The Moderating Effect of Vaccine Hesitancy on the Relationship between the COVID-19 Vaccine Coverage Index and Vaccine Coverage
Abstract
1. Introduction
Study Aims
2. Materials and Methods
2.1. Data
2.2. Measures
2.3. Data Analysis Plan
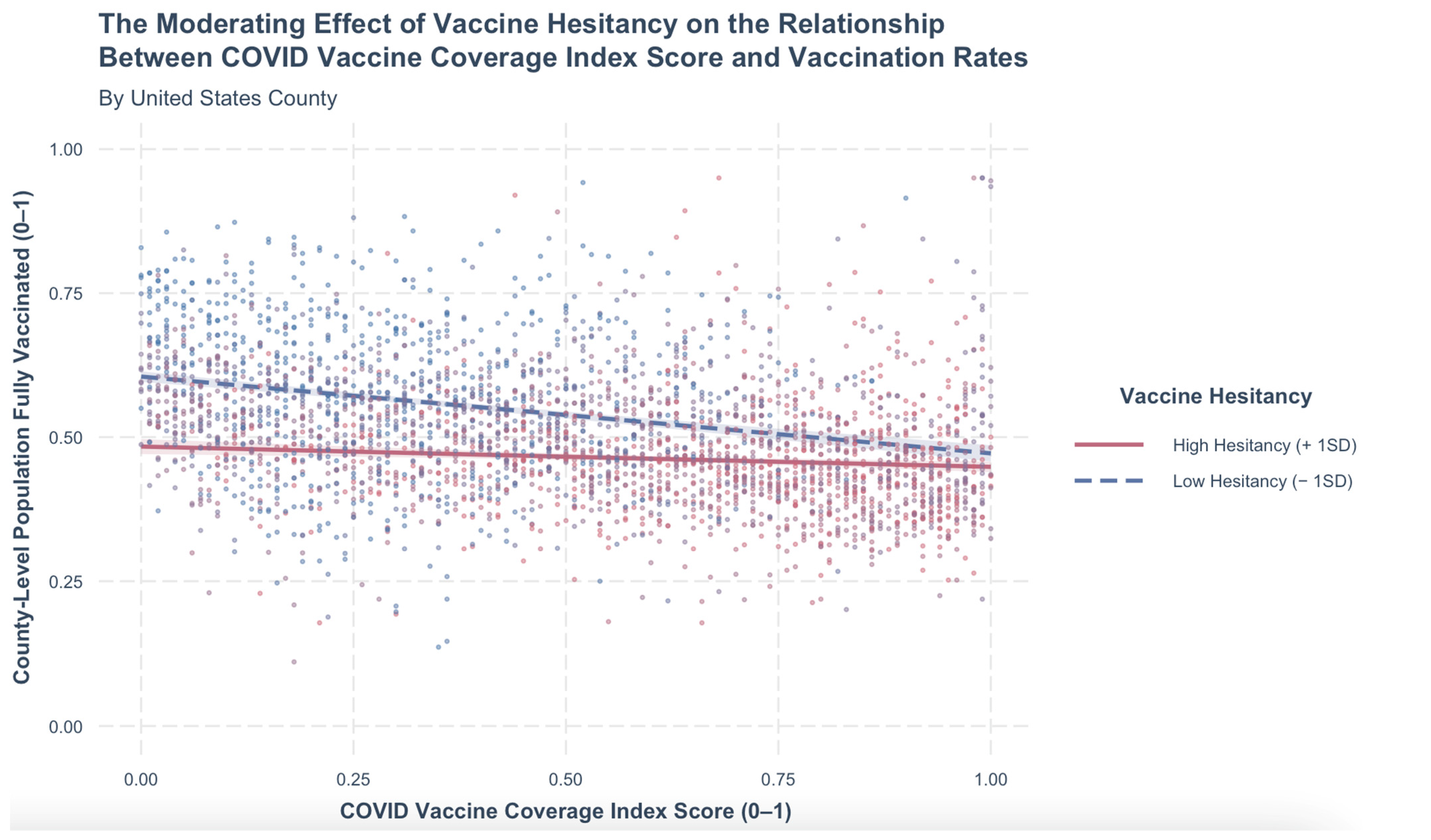
3. Results
4. Discussion
4.1. Implications for Health Equity
4.2. Implications for Practice
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Vanderslott, S. How is the World Doing in its Fight Against Vaccine Preventable Diseases? Our World Data 2018. Available online: https://ourworldindata.org/vaccine-preventable-diseases (accessed on 25 May 2023).
- Zhou, F.; Shefer, A.; Wenger, J.; Messonnier, M.; Wang, L.Y.; Lopez, A.; Moore, M.; Murphy, T.V.; Cortese, M.; Rodewald, L. Economic Evaluation of the Routine Childhood Immunization Program in the United States, 2009. Pediatrics 2014, 133, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Kawai, A.T. Racial/Ethnic and Socioeconomic Disparities in Adult Vaccination Coverage. Am. J. Prev. Med. 2021, 61, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Lindley, M.C.; Wortley, P.M.; Winston, C.A.; Bardenheier, B.H. The Role of Attitudes in Understanding Disparities in Adult Influenza Vaccination. Am. J. Prev. Med. 2006, 31, 281–285. [Google Scholar] [CrossRef]
- Lu, P.; O’Halloran, A.; Williams, W.W.; Lindley, M.C.; Farrall, S.; Bridges, C.B. Racial and Ethnic Disparities in Vaccination Coverage Among Adult Populations in the U.S. Vaccine 2015, 33, D83–D91. [Google Scholar] [CrossRef]
- Singleton, J.A.; Santibanez, T.A.; Wortley, P.M. Influenza and Pneumococcal Vaccination of Adults Aged ≥65: Racial/Ethnic Differences. Am. J. Prev. Med. 2005, 29, 412–420. [Google Scholar] [CrossRef]
- Kriss, J.L.; Hung, M.-C.; Srivastav, A.; Black, C.L.; Lindley, M.C.; Lee, J.T.; Koppaka, R.; Tsai, Y.; Lu, P.-J.; Yankey, D.; et al. COVID-19 Vaccination Coverage, by Race and Ethnicity—National Immunization Survey Adult COVID Module, United States, December 2020–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Indian Health Service. Coronavirus (COVID-19). U.S. Department of Health and Human Services. Available online: https://www.ihs.gov/coronavirus/ (accessed on 10 April 2022).
- Gravlee, C.C. Systemic Racism, Chronic Health Inequities, and COVID-19: A Syndemic in the Making? Am. J. Hum. Biol. 2020, 32, e23482. [Google Scholar] [CrossRef]
- Hill, L.; Artiga, S. COVID-19 Cases and Deaths by Race/Ethnicity: Current Data and Changes Over Time. KFF. Available online: https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-cases-and-deaths-by-race-ethnicity-current-data-and-changes-over-time/ (accessed on 10 April 2022).
- Mishra, A.; Sutermaster, S.; Smittenaar, P.; Stewart, N.; Sgaier, S.K. COVID-19 Vaccine Coverage Index: Identifying Barriers to COVID-19 Vaccine Uptake Across U.S. Counties. medRxiv 2021. [Google Scholar] [CrossRef]
- Surgo Ventures. The U.S. COVID-19 Vaccine Coverage Index (CVAC) Methodology. 2021. Available online: https://cvi-data-output.s3.amazonaws.com/assets/CVAC_Methodology_Feb2021.pdf (accessed on 26 May 2023).
- MacDonald, N.E. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Peters, M.D.J. Addressing Vaccine Hesitancy and Resistance for COVID-19 Vaccines. Int. J. Nurs. Stud. 2022, 131, 104241. [Google Scholar] [CrossRef] [PubMed]
- Romate, J.; Rajkumar, E.; Gopi, A.; Abraham, J.; Rages, J.; Lakshmi, R.; Jesline, J.; Bhogle, S. What Contributes to COVID-19 Vaccine Hesitancy? A Systematic Review of the Psychological Factors Associated with COVID-19 Vaccine Hesitancy. Vaccines 2022, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Simione, L.; Vagni, M.; Gnagnarella, C.; Bersani, G.; Pajardi, D. Mistrust and Beliefs in Conspiracy Theories Differently Mediate the Effects of Psychological Factors on Propensity for COVID-19 Vaccine. Front. Psychol. 2021, 12, 683684. [Google Scholar] [CrossRef]
- Simione, L.; Vagni, M.; Maiorano, T.; Giostra, V.; Pajardi, D. How Implicit Attitudes toward Vaccination Affect Vaccine Hesitancy and Behaviour: Developing and Validating the V-IRAP. Int. J. Environ. Res. Public. Health 2022, 19, 4205. [Google Scholar] [CrossRef] [PubMed]
- Goin-Kochel, R.P.; Mire, S.S.; Dempsey, A.G. Emergence of Autism Spectrum Disorder in Children from Simplex Families: Relations to Parental Perceptions of Etiology. J. Autism Dev. Disord. 2015, 45, 1451–1463. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Ahmed, M.; Hussain, S. The Anti-Vaccination Movement: A Regression in Modern Medicine. Cureus 2018, 10, e2919. [Google Scholar] [CrossRef]
- Mensah-Bonsu, N.E.M.-; Mire, S.S.; Sahni, L.C.; Berry, L.N.; Dowell, L.R.; Minard, C.G.; Cunningham, R.M.; Boom, J.A.; Voigt, R.G.; Goin-Kochel, R.P. Understanding Vaccine Hesitancy Among Parents of Children With Autism Spectrum Disorder and Parents of Children With Non-Autism Developmental Delays. J. Child Neurol. 2021, 36, 911–918. [Google Scholar] [CrossRef]
- Rao, T.S.S.; Andrade, C. The MMR Vaccine and Autism: Sensation, Refutation, Retraction, and Fraud. Indian J. Psychiatry 2011, 53, 95–96. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J.A. Vaccine Hesitancy. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef]
- Fridman, A.; Gershon, R.; Gneezy, A. COVID-19 and Vaccine Hesitancy: A Longitudinal Study. PLoS ONE 2021, 16, e0250123. [Google Scholar] [CrossRef] [PubMed]
- Lasher, E.; Fulkerson, G.; Seale, E.; Thomas, A.; Gadomski, A. COVID-19 Vaccine Hesitancy and Political Ideation among College Students in Central New York: The Influence of Differential Media Choice. Prev. Med. Rep. 2022, 27, 101810. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.K.; Wolfe, R.M.; Fox, D.E.; Fox, J.R.; Nowalk, M.P.; Troy, J.A.; Sharp, L.K. Vaccine Criticism on the World Wide Web. J. Med. Internet Res. 2005, 7, e17. [Google Scholar] [CrossRef]
- Lee, C.; Whetten, K.; Omer, S.; Pan, W.; Salmon, D. Hurdles to Herd Immunity: Distrust of Government and Vaccine Refusal in the US, 2002–2003. Vaccine 2016, 34, 3972–3978. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, P.G.; Thomas, K.; Shah, M.D.; Vizueta, N.; Cui, Y.; Vangala, S.; Fox, C.; Kapteyn, A. The Role of Trust in the Likelihood of Receiving a COVID-19 Vaccine: Results from a National Survey. Prev. Med. 2021, 153, 106727. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.R.; Alzubaidi, M.S.; Shah, U.; Abd-Alrazaq, A.A.; Shah, Z. A Scoping Review to Find Out Worldwide COVID-19 Vaccine Hesitancy and Its Underlying Determinants. Vaccines 2021, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.M.; St. Sauver, J.L.; Finney Rutten, L.J. Vaccine Hesitancy. Mayo Clin. Proc. 2015, 90, 1562–1568. [Google Scholar] [CrossRef]
- Kwok, K.O.; Li, K.-K.; Wei, W.I.; Tang, A.; Wong, S.Y.S.; Lee, S.S. Influenza Vaccine Uptake, COVID-19 Vaccination Intention and Vaccine Hesitancy among Nurses: A Survey. Int. J. Nurs. Stud. 2021, 114, 103854. [Google Scholar] [CrossRef] [PubMed]
- Latkin, C.; Dayton, L.; Miller, J.; Yi, G.; Balaban, A.; Boodram, B.; Uzzi, M.; Falade-Nwulia, O. A Longitudinal Study of Vaccine Hesitancy Attitudes and Social Influence as Predictors of COVID-19 Vaccine Uptake in the US. Hum. Vaccines Immunother. 2022, 18, 2043102. [Google Scholar] [CrossRef]
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.-L. Barriers of Influenza Vaccination Intention and Behavior—A Systematic Review of Influenza Vaccine Hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar] [CrossRef]
- [Dataset] Centers for Disease Control and Prevention. Estimates of Vaccine Hesitancy for COVID-19. U.S. Department of Health and Human Services. Available online: https://data.cdc.gov/stories/s/Vaccine-Hesitancy-for-COVID-19/cnd2-a6zw/ (accessed on 15 April 2022).
- [Dataset] Centers for Disease Control and Prevention. COVID-19 Vaccinations in the United States, County. U.S. Department of Health and Human Services. Available online: https://data.cdc.gov/Vaccinations/COVID-19-Vaccinations-in-the-United-States-County/8xkx-amqh (accessed on 15 April 2022).
- Centers for Disease Control and Prevention. COVID-19 County Hesitancy. U.S. Department of Health and Human Services. Available online: https://data.cdc.gov/Vaccinations/COVID-19-County-Hesitancy/c4bi-8ytd (accessed on 15 April 2022).
- Arizona Department of Health Services. Vaccine Administration. Available online: https://www.azdhs.gov/covid19/data/index.php#vaccine-admin (accessed on 15 April 2022).
- Reno Gazette Journal. COVID-19 Vaccine Tracker. Available online: https://data.rgj.com/covid-19-vaccine-tracker/ (accessed on 15 April 2022).
- Georgia Department of Public Health Vaccine Distribution Dashboard. COVID-19 Vaccine Dashboard. Available online: https://experience.arcgis.com/experience/3d8eea39f5c1443db1743a4cb8948a9c (accessed on 15 April 2022).
- Daily Advisor. Irion County, TX COVID-19 Vaccine Tracker. Available online: https://data.theadvertiser.com/covid-19-vaccine-tracker/texas/irion-county/48235/ (accessed on 15 April 2022).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum: Mahwah, NJ, USA, 1988. [Google Scholar]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M.; Danks, N.P.; Ray, S. Moderation analysis. In Partial Least Squares Structural Equation Modeling (PLS-SEM) Using R: A Workbook; Hair, J.F., Jr., Hult, G.T.M., Ringle, C.M., Sarstedt, M., Danks, N.P., Ray, S., Eds.; Classroom Companion: Business; Springer International Publishing: Cham, Switzerland, 2021; pp. 155–172. [Google Scholar] [CrossRef]
- Aguinis, H.; Beaty, J.C.; Boik, R.J.; Pierce, C.A. Effect size and power in assessing moderating effects of categorical variables using multiple regression: A 30-year review. J. Appl. Psychol. 2005, 90, 94–107. [Google Scholar] [CrossRef]
- Cutler, D.M.; Summers, L.H. The COVID-19 Pandemic and the $16 Trillion Virus. JAMA 2020, 324, 1495–1496. [Google Scholar] [CrossRef] [PubMed]
- Bruns, R.; Teran, N. Weighing the Cost of the Pandemic. Institute for Progress. 2022. Available online: https://progress.institute/weighing-the-cost-of-the-pandemic/ (accessed on 1 April 2022).
- Kawachi, I. COVID-19 and the ‘Rediscovery’ of Health Inequities. Int. J. Epidemiol. 2020, 49, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y. Racial and ethnic and income disparities in COVID-19 vaccination among Medicare beneficiaries. J. Am. Geriatr. Soc. 2022, 70, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.M.; Wang, A.; Grossman, M.K.; Pun, E.; Whiteman, A.; Deng, L.; Hallisey, E.; Sharpe, J.D.; Ussery, E.N.; Stokley, S.; et al. County-level COVID-19 vaccination coverage and social vulnerability—United States, December 14, 2020–March 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Joshi, A.D.; Drew, D.A.; Merino, J.; Ma, W.; Lo, C.-H.; Kwon, S.; Wang, K.; Graham, M.S.; Polidori, L.; et al. Racial and ethnic differences in COVID-19 vaccine hesitancy and uptake. medRxiv 2021. [Google Scholar] [CrossRef]
- Wu, C. Racial concentration and dynamics of COVID-19 vaccination in the United States. SSM—Popul. Health 2022, 19, 101198. [Google Scholar] [CrossRef] [PubMed]
- Andrasik, M.P.; Maunakea, A.K.; Oseso, L.; Rodriguez-Diaz, C.E.; Wallace, S.; Walters, K.; Yukawa, M. Awakening: The Unveiling of Historically Unaddressed Social Inequities During the COVID-19 Pandemic in the United States. Infect. Dis. Clin. 2022, 36, 295–308. [Google Scholar] [CrossRef]
- Barry, V. Patterns in COVID-19 vaccination coverage, by social vulnerability and urbanicity—United States, December 14, 2020–May 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 818–824. [Google Scholar] [CrossRef]
- Hernandez, I.; Dickson, S.; Tang, S.; Gabriel, N.; Berenbrok, L.A.; Guo, J. Disparities in distribution of COVID-19 vaccines across US counties: A geographic information system–based cross-sectional study. PLoS Med. 2022, 19, e1004069. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Critchfield-Jain, I.; Boykin, M.; Owens, A.; Muratore, R.; Nunn, T.; Oh, J. Racial/ethnic disparities in state-level COVID-19 vaccination rates and their association with structural racism. J. Racial Ethn. Health Disparities 2022, 9, 2361–2374. [Google Scholar] [CrossRef]
- Hu, S.; Xiong, C.; Li, Q.; Wang, Z.; Jiang, Y. COVID-19 vaccine hesitancy cannot fully explain disparities in vaccination coverage across the contiguous United States. Vaccine 2022, 40, 5471–5482. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.M.; Camara, K.S.; Adams, L.S.; Sarpong, A.P.; Fuller, D.G.; Peck, S.E.; Ramos, A.S.; Acevedo, A.L.; Badume, M.A.; Briggs, S.A.; et al. Profiles of COVID-19 Vaccine Hesitancy by Race and Ethnicity in Eastern Pennsylvania. PLoS ONE 2023, 18, e0280245. [Google Scholar] [CrossRef] [PubMed]
- Khubchandani, J.; Sharma, S.; Price, J.H.; Wiblishauser, M.J.; Sharma, M.; Webb, F.J. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Sssessment. J. Community Health 2021, 46, 270–277. [Google Scholar] [CrossRef]
- Quinn, S.C.; Jamison, A.; Freimuth, V.S.; An, J.; Hancock, G.R.; Musa, D. Exploring Racial Influences on Flu Vaccine Attitudes and Behavior: Results of a National Survey of African American and White Adults. Vaccine 2017, 35, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.; Martinez, B.; Shin, M.B.; Allee-Munoz, A.; Rodriguez, I.; Navarro, J.; Thomas-Barrios, K.R.; Kast, W.M.; Baezconde-Garbanati, L. Understanding Medical Mistrust and HPV Vaccine Hesitancy among Multiethnic Parents in Los Angeles. J. Behav. Med. 2023, 46, 100–115. [Google Scholar] [CrossRef]
- Willis, D.E.; Andersen, J.A.; Bryant-Moore, K.; Selig, J.P.; Long, C.R.; Felix, H.C.; Curran, G.M.; McElfish, P.A. COVID-19 Vaccine Hesitancy: Race/Ethnicity, Trust, and Fear. Clin. Transl. Sci. 2021, 14, 2200–2207. [Google Scholar] [CrossRef]
- Reimer, N.K.; Atari, M.; Karimi-Malekabadi, F.; Trager, J.; Kennedy, B.; Graham, J.; Dehghani, M. Moral values predict county-level COVID-19 vaccination rates in the United States. Am. Psychol. 2022, 77, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.B.; Bell, R.A. Predictors of intention to vaccinate against COVID-19: Results of a nationwide survey. Vaccine 2021, 39, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.V. Long-Term Cultural Barriers to Sustaining Collective Effort in Vaccination against COVID-19; University of Otago: Dunedin, New Zealand, 2021; Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3943011 (accessed on 22 May 2023). [CrossRef]
- Williams, D.R.; Cooper, L.A. COVID-19 and health equity—A new kind of “herd immunity”. JAMA 2020, 323, 2478–2480. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. CDC COVID-19 Response Health Equity Strategy: Accelerating Progress towards Reducing COVID-19 Disparities and Achieving Health Equity. The U.S. Department of Health and Human Services. Available online: https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/cdc-strategy.html (accessed on 23 May 2023).
- Surgo Ventures. How Do We Get America Vaccinated? Available online: https://surgoventures.org/vaccine-persona-explainer (accessed on 22 May 2023).
- Surgo Ventures; Mary’s Center. COVID-19 Vaccine Ambassador Toolkit. 2022. Available online: https://precisionforcovid.org/ambassador (accessed on 22 May 2023).
- Ogunwole, S.U.; Rabe, M.A.; Roberts, A.W.; Caplan, Z. Population under Age 18 Declined Last Decade. US Census Bureau. 2021. Available online: https://www.census.gov/library/stories/2021/08/united-states-adult-population-grew-faster-than-nations-total-population-from-2010-to-2020.html (accessed on 23 May 2023).
- Mackun, P.; Comenetz, J.; Spell, L. Around Four-Fifths of All U.S. Metro Areas Grew between 2010 and 2020. US Census Bureau. 2021. Available online: https://www.census.gov/library/stories/2021/08/more-than-half-of-united-states-counties-were-smaller-in-2020-than-in-2010.html (accessed on 23 May 2023).
- Bosman, J.; Hoffman, J.; Sanger-Katz, M.; Arango, T. Who Are the Unvaccinated in America? There’s No One Answer. The New York Times 2021. Available online: https://www.nytimes.com/2021/07/31/us/virus-unvaccinated-americans.html (accessed on 22 May 2023).
- Johns Hopkins. Understanding Vaccination Progress by Country. Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/vaccines/international (accessed on 22 May 2023).
- Bolotin, S.; Wilson, S.; Murti, M. Achieving and Sustaining Herd Immunity to SARS-CoV-2. CMAJ Can. Med. Assoc. J. 2021, 193, E1089. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Percentages of Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2. Vaccines 2022, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- USAFacts. US Coronavirus Vaccine Tracker. Available online: https://usafacts.org/visualizations/covid-vaccine-tracker-states (accessed on 22 May 2023).
- Centers for Disease Control and Prevention. COVID Data Tracker. U.S. Department of Health and Human Services. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 22 May 2023).
- Adhikari, S.; Pantaleo, N.P.; Feldman, J.M.; Ogedegbe, O.; Thorpe, L.; Troxel, A.B. Assessment of Community-Level Disparities in Coronavirus Disease 2019 (COVID-19) Infections and Deaths in Large US Metropolitan Areas. JAMA Netw. Open 2020, 3, e2016938. [Google Scholar] [CrossRef] [PubMed]
- Ndugga, N.; Hill, L.; Artiga, S.; Haldar, H. Latest Data on COVID-19 Vaccinations by Race/Ethnicity. KFF. 2022. Available online: https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-by-race-ethnicity/ (accessed on 4 October 2022).
- Sepulveda, E.R.; Brooker, A.-S. Income Inequality and COVID-19 Mortality: Age-Stratified Analysis of 22 OECD Countries. SSM—Popul. Health 2021, 16, 100904. [Google Scholar] [CrossRef] [PubMed]
| Barriers * | Definition |
|---|---|
| Historic Undervaccination | Lower coverage and higher refusal rates based on the proportion of children and adults that have routine vaccinations, such as MMR, polio, HPV, flu |
| Sociodemographic Barriers | Proportion of the population socio-economically disadvantaged based on census data such as unemployment, household income, education, proportion of racial and ethnic minority groups |
| Resource-Constrained Health System | Healthcare system has less available healthcare staff and funding, lower quality of care, and less infrastructure of vaccine administration per capita |
| Healthcare Accessibility Barriers | Proportion of population with barriers to healthcare such as cost and transportation |
| Irregular Care-Seeking Behavior | Proportion of population without routine access to providers and/or healthcare systems |
| Mean (SD) | Min. | Max. | |
|---|---|---|---|
| CVAC Score | 0.5 (0.29) | 0.0 | 1.0 |
| % Vaccinated (April 2022) * | 50.8 (11.9) | 11.1 | 95.0 |
| % Vaccine Hesitant * | 19.2 (5.3) | 5.0 | 32.3 |
| Racial/Ethnic Demographics * | |||
| % Hispanic/Latino | 9.4 (13.9) | 0.0 | 99.2 |
| % American Indian/Alaskan Native | 1.8 (7.6) | 0.0 | 91.9 |
| % Asian | 1.4 (2.7) | 0.0 | 41.7 |
| % Black | 9.0 (14.4) | 0.0 | 87.2 |
| % Native Hawaiian/Pacific Islander | 1.0 (0.4) | 0.0 | 11.1 |
| % White | 76.3 (20.2) | 7.0 | 100.0 |
| N = 3130 |
| (1) | (2) | (3) | |
|---|---|---|---|
| CVAC | −0.14 *** | −0.26 *** | −0.28 *** |
| (0.01) | (0.03) | (0.03) | |
| Vaccine Hesitant | −1.14 *** | −0.65 *** | |
| (0.07) | (0.07) | ||
| CVAC: Hesitant | 0.92 *** | 0.50 *** | |
| (0.13) | (0.13) | ||
| Racial/Ethnic Demographics | |||
| Hispanic/Latino | −0.01 | ||
| (0.12) | |||
| American Indian/Alaska Native | 0.09 | ||
| (0.12) | |||
| Asian | 0.77 *** | ||
| (0.15) | |||
| Black | −0.10 | ||
| (0.12) | |||
| Native Hawaiian/Pacific Islander | −3.22 *** | ||
| (0.55) | |||
| White | −0.27 * | ||
| (0.12) | |||
| Constant | 0.58 *** | 0.76 *** | 0.93 *** |
| (0.00) | (0.01) | (0.11) | |
| F-Statistic | 397.2 *** | 269.5 *** | 220.4 *** |
| Adjusted R-Squared | 0.11 | 0.21 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolley, A.J.; Scott, V.C.; Mitsdarffer, M.L.; Scaccia, J.P. The Moderating Effect of Vaccine Hesitancy on the Relationship between the COVID-19 Vaccine Coverage Index and Vaccine Coverage. Vaccines 2023, 11, 1231. https://doi.org/10.3390/vaccines11071231
Tolley AJ, Scott VC, Mitsdarffer ML, Scaccia JP. The Moderating Effect of Vaccine Hesitancy on the Relationship between the COVID-19 Vaccine Coverage Index and Vaccine Coverage. Vaccines. 2023; 11(7):1231. https://doi.org/10.3390/vaccines11071231
Chicago/Turabian StyleTolley, Annalise Julia, Victoria C. Scott, Mary Louise Mitsdarffer, and Jonathan P. Scaccia. 2023. "The Moderating Effect of Vaccine Hesitancy on the Relationship between the COVID-19 Vaccine Coverage Index and Vaccine Coverage" Vaccines 11, no. 7: 1231. https://doi.org/10.3390/vaccines11071231
APA StyleTolley, A. J., Scott, V. C., Mitsdarffer, M. L., & Scaccia, J. P. (2023). The Moderating Effect of Vaccine Hesitancy on the Relationship between the COVID-19 Vaccine Coverage Index and Vaccine Coverage. Vaccines, 11(7), 1231. https://doi.org/10.3390/vaccines11071231





