Influence of SARS-CoV-2 mRNA Vaccine Booster among Cancer Patients on Active Treatment Previously Immunized with Inactivated versus mRNA Vaccines: A Prospective Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
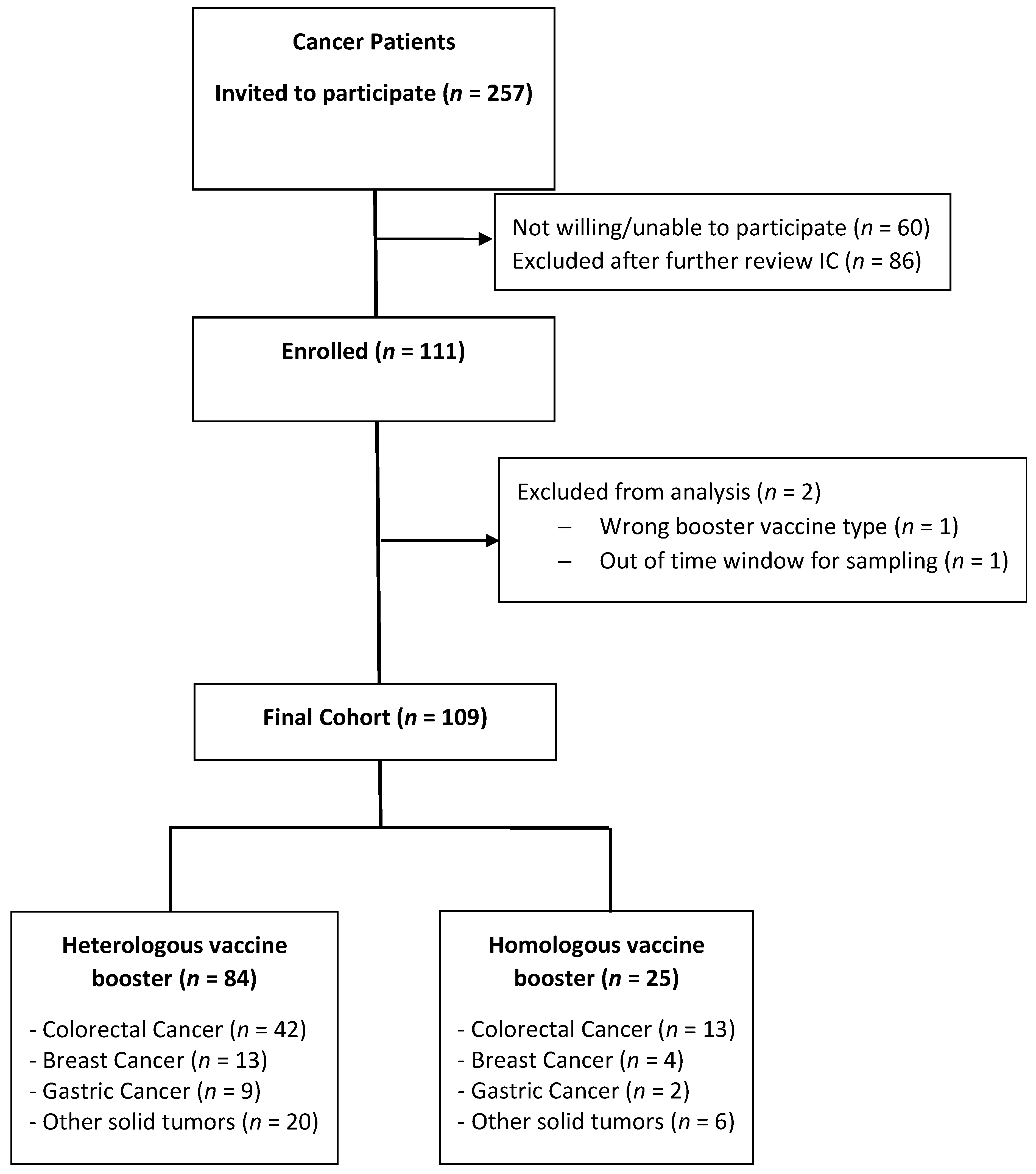
2.1. Patients
2.2. Determination of Anti-SARS-CoV-2 IgG Antibodies
2.3. Determination of Neutralizing Antibodies against SARS-CoV-2
2.4. T-Cell Immune Response
2.5. Statistical Analyses
3. Results
3.1. Patients
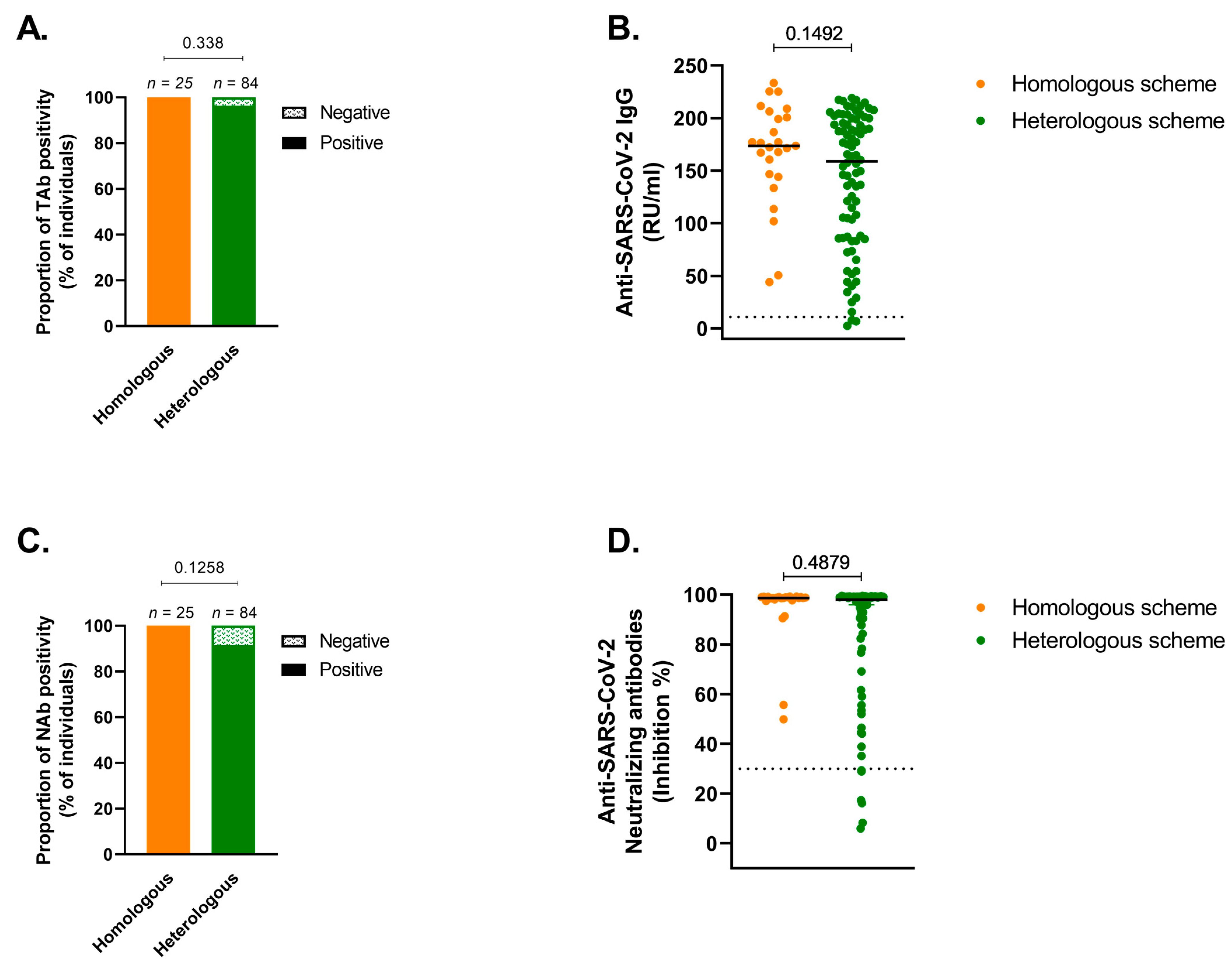
3.2. Humoral Response
3.3. T-Cell Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Akil, L.; Barner, Y.M.; Bisht, A.; Okoye, E.; Ahmad, H.A. COVID-19 incidence and death rates in the southern region of the United States: A racial and ethnic association. Int. J. Environ. Res. Public Health 2022, 19, 13990. [Google Scholar] [CrossRef]
- Giannakoulis, V.G.; Papoutsi, E.; Siempos, I.I. Effect of Cancer on Clinical Outcomes of Patients with COVID-19: A Meta-Analysis of Patient Data. JCO Glob. Oncol. 2020, 6, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, N.; Bates, B.; Song, Q.; Madhira, V.; Yan, Y.; Dong, S.; Lee, E.; Kuhrt, N.; Shao, Y.R.; Liu, F.; et al. Outcomes of COVID-19 in Patients With Cancer: Report From the National COVID Cohort Collaborative (N3C). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2232–2246. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Busubaih, J.S.; Al Dossar, N.; Alsuliman, M.; Baltyour, S.A.; Alissa, I.; Al Hassar, H.I.; Al Aithan, N.A.; Albassri, H.A.; et al. Colorectal cancer in patients with SARS-CoV-2: A systemic review and meta-analysis. Infect. Agents Cancer 2022, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Gonzalez, M.A.; Ruthrich, M.M.; Sharon, E.; von Lilienfeld-Toal, M. COVID-19 and Cancer: Special Considerations for Patients Receiving Immunotherapy and Immunosuppressive Cancer Therapies. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 176–188. [Google Scholar] [CrossRef]
- Strang, P.; Schultz, T. Dying with cancer and COVID-19, with special reference to lung cancer: Frailty as a risk factor. Cancers 2022, 14, 6002. [Google Scholar] [CrossRef]
- Grivas, P.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.Y.; Shyr, Y.; Li, X.; Choueiri, T.K.; Painter, C.A.; et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: A report from the COVID-19 and cancer consortium. Ann. Oncol. 2021, 32, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Hulme, W.J.; Williamson, E.J.; Green, A.C.A.; Bhaskaran, K.; McDonald, H.I.; Rentsch, C.T.; Schultze, A.; Tazare, J.; Curtis, H.J.; Walker, A.J.; et al. Comparative effectiveness of ChAdOx1 versus BNT162b2 COVID-19 vaccines in health and social care workers in England: Cohort study using OpenSAFELY. BMJ 2022, 378, e068946. [Google Scholar] [CrossRef]
- Shmueli, E.S.; Itay, A.; Margalit, O.; Berger, R.; Halperin, S.; Jurkowicz, M.; Levin, E.G.; Levy, I.; Olmer, L.; Regev-Yochay, G.; et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy—A single centre prospective study. Eur. J. Cancer 2021, 157, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [PubMed]
- Becerril-Gaitan, A.; Vaca-Cartagena, B.F.; Ferrigno, A.S.; Mesa-Chavez, F.; Barrientos-Gutierrez, T.; Tagliamento, M.; Lambertini, M.; Villarreal-Garza, C. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2022, 160, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Balcells, M.E.; Le Corre, N.; Duran, J.; Ceballos, M.E.; Vizcaya, C.; Mondaca, S.; Dib, M.; Rabagliati, R.; Sarmiento, M.; Burgos, P.I.; et al. Reduced immune response to inactivated SARS-CoV-2 vaccine in a cohort of immunocompromised patients in Chile. Clin. Infect. Dis. 2022, 75, e594–e602. [Google Scholar] [CrossRef]
- Saure, D.; O’Ryan, M.; Torres, J.P.; Zuniga, M.; Santelices, E.; Basso, L.J. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: A sentinel surveillance study. Lancet Infect. Dis. 2022, 22, 56–63. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Munoz-Ruiz, M.; McKenzie, D.R.; Del Molino Del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Tang, K.; Wei, Z.; Wu, X. Impaired serological response to COVID-19 vaccination following anticancer therapy: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 4860–4868. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Cerqueira-Silva, T.; de Araujo Oliveira, V.; Paixao, E.S.; Junior, J.B.; Penna, G.O.; Werneck, G.L.; Pearce, N.; Barreto, M.L.; Boaventura, V.S.; Barral-Netto, M. Duration of protection of CoronaVac plus heterologous BNT162b2 booster in the Omicron period in Brazil. Nat. Commun. 2022, 13, 4154. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Wang, M.; Islam, M.S.; Liao, P.; Hu, Y.; Chen, X. Humoral and Cellular Immune Responses of COVID-19 vaccines against SARS-Cov-2 Omicron variant: A systemic review. Int. J. Biol. Sci. 2022, 18, 4629–4641. [Google Scholar] [CrossRef]
- Rahman, S.; Hossain, J.; Nahar, Z.; Shahriar, M.; Bhuiyan, M.A.; Islam, R. Emerging SARS-CoV-2 variants and subvariants: Challenges and opportunities in the context of COVID-19 pandemic. Environ. Health Insights 2022, 16, 1–7. [Google Scholar]
- Poparn, H.; Srichumpuang, C.; Sosothikul, D.; Jantarabenjakul, W.; Lauhasurayotin, S.; Techavichit, P.; Chiangthong, K.; Poovorawan, Y. Immune Response after 2 Doses of BNT162b2 mRNA COVID-19 Vaccinations in Children and Adolescents with Cancer and Hematologic Diseases. Asian Pac. J. Cancer Prev. 2022, 23, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, A.M.; Giacobini, G.; Anichini, G.; Gandolfo, C.; D’Alonzo, V.; Calabro, L.; Lofiego, M.F.; Cusi, M.G.; Maio, M. SARS-CoV-2 infection in cancer patients on active therapy after the booster dose of mRNA vaccines. Eur. J. Cancer 2022, 171, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, S.J.; Yu, J.; Olson, D.; Zha, Y.; Pezeshk, A.; Cabanov, A.; Pyzer, A.R.; Trujillo, J.; Derman, B.A.; O’Donnell, P.; et al. Antibody and T cell responses to COVID-19 vaccination in patients receiving anticancer therapies. J. Immunother. Cancer 2022, 10, e004766. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kainulainen, M.H.; Jiang, N.; Di, H.; Bonenfant, G.; Mills, L.; Currier, M.; Shrivastava-Ranjan, P.; Calderon, B.M.; Sheth, M.; et al. Differential neutralization and inhibition of SARS-CoV-2 variants by antibodies elicited by COVID-19 mRNA vaccines. Nat. Commun. 2022, 13, 4350. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Stemmer, A.; Ghantous, N.; Ness, A.; Awwad, M.; Leibovici-Weisman, Y.; Stemmer, S.M. Antibody Titers After a Third and Fourth SARS-CoV-2 BNT162b2 Vaccine Dose in Older Adults. JAMA Netw. Open 2022, 5, e2223090. [Google Scholar] [CrossRef]

| Homologous Vaccine Scheme (n = 25) | Heterologous Vaccine Scheme (n = 84) | p Value | |
|---|---|---|---|
| Median Age (range) | 52 (10) | 58 (13) | 0.051 |
| Sex (%) Female Male | 12 (48) 13 (52) | 45 (53) 39 (45) | 0.147 |
| Comorbidities (%) Hypertension Diabetes Mellitus Asthma/COPD Chronic Liver disease | 5 (20) 1 (4) 0 0 | 30 (36) 19 (22) 4 (5) 3 (4) | 0.22 0.04 0.57 1.00 |
| Diagnoses (%) Colorectal Cancer Breast Cancer Gastric Cancer Other solid tumors | 13 (52) 4 (16) 2 (8) 6 (24) | 42 (50) 13 (15) 9 (10) 20 (24) | 0.457 |
| Stage Localized Metastatic | 8 (32) 17 (68) | 29 (35) 55 (65) | 0.815 |
| Median Lymphocyte blood count (IQR) | 1260 (1005 –1855) | 1410 (1020 –1910) | 0.69 |
| Chemotherapy before first COVID19 vaccination Yes No | 9 (36) 16 (64) | 32 (38) 52 (62) | 0.849 |
| Nab a | Tab b | |||
|---|---|---|---|---|
| Variable | Coef. (95% CI) | p Value | GMR (95% CI) | p Value |
| Vaccine group | ||||
| Heterologous group | Reference | Reference | ||
| Homologous group | 0.26 (0.029–0.50) | 0.027 | 1.41 (1.009–1.989) | 0.044 |
| Lymphocyte count | 0.0001 (0.00005–0.0002) | 0.005 | 1.00025 (1.0000–1.0004) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondaca, S.; Walbaum, B.; Le Corre, N.; Ferrés, M.; Valdés, A.; Martínez-Valdebenito, C.; Ruiz-Tagle, C.; Macanas-Pirard, P.; Ross, P.; Cisternas, B.; et al. Influence of SARS-CoV-2 mRNA Vaccine Booster among Cancer Patients on Active Treatment Previously Immunized with Inactivated versus mRNA Vaccines: A Prospective Cohort Study. Vaccines 2023, 11, 1193. https://doi.org/10.3390/vaccines11071193
Mondaca S, Walbaum B, Le Corre N, Ferrés M, Valdés A, Martínez-Valdebenito C, Ruiz-Tagle C, Macanas-Pirard P, Ross P, Cisternas B, et al. Influence of SARS-CoV-2 mRNA Vaccine Booster among Cancer Patients on Active Treatment Previously Immunized with Inactivated versus mRNA Vaccines: A Prospective Cohort Study. Vaccines. 2023; 11(7):1193. https://doi.org/10.3390/vaccines11071193
Chicago/Turabian StyleMondaca, Sebastián, Benjamín Walbaum, Nicole Le Corre, Marcela Ferrés, Alejandro Valdés, Constanza Martínez-Valdebenito, Cinthya Ruiz-Tagle, Patricia Macanas-Pirard, Patricio Ross, Betzabé Cisternas, and et al. 2023. "Influence of SARS-CoV-2 mRNA Vaccine Booster among Cancer Patients on Active Treatment Previously Immunized with Inactivated versus mRNA Vaccines: A Prospective Cohort Study" Vaccines 11, no. 7: 1193. https://doi.org/10.3390/vaccines11071193
APA StyleMondaca, S., Walbaum, B., Le Corre, N., Ferrés, M., Valdés, A., Martínez-Valdebenito, C., Ruiz-Tagle, C., Macanas-Pirard, P., Ross, P., Cisternas, B., Pérez, P., Cabrera, O., Cerda, V., Ormazábal, I., Barrera, A., Prado, M. E., Venegas, M. I., Palma, S., Broekhuizen, R., ... Nervi, B. (2023). Influence of SARS-CoV-2 mRNA Vaccine Booster among Cancer Patients on Active Treatment Previously Immunized with Inactivated versus mRNA Vaccines: A Prospective Cohort Study. Vaccines, 11(7), 1193. https://doi.org/10.3390/vaccines11071193








