Urinary Tract Infection in the Past 2 Years. Available online: https://clinicaltrials.gov/ct2/show/NCT04899336 (accessed on 21 February 2023).
- NIH. A Study of Three Different Doses of VAC52416 (ExPEC10V) in Adults Aged 60 to 85 Years in Stable Health. Available online: https://clinicaltrials.gov/ct2/show/NCT03819049 (accessed on 21 February 2023).
- Doua, J.; Fierro, C.; Sarnecki, M.; Spiessens, B.; Go, O.; Davies, T.; van den Dobbelsteen, G.; Poolman, J.; Haazen, W. 120. safety, reactogenicity, and immunogenicity of three different doses of VAC52416 (ExPEC10V) in adults aged 60–85 years in a randomized, multicenter, interventional, first-in-human, phase 1/2a study. Open Forum Infect. Dis. 2022, 9, ofac492.198. [Google Scholar] [CrossRef]
- Eldridge, G.R.; Hughey, H.; Rosenberger, L.; Martin, S.M.; Shapiro, A.M.; D’Antonio, E.; Krejci, K.G.; Shore, N.; Peterson, J.; Lukes, A.S.; et al. Safety and immunogenicity of an adjuvanted Escherichia coli adhesin vaccine in healthy women with and without histories of recurrent urinary tract infections: Results from a first-in-human phase 1 study. Hum. Vaccin. Immunother. 2021, 17, 1262–1270. [Google Scholar] [CrossRef]
- Wade, D.; Cooper, J.; Derry, F.; Taylor, J. Uro-Vaxom® versus placebo for the prevention of recurrent symptomatic urinary tract infections in participants with chronic neurogenic bladder dysfunction: A randomised controlled feasibility study. Trials 2019, 20, 223. [Google Scholar] [CrossRef] [PubMed]
- Brodie, A.; El-Taji, O.; Jour, I.; Foley, C.; Hanbury, D. A retrospective study of immunotherapy treatment with Uro-Vaxom (OM-89®) for prophylaxis of recurrent urinary tract infections. Curr. Urol. 2020, 14, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.F.; Wacker, M.; Hernandez, M.; Hitchen, P.G.; Marolda, C.L.; Kowarik, M.; Morris, H.R.; Dell, A.; Valvano, M.A.; Aebi, M. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. USA 2005, 102, 3016–3021. [Google Scholar] [CrossRef]
- Poolman, J.T.; Wacker, M. Extraintestinal pathogenic Escherichia coli, a common human pathogen: Challenges for vaccine development and progress in the field. J. Infect. Dis. 2016, 213, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Baliban, S.M.; Lu, Y.J.; Malley, R. Overview of the nontyphoidal and paratyphoidal Salmonella vaccine pipeline: Current status and future prospects. Clin. Infect. Dis. 2020, 71, S151–S154. [Google Scholar] [CrossRef]
- Wahid, R.; Kotloff, K.L.; Levine, M.M.; Sztein, M.B. Cell mediated immune responses elicited in volunteers following immunization with candidate live oral Salmonella enterica serovar Paratyphi A attenuated vaccine strain CVD 1902. Clin. Immunol. 2019, 201, 61–69. [Google Scholar] [CrossRef]
- Shakya, M.; Neuzil, K.M.; Pollard, A.J. Prospects of future typhoid and paratyphoid vaccines in endemic countries. J. Infect. Dis. 2021, 224, S770–S774. [Google Scholar] [CrossRef]
- NIH. Understanding Typhoid Disease after Vaccination. Available online: https://clinicaltrials.gov/ct2/show/NCT01405521 (accessed on 14 July 2023).
- Xie, L.; Ming, L.; Ding, M.; Deng, L.; Liu, M.; Cong, Y. Paratyphoid fever A: Infection and prevention. Front. Microbiol. 2022, 13, 945235. [Google Scholar] [CrossRef]
- Xiong, K.; Zhu, C.; Chen, Z.; Zheng, C.; Tan, Y.; Rao, X.; Cong, Y. Vi Capsular polysaccharide produced by recombinant Salmonella enterica Serovar Paratyphi A confers immunoprotection against infection by Salmonella enterica Serovar Typhi. Front. Cell Infect. Microbiol. 2017, 7, 135. [Google Scholar] [CrossRef]
- Dobinson, H.C.; Gibani, M.M.; Jones, C.; Thomaides-Brears, H.B.; Voysey, M.; Darton, T.C.; Waddington, C.S.; Campbell, D.; Milligan, I.; Zhou, L.; et al. Evaluation of the clinical and microbiological response to Salmonella Paratyphi A infection in the first paratyphoid human challenge model. Clin. Infect. Dis. 2017, 64, 1066–1073. [Google Scholar] [CrossRef]
- NIH. Safety and Immunogenicity Study of GSK’s Clostridium Difficile Vaccine 2904545A when Administered in Healthy Adults Aged 18–45 Years and 50–70 Years. Available online: https://clinicaltrials.gov/ct2/show/NCT04026009 (accessed on 21 February 2023).
- Inoue, M.; Yonemura, T.; de Solom, R.; Yamaji, M.; Aizawa, M.; Knirsch, C.; Pride, M.W.; Jansen, K.U.; Gruber, W.; Webber, C. A phase 1 randomized study assessing safety and immunogenicity of two 3-dose regimens of a Clostridium difficile vaccine in healthy older Japanese adults. Vaccine 2019, 37, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, N.; Remich, S.A.; Peterson, J.; Peng, Y.; Gruber, W.C.; Jansen, K.U.; Pride, M.W.; Anderson, A.S.; Knirsch, C.; Webber, C. A Phase 2 Study evaluating the safety, tolerability, and immunogenicity of two 3-dose regimens of a Clostridium difficile vaccine in healthy US adults aged 65 to 85 years. Clin. Infect. Dis. 2020, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, G.; Gordon, D.L.; Steiner, T.; Tambyah, P.; Cosgrove, C.; Martens, M.; Bassily, E.; Chan, E.S.; Patel, D.; Chen, J.; et al. Safety, immunogenicity, and efficacy of a Clostridioides difficile toxoid vaccine candidate: A phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infect. Dis. 2021, 21, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Bézay, N.; Ayad, A.; Dubischar, K.; Firbas, C.; Hochreiter, R.; Kiermayr, S.; Kiss, I.; Pinl, F.; Jilma, B.; Westritschnig, K. Safety, immunogenicity and dose response of VLA84, a new vaccine candidate against Clostridium difficile, in healthy volunteers. Vaccine 2016, 34, 2585–2592. [Google Scholar] [CrossRef]
- Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther. Adv. Infect. Dis. 2016, 3, 23–42. [Google Scholar] [CrossRef]
- Riley, T.V.; Lyras, D.; Douce, G.R. Status of vaccine research and development for Clostridium difficile. Vaccine 2019, 37, 7300–7306. [Google Scholar] [CrossRef]
- Humphreys, D.P.; Wilcox, M.H. Antibodies for treatment of Clostridium difficile infection. Clin. Vaccine Immunol. 2014, 21, 913–923. [Google Scholar] [CrossRef]
- Semchenko, E.A.; Seib, K.L. Outer membrane vesicle vaccines for Neisseria gonorrhoeae. Nat. Rev. Urol. 2022, 19, 5–6. [Google Scholar] [CrossRef]
- Mahase, E. Meningitis vaccine could protect against gonorrhoea, studies find. BMJ 2022, 377, o997. [Google Scholar] [CrossRef]
- Edwards, J.L.; Jennings, M.P.; Apicella, M.A.; Seib, K.L. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit. Rev. Microbiol. 2016, 42, 928–941. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Johnston, C. Future prospects for new vaccines against sexually transmitted infections. Curr. Opin. Infect. Dis. 2017, 30, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Sikora, A.E. Infection: Proof of principle for effectiveness of a gonorrhoea vaccine. Nat. Rev. Urol. 2017, 14, 643–644. [Google Scholar] [CrossRef] [PubMed]
- van Deuren, M.; Brandtzaeg, P.; van der Meer, J.W. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 2000, 13, 144–166. [Google Scholar] [CrossRef] [PubMed]
- Booy, R.; Gentile, A.; Nissen, M.; Whelan, J.; Abitbol, V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum. Vaccin. Immunother. 2019, 15, 470–480. [Google Scholar] [CrossRef]
- Spinosa, M.R.; Progida, C.; Talà, A.; Cogli, L.; Alifano, P.; Bucci, C. The Neisseria meningitidis capsule is important for intracellular survival in human cells. Infect. Immun. 2007, 75, 3594–3603. [Google Scholar] [CrossRef] [PubMed]
- Pace, D. Quadrivalent meningococcal ACYW-135 glycoconjugate vaccine for broader protection from infancy. Expert. Rev. Vaccines 2009, 8, 529–542. [Google Scholar] [CrossRef]
- Serruto, D.; Bottomley, M.J.; Ram, S.; Giuliani, M.M.; Rappuoli, R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: Immunological, functional and structural characterization of the antigens. Vaccine 2012, 30 (Suppl. S2), B87–B97. [Google Scholar] [CrossRef]
- Rivero-Calle, I.; Raguindin, P.F.; Gómez-Rial, J.; Rodriguez-Tenreiro, C.; Martinón-Torres, F. Meningococcal group B vaccine for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroup B. Infect. Drug Resist. 2019, 12, 3169–3188. [Google Scholar] [CrossRef]
- Masignani, V.; Pizza, M.; Moxon, E.R. The development of a vaccine against Meningococcus B using reverse vaccinology. Front. Immunol. 2019, 10, 751. [Google Scholar] [CrossRef]
- McNeil, L.K.; Zagursky, R.J.; Lin, S.L.; Murphy, E.; Zlotnick, G.W.; Hoiseth, S.K.; Jansen, K.U.; Anderson, A.S. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol. Mol. Biol. Rev. 2013, 77, 234–252. [Google Scholar] [CrossRef]
- Kleinschmidt, A.; Vadivelu, K.; Serino, L.; Neidig, N.; de Wergifosse, B. Endogenous complement human serum bactericidal assay (enc-hSBA) for vaccine effectiveness assessments against meningococcal serogroup B. NPJ Vaccines 2021, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Østergaard, L.; Diez-Domingo, J.; Wysocki, J.; Flodmark, C.E.; Beeslaar, J.; Eiden, J.; Jiang, Q.; Jansen, K.U.; Jones, T.R.; et al. Meningococcal serogroup B bivalent rLP2086 vaccine elicits broad and robust serum bactericidal responses in healthy adolescents. J. Pediatric Infect. Dis. Soc. 2016, 5, 152–160. [Google Scholar] [CrossRef]
- Svennerholm, A.M.; Lundgren, A.; Leach, S.; Akhtar, M.; Qadri, F. Mucosal immune responses against an oral enterotoxigenic Escherichia coli vaccine evaluated in clinical trials. J. Infect. Dis. 2021, 224, S821–S828. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chowdhury, M.I.; Bhuiyan, T.R.; Kaim, J.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Begum, Y.A.; et al. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine 2019, 37, 5645–5656. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Akhtar, M.; Bhuiyan, T.R.; Chowdhury, M.I.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Lundgren, A.; et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: A double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2020, 20, 208–219. [Google Scholar] [CrossRef]
- NIH. A Phase 2 Bridging Study to Assess the New Formulation of ETVAX. Available online: https://clinicaltrials.gov/ct2/show/NCT05178134 (accessed on 21 February 2023).
- Steele, D.; Riddle, M.; Van De Verg, L.; Bourgeois, L. Vaccines for enteric diseases: A meeting summary. Expert. Rev. Vaccines 2012, 11, 407–409. [Google Scholar] [CrossRef]
- NIH. Study Confirming a Human Challenge Model and Investigating the Safety of VLA1701. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03576183 (accessed on 14 July 2023).
- Harro, C.; Chakraborty, S.; Feller, A.; DeNearing, B.; Cage, A.; Ram, M.; Lundgren, A.; Svennerholm, A.-M.; Bourgeois, A.L.; Walker, R.I. Refinement of a human challenge model for evaluation of enterotoxigenic Escherichia coli vaccines. Clin. Vaccine Immunol. 2011, 18, 1719–1727. [Google Scholar] [CrossRef]
- Chakraborty, S.; Randall, A.; Vickers, T.J.; Molina, D.; Harro, C.D.; DeNearing, B.; Brubaker, J.; Sack, D.A.; Bourgeois, A.L.; Felgner, P.L.; et al. Interrogation of a live-attenuated enterotoxigenic Escherichia coli vaccine highlights features unique to wild-type infection. NPJ Vaccines 2019, 4, 37. [Google Scholar] [CrossRef]
- Wolf, M.K. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 1997, 10, 569–584. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Lin, T.-L.; Yang, F.-L.; Ren, C.-T.; Pan, Y.-J.; Liao, K.-S.; Tu, I.-F.; Chang, Y.-P.; Cheng, Y.-Y.; Wu, C.-Y.; Wu, S.-H.; et al. Development of Klebsiella pneumoniae capsule polysaccharide-conjugated vaccine candidates using phage depolymerases. Front. Immunol. 2022, 13, 843183. [Google Scholar] [CrossRef] [PubMed]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal capsules and their types: Past, present, and future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Cryz, S.J., Jr.; Fürer, E.; Germanier, R. Safety and immunogenicity of Klebsiella pneumoniae K1 capsular polysaccharide vaccine in humans. J. Infect. Dis. 1985, 151, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Cryz, S.J., Jr.; Cross, A.S.; Sadoff, G.C.; Que, J.U. Human IgG and IgA subclass response following immunization with a polyvalent Klebsiella capsular polysaccharide vaccine. Eur. J. Immunol. 1988, 18, 2073–2075. [Google Scholar] [CrossRef]
- Cryz, S.J., Jr.; Mortimer, P.; Cross, A.S.; Fürer, E.; Germanier, R. Safety and immunogenicity of a polyvalent Klebsiella capsular polysaccharide vaccine in humans. Vaccine 1986, 4, 15–20. [Google Scholar] [CrossRef]
- Dintzis, R.Z. Rational design of conjugate vaccines. Pediatr. Res. 1992, 32, 376–385. [Google Scholar] [CrossRef]
- Trautmann, M.; Ruhnke, M.; Rukavina, T.; Held, T.K.; Cross, A.S.; Marre, R.; Whitfield, C. O-antigen seroepidemiology of Klebsiella clinical isolates and implications for immunoprophylaxis of Klebsiella infections. Clin. Diagn. Lab. Immunol. 1997, 4, 550–555. [Google Scholar] [CrossRef]
- Hansen, D.S.; Mestre, F.; Alberti, S.; Hernández-Allés, S.; Alvarez, D.; Doménech-Sánchez, A.; Gil, J.; Merino, S.; Tomás, J.M.; Benedí, V.J. Klebsiella pneumoniae lipopolysaccharide O typing: Revision of prototype strains and O-group distribution among clinical isolates from different sources and countries. J. Clin. Microbiol. 1999, 37, 56–62. [Google Scholar] [CrossRef]
- Choi, M.; Hegerle, N.; Nkeze, J.; Sen, S.; Jamindar, S.; Nasrin, S.; Sen, S.; Permala-Booth, J.; Sinclair, J.; Tapia, M.D.; et al. The diversity of lipopolysaccharide (O) and capsular polysaccharide (K) antigens of invasive Klebsiella pneumoniae in a multi-country collection. Front. Microbiol. 2020, 11, e1249. [Google Scholar] [CrossRef]
- Bulati, M.; Busà, R.; Carcione, C.; Iannolo, G.; Di Mento, G.; Cuscino, N.; Di Gesù, R.; Piccionello, A.P.; Buscemi, S.; Carreca, A.P.; et al. Klebsiella pneumoniae lipopolysaccharides serotype O2afg induce poor inflammatory immune responses ex vivo. Microorganisms 2021, 9, 1317. [Google Scholar] [CrossRef]
- Choi, M.; Tennant, S.M.; Simon, R.; Cross, A.S. Progress towards the development of Klebsiella vaccines. Expert. Rev. Vaccines 2019, 18, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Rani, M.; Vanashree, Y. Immunoprotective potential of polysaccharide-tetanus toxoid conjugate in Klebsiella pneumoniae induced lobar pneumonia in rats. Indian. J. Exp. Biol. 2005, 43, 40–45. [Google Scholar] [PubMed]
- Hegerle, N.; Choi, M.; Sinclair, J.; Amin, M.N.; Ollivault-Shiflett, M.; Curtis, B.; Laufer, R.S.; Shridhar, S.; Brammer, J.; Toapanta, F.R.; et al. Development of a broad spectrum glycoconjugate vaccine to prevent wound and disseminated infections with Klebsiella pneumoniae and Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0203143. [Google Scholar] [CrossRef] [PubMed]
- NIH. Evaluation of the Efficacy and Safety of MV140 (MV140). Available online: https://clinicaltrials.gov/ct2/show/NCT02543827 (accessed on 14 July 2023).
- NIH. Uromune in Treating Recurrent Urinary Tract Infections in Women. Available online: https://clinicaltrials.gov/ct2/show/NCT04096820 (accessed on 14 July 2023).
- Nickel, J.C.; Saz-Leal, P.; Doiron, R.C. Could sublingual vaccination be a viable option for the prevention of recurrent urinary tract infection in Canada? A systematic review of the current literature and plans for the future. Can. Urol. Assoc. J. 2020, 14, 281–287. [Google Scholar] [CrossRef]
- Yang, B.; Foley, S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int. 2018, 121, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Kunz Coyne, A.J.; El Ghali, A.; Holger, D.; Rebold, N.; Rybak, M.J. Therapeutic strategies for emerging multidrug-resistant Pseudomonas aeruginosa. Infect. Dis. Ther. 2022, 11, 661–682. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Colmer-Hamood, J.A.; Kopel, J.; San Francisco, M.J.; Hamood, A.N. Anti-Pseudomonas aeruginosa vaccines and therapies: An assessment of clinical trials. Microorganisms 2023, 11, 916. [Google Scholar] [CrossRef]
- de Sousa, T.; Hébraud, M.; Dapkevicius, M.; Maltez, L.; Pereira, J.E.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Genomic and metabolic characteristics of the pathogenicity in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 12892. [Google Scholar] [CrossRef]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Cabral, M.P.; Correia, A.; Vilanova, M.; Gärtner, F.; Moscoso, M.; García, P.; Vallejo, J.A.; Pérez, A.; Francisco-Tomé, M.; Fuentes-Valverde, V.; et al. A live auxotrophic vaccine confers mucosal immunity and protection against lethal pneumonia caused by Pseudomonas aeruginosa. PLOS Pathog. 2020, 16, e1008311. [Google Scholar] [CrossRef]
- Cryz, S.J., Jr.; Fürer, E.; Cross, A.S.; Wegmann, A.; Germanier, R.; Sadoff, J.C. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J. Clin. Investig. 1987, 80, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Killough, M.; Rodgers, A.M.; Ingram, R.J. Pseudomonas aeruginosa: Recent Advances in Vaccine Development. Vaccines 2022, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, E.; Gabelsberger, J.; Knapp, B.; Hundt, E.; Lenz, U.; Hungerer, K.D.; Gilleland, H.E., Jr.; Staczek, J.; Domdey, H.; von Specht, B.U. Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Infect. Immun. 1999, 67, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Adlbrecht, C.; Wurm, R.; Depuydt, P.; Spapen, H.; Lorente, J.A.; Staudinger, T.; Creteur, J.; Zauner, C.; Meier-Hellmann, A.; Eller, P.; et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients—A randomized clinical trial. Crit. Care 2020, 24, 74. [Google Scholar] [CrossRef]
- Shaikh, M.O.F.; Schaefers, M.M.; Merakou, C.; DiBlasi, M.; Bonney, S.; Liao, T.; Zurakowski, D.; Kehl, M.; Tabor, D.E.; DiGiandomenico, A.; et al. Multicomponent Pseudomonas aeruginosa vaccines eliciting Th17 cells and functional antibody responses confer enhanced protection against experimental acute pneumonia in Mice. Infect. Immun. 2022, 90, e0020322. [Google Scholar] [CrossRef]
- Sabzehali, F.; Goudarzi, H.; Salimi Chirani, A.; Yoosefi Izad, M.H.; Goudarzi, M. Development of multi-epitope subunit vaccine against Pseudomonas aeruginosa using OprF/OprI and PopB proteins. Arch. Clin. Infect. Dis. 2021, 16, e118243. [Google Scholar] [CrossRef]
- Dey, J.; Mahapatra, S.R.; Patnaik, S.; Lata, S.; Kushwaha, G.S.; Panda, R.K.; Misra, N.; Suar, M. Molecular characterization and designing of a novel multiepitope vaccine construct against Pseudomonas aeruginosa. Int. J. Pept. Res. Ther. 2022, 28, 49. [Google Scholar] [CrossRef]
- Elhag, M.; Alaagib, R.M.; Ahmed, N.M.; Abubaker, M.; Haroun, E.M.; Albagi, S.O.A.; Hassan, M.A. Design of epitope-based peptide vaccine against Pseudomonas aeruginosa fructose bisphosphate aldolase protein using immunoinformatics. J. Immunol. Res. 2020, 2020, 9475058. [Google Scholar] [CrossRef]
- Beg, A.Z.; Farhat, N.; Khan, A.U. Designing multi-epitope vaccine candidates against functional amyloids in Pseudomonas aeruginosa through immunoinformatic and structural bioinformatics approach. Infect. Genet. Evol. 2021, 93, 104982. [Google Scholar] [CrossRef]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus vaccine research and development: The past, present and future, including novel therapeutic strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Katayama, Y.; Matsuo, M.; Sasaki, T.; Morimoto, Y.; Sekiguchi, A.; Baba, T. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J. Infect. Chemother. 2014, 20, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Jahantigh, H.R.; Faezi, S.; Habibi, M.; Mahdavi, M.; Stufano, A.; Lovreglio, P.; Ahmadi, K. The candidate antigens to achieving an effective vaccine against Staphylococcus aureus. Vaccines 2022, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Miller, A.A.; Donald, R.G.K.; Scully, I.L.; Nanra, J.S.; Cooper, D.; Jansen, K.U. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum. Vaccines Immunother. 2012, 8, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Scully, I.L.; Timofeyeva, Y.; Illenberger, A.; Lu, P.; Liberator, P.A.; Jansen, K.U.; Anderson, A.S. Performance of a four-antigen Staphylococcus aureus vaccine in preclinical models of invasive S. aureus disease. Microorganisms 2021, 9, 177. [Google Scholar] [CrossRef]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–751. [Google Scholar] [CrossRef]
- Schmidt, C.S.; White, C.J.; Ibrahim, A.S.; Filler, S.G.; Fu, Y.; Yeaman, M.R.; Edwards, J.E., Jr.; Hennessey, J.P., Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012, 30, 7594–7600. [Google Scholar] [CrossRef]
- NIH. Non-Inferiority and Safety Study of EuTCV Compared to Typbar-TCV in Healthy 6 Months-45 Years Aged Participants. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04830371 (accessed on 14 July 2023).
- NIH. Immunogenicity and Safety of Vi-DT (Diphtheria toxoid) Typhoid Conjugate Vaccine (Phase III). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04051268 (accessed on 14 July 2023).
- NIH. Salmonella Conjugates CVD 1000: Study of Responses to Vaccination with Trivalent Invasive Salmonella Disease Vaccine. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03981952 (accessed on 14 July 2023).
- NIH. Study of DTwP-HepB-Hib-IPV (SHAN6™) Vaccine Administered Concomitantly with Routine Pediatric Vaccines to Healthy Infants and Toddlers in Thailand. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04429295 (accessed on 14 July 2023).
- NIH. Confirmatory Study of BK1310 in Healthy Infants. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03891758 (accessed on 14 July 2023).
- NIH. Immunogenicity and Safety of 23-Valent Pneumococcal Polysaccharide Vaccine in Healthy Volunteers Aged 2 Years and Above. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04278248 (accessed on 14 July 2023).
- NIH. Immunogenicity and Safety Study of 15-Valent Pneumococcal Conjugate Vaccine in 2-Month-Old and 3-Month-Old Healthy Volunteers. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04357522 (accessed on 14 July 2023).
- NIH. A Phase III Clinical Trial of a 13-Valent Pneumococcal Conjugate Vaccine in Healthy Infants. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02494999 (accessed on 14 July 2023).
- NIH. A Single Ascending Dose Study in Adults (Stage 1) and Single Ascending Dose-Finding Study (Stage 2) in Elderly Subjects with ASP3772, a Pneumococcal Vaccine. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03803202 (accessed on 14 July 2023).
- NIH. A Study to Evaluate the Safety and Immunogenecity of LBVE(Multivalent Pneumococcal Conjugate Vaccine) in Healthy Infants. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03467984 (accessed on 14 July 2023).
- NIH. A Phase 1/Phase 2 Study of Polyvalent Pneumococcal Conjugate Vaccine (V116) in Adults (V116-001). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04168190 (accessed on 14 July 2023).
- NIH. Study of Investigational Pneumococcal Vaccine in Healthy Adults, Toddlers and Infants. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01446926 (accessed on 14 July 2023).
- NIH. Study of a Pneumococcal Conjugate Vaccine in Adults Aged 50 to 84 Years. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04583618 (accessed on 14 July 2023).
- NIH. Phase I Clinical Trial of a Candidate PCV13 in Healthy People Aged 6 Weeks and Above (PICTPCV13i). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04100772 (accessed on 14 July 2023).
- NIH. Phase Ⅰa Clinical Trial of a Pneumococcal Vaccine (PⅠCTPV). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04087460 (accessed on 14 July 2023).
- NIH. Safety and Immunogenicity of the ‘EuPCV15’ in Healthy Korean Adults. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04830358 (accessed on 14 July 2023).
- NIH. Preventing UTIs in Chronic Neurogenic Bladder Dysfunction (Mix Methods) (PReSuTINeB). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02591901 (accessed on 14 July 2023).
- NIH. Safety and Immunogenicity of CVD 1902 Oral Attenuated Vaccine to Prevent S. Paratyphi A infection. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01129453 (accessed on 14 July 2023).
- NIH. Clostridium Difficile Vaccine Efficacy Trial (Clover). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03090191 (accessed on 14 July 2023).
- NIH. Efficacy Study of 4CMenB (Bexsero®) to Prevent Gonorrhoea Infection in Gay and Bisexual Men (GoGoVax). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04415424 (accessed on 14 July 2023).
- NIH. Challenge Study of an ETEC Vaccine. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01922856 (accessed on 14 July 2023).
- NIH. Shigella CVD 31000: Study of Responses with Shigella-ETEC Vaccine Strain CVD 1208S-122. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04634513 (accessed on 14 July 2023).
- NIH. A Double-Blind Placebo-Control Dose Escalating Study to Evaluate the Safety and Immunogenicity of dmLT by Oral, Sublingual and Intradermal Vaccination in Adults Residing in an Endemic Area. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03548064 (accessed on 14 July 2023).
- NIH. Safety and Immunogenicity of a Klebsiella Pneumoniae Tetravalent Bioconjugate Vaccine (Kleb4V). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04959344 (accessed on 14 July 2023).
- NIH. Confirmatory Phase II/III Study Assessing Efficacy, Immunogenicity and Safety of IC43. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01563263 (accessed on 14 July 2023).
- NIH. Clinical Trial of the Biomed rTSST-1 Variant Vaccine in Healthy Adults. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02814708 (accessed on 14 July 2023).
- NIH. Safety, Immunogenicity and Efficacy of GSK S. Aureus Candidate Vaccine (GSK3878858A) when Administered to Healthy Adults (Dose-Escalation) and to Adults 18 to 64 Years of Age with a Recent S. Aureus Skin and Soft Tissue Infection (SSTI). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04420221 (accessed on 14 July 2023).
- Ong, E.; Wong, M.U.; Huffman, A.; He, Y. COVID-19 Coronavirus vaccine design using reverse vaccinology and machine learning. Front. Immunol. 2020, 11, e1581. [Google Scholar] [CrossRef]
- Soltan, M.A.; Magdy, D.; Solyman, S.M.; Hanora, A. Design of Staphylococcus aureus new vaccine candidates with b and t cell epitope mapping, reverse vaccinology, and immunoinformatics. Omics 2020, 24, 195–204. [Google Scholar] [CrossRef]
- Bianconi, I.; Alcalá-Franco, B.; Scarselli, M.; Dalsass, M.; Buccato, S.; Colaprico, A.; Marchi, S.; Masignani, V.; Bragonzi, A. Genome-based approach delivers vaccine candidates against Pseudomonas aeruginosa. Front. Immunol. 2018, 9, 3021. [Google Scholar] [CrossRef]
| Vaccine | Advantages | Limitations | References |
|---|---|---|---|
| LAVs |
|
| [41,42,43,44] |
| IVs |
|
| [40,45,46,47] |
| Recombinant protein vaccines |
|
| [50,51,52,53,54,55,56] |
| DNA vaccines |
|
| [71,72,73,74,75,76,77] |
| mRNA vaccines |
|
| [81,82,83,84,85,86] |
| Bacterium | Vaccine Candidate | Number of Participants | Primary Outcome Measures and Data on Safety and Immunogenicity | Phase of the Study | Target Population | References |
|---|---|---|---|---|---|---|
| S. enterica serovar typhi | EuTCV | 444 | Seroconversion rate; solicited local and systemic AEs. Seroconversion in 99.4% of immunized individuals; reasonable safety profile. | Phase III NCT04830371 | 6 Months–45 Years | [90,219] |
| Vi-DT | 3071 | Immunogenicity (seroconversion rate). Anti-Vi-IgG seroconversion rate of 99.33%; good safety profile. | Phase III NCT04051268 | 6 months–60 years | [90,220] | |
| Typhoid Vi conjugate vaccine | NR | Anti-Vi IgG geometric mean titers increased by 502 times, from 4·2 EU/mL to 2383·7 EU/mL at day 28; safe and tolerable. | Phase III | NR | [90] | |
| Entervax | 99 | Diagnosis of typhoid fever. No results posted. | Phase IIb NCT01405521 | Adults 18–60 Years | [90,144] | |
| CVD 1000 | 96 | Frequency and severity of solicited local and systemic AEs. No results posted. | Phase I NCT03981952a | Adults 18–45 Years | [90,221] | |
| H. influenzae type b | Shan 6 | 460 | Geometric mean concentrations (aGMCs) of Abs against pertussis antigens; potent immunogenicity; good safety profile. | Phase III NCT04429295 | Healthy Infants and Toddlers in Thailand | [90,222] |
| Freeze-dried Haemophilus influenzae type b (Hib) combined vaccine | NR | NR | Phase III Chi-CTR2000032281 | NR | [90] | |
| MT-2355 (BK1310) | 267 | Antibody prevalence rate against anti-PRP with 1 μg/mL or higher, diphtheria toxin, pertussis, tetanus toxin, and polio virus. No results posted. | Phase III NCT03891758 | Healthy Infants | [90,223] | |
| LBVD | 460 | Number of participants with antibodies (Abs) above a predefined threshold against diphtheria (D), tetanus (T), hepatitis B (Hep B), Haemophilus influenzae type b (Hib), and poliovirus (Polio) antigens. Safe and immunogenic. | Phase I NCT04429295 | Healthy Infants and Toddlers in Thailand | [90,222] | |
| S. pneumoniae | 23-Valent pneumococcal polysaccharide vaccine (PPSV23) | 1940 | Immunogenicity study endpoint. Safety study endpoint. No results posted. | Phase III NCT04278248 | healthy volunteers aged 2 Years and above | [90,224] |
| 15-Valent pneumococcal conjugate vaccine (PCV) | 1950 | Immunogenicity study endpoint. Safety study endpoint. No results posted. | Phase III NCT04357522 | 2 and 3-month-old Healthy Volunteers | [90,225] | |
| 14-Valent PCV (adsorbed) | NR | NR | Phase III | NR | [90] | |
| 13-Valent PCV | 1200 | Geometric mean concentration (GMC) of serotype-specific pneumococcal IgG antibody concentration ≥ 0.35 ug/mL at 30 days after primary vaccination. No results posted. | Phase III NCT02494999 | healthy infants aged 2 months | [90,226] | |
| ASP3772 | 630 | Safety and immunological response of PCV13, ASP3772, and PPSV23. No results posted. | Phase II NCT03803202 | elderly 65 to 85 | [90,227] | |
| Multivalent PCV | 230 | Pneumococcal serotype-specific IgG GMC ratios. No results posted. | Phase II NCT03467984 | healthy infants | [90,228] | |
| Polyvalent PCV V116 | 600 | Adverse effects and serotype-specific opsonophagocytic activity (OPA); geometric mean titers (GMTs) for the common serotypes in V116 and Pneumovax™ 23. No results posted. | Phase II NCT04168190 | Healthy adults | [90,229] | |
| Nucovac | 48 | NR | Phase II CTRI/2013/ 05/003711 | Healthy adults 18–65 | [90] | |
| Pneumococcal recombinant protein vaccine (PPrV) (35) | 280 | Immunogenicity and adverse effects. No results posted. | Phase II NCT01446926 | Healthy Adults, Toddlers and Infants | [90,230] | |
| SP0202, SKYPAC | 750 | Geometric mean (GM) of serotype-specific opsonophagocytic (OPA) titers for all pneumococcal serotypes included in the SP0202 formulations. No results posted. | Phase II NCT04583618 | Adults Aged 50 to 84 | [90,231] | |
| 15-Valent PCV | 140 | NR | Phase II CTRI2019-02-017527 | healthy subjects 2–5 years | [90] | |
| PF-06842433 | NR | NR | Phase II EudraCT 2020-005039-59 | NR | [90] | |
| 13-Valent PCV | 237 | Adverse reactions and immunogenicity. No results posted. | Phase I NCT04100772 | Healthy People Aged 6 Weeks and Above | [90,232] | |
| Protein-based pneumococcal vaccine (PBPV) | 120 | Solicited and unsolicited adverse reactions; immunogenicity. No results posted. | Phase I NCT04087460 | Elderly 18 to 49 years of age | [90,233] | |
| 13-Valent PCV | NR | NR | Phase I | NR | [90] | |
| euPCV15 | 60 | Incidence of solicited AEs. No results posted. | Phase I NCT04830358 | Healthy Koreans 19–50 Years | [90,234] | |
| ExPEC | ExPEC9V | 18556 | Participants with first invasive extraintestinal pathogenic E. coli disease. No results posted. | Phase III NCT04899336 | Adults Aged 60 Years and Older | [90,133] |
| UTI Vx, FimH vaccine (FimCH) | NR | NR | Phase II | NR | [90] | |
| Uro-Vaxom (OM-89) | 48 | Checklist or consensus guidelines that can be used to measure a symptomatic urinary tract infection and practicality of carrying out a definitive randomized controlled clinical study. Urinary tract infection rates varied between different catheterization methods: male indwelling (2.72), clean intermittent (0.41), condom (0.36), female suprapubic (0.34), and normal voiding (0.06), with an overall incidence of 0.68. | Phase II NCT02591901 | Adults 18–75 Years | [90,235] | |
| ExPEC10V (VAC52416, JNJ-69968054) | 836 | Safety and antibody titers. No results posted. | Phase I/II NCT03819049 | Adults 60–85 Years | [90,134] | |
| Salmonella enterica serovar Paratyphi A | O:2,12-TT | NR | NR | Phase III | NR | [90] |
| Entervax | 99 | Diagnosis of typhoid fever. No results posted. | Phase IIb NCT01405521 | Adults 18–60 Years | [90,144] | |
| CVD 1902 | 51 | Safety and serum antibodies. No results posted. | Phase I NCT01129453 | Adults 18–45 Years | [90,236] | |
| C. difficile | PF-06425090 (+/− adjuvant) (62–64, 68) | 17535 | Number of first primary episodes of CDI. No results posted. | Phase III NCT03090191 | Adults 50 and older | [90,237] |
| PF-06425090 (+/− adjuvant) (62–64, 68) | 140 | Adverse effects. No results posted. | Phase I NCT04026009 | Adults 18–70 | [90,148] | |
| N.gonorrhoeae | 4CMenB (Bexsero) | 652 | Change in the incidence of the first episode of N. gonorrhoeae infection. Overall incidence of all episodes of N. gonorrhoeae infection. No results posted. | Phase III NCT04415424 | Gay and Bisexual Men 18 to ≤50 years of age | [90,238] |
| ETEC | ETVAX/dmLT; ETVAX (OEV-122); ETVAX (OEV-123); ETVAX (OEV-121); (OEV-124) | NR | NR | Phase IIb PACTR202010819218562 | NR | [90] |
| Phase I: CfaE + mLT (ID); CssBA + dmLT; Phase II: CfaE + mLT | 56 | Number of adverse events and prevented diarrhea episodes. No results posted. | Phase II NCT01922856 | Adults 18–50 Years | [90,239] | |
| Shigella-ETEC | NR | NR | Phase I | NR | [90] | |
| ShigETEC | NR | NR | Phase I EudraCT: 2020-000248-79 | NR | [90] | |
| CVD 31000 (CVD 1208S-122) | 54 | Adverse reactions. No results posted. | Phase I NCT04634513 | Adults 18–49 Years | [90,240] | |
| dmLT (LTR192G/L211A) (77) | 75 | Adverse effects and reactogenicity. No results posted. | Phase I NCT03548064 | Adults 18–45 Years | [90,241] | |
| K. pneumoniae | KlebV4 | 166 | Adverse effects and IgG titers against K. pneumoniae O serotypes. No results posted. | Phase I/II NCT04959344 | Adults 18–70 Years | [90,242] |
| P. aeruginosa | VLA43 (IC43) | 803 | Number of deaths until day 28. No results posted. | Phase II/III NCT01563263 (Discontinued) | Adults 18–80 Years | [90,243] |
| S. aureus | rTSST-1 variant vaccine (ORG28077) | 140 | Adverse events and fold increase of ELISA IgGs against rTSST-1. No results posted. | Phase II NCT02814708 | Adults 18–64 Years | [90,244] |
| GSK3878858A | 632 | Number of participants with solicited local adverse events. No results posted. | Phase I/II NCT04420221 | Adults 18–64 Years | [90,245] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, K.; Poh, C.L. The Promising Potential of Reverse Vaccinology-Based Next-Generation Vaccine Development over Conventional Vaccines against Antibiotic-Resistant Bacteria. Vaccines 2023, 11, 1264. https://doi.org/10.3390/vaccines11071264
Khalid K, Poh CL. The Promising Potential of Reverse Vaccinology-Based Next-Generation Vaccine Development over Conventional Vaccines against Antibiotic-Resistant Bacteria. Vaccines. 2023; 11(7):1264. https://doi.org/10.3390/vaccines11071264
Chicago/Turabian StyleKhalid, Kanwal, and Chit Laa Poh. 2023. "The Promising Potential of Reverse Vaccinology-Based Next-Generation Vaccine Development over Conventional Vaccines against Antibiotic-Resistant Bacteria" Vaccines 11, no. 7: 1264. https://doi.org/10.3390/vaccines11071264
APA StyleKhalid, K., & Poh, C. L. (2023). The Promising Potential of Reverse Vaccinology-Based Next-Generation Vaccine Development over Conventional Vaccines against Antibiotic-Resistant Bacteria. Vaccines, 11(7), 1264. https://doi.org/10.3390/vaccines11071264






