Abstract
There is growing concern among healthcare providers worldwide regarding the prevalence of multidrug-resistant Acinetobacter baumannii (A. baumannii). Some of the worst hospital-acquired infections, often in intensive care units (ICUs), are caused by this bacterial pathogen. In recent years, the rise in multidrug-resistant A. baumannii has been linked to the overuse of antimicrobial drugs and the lack of adequate infection control measures. Infections caused by this bacterial pathogen are the result of prolonged hospitalization and ICU stays, and they are associated with increased morbidity and mortality. This review outlines the epidemiology, risk factors, and antimicrobial resistance associated with A. baumannii in various countries, with a special focus on the Kingdom of Saudi Arabia. In response to the growing concern regarding this drug-resistant bacteria, fundamental information about its pathology has been incorporated into the development of vaccines. Although these vaccines have been successful in animal models, their effectiveness in humans remains unproven. The review will discuss the development of A. baumannii vaccines, potential related obstacles, and efforts to find an effective strategy against this pathogen.
1. Introduction
Acinetobacter baumannii is a widely distributed Gram-negative, non-fermentative, strictly non-motile, and oxidase-negative microbial pathogen that has been found in both natural and clinical environments [1,2]. It is also one of the six most significant pathogens in the ESKAPE group, which includes Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa, and Enterobacter species [3,4]. According to the Infectious Disease Society of America, A. baumannii is a serious global nosocomial concern [3,5]. It is a primary cause of various infections, particularly in immunosuppressed individuals or those receiving mechanical ventilation in intensive care units (ICUs) [6]. The most common infections associated with A. baumannii include pneumonia, bacteremia, meningitis, respiratory tract infection, and urinary tract infection [5]. Over the past decade, mortality rates due to A. baumannii infections have increased in many regions worldwide, ranging from 30% to 75% [7,8,9]. Infections caused by A. baumannii are associated with various risk factors, including burns, preterm birth, prolonged hospital stays, mechanical ventilation, indwelling devices, and the extensive use of antibiotic therapy [10,11,12].
According to the World Health Organization, multidrug-resistant A. baumannii is one of the most critical pathogens due to its resistance to numerous antibiotic classes, particularly carbapenems and third-generation cephalosporins [7]. Several studies conducted in the Kingdom of Saudi Arabia have indicated the increasing resistance of A. baumannii isolates to various antibiotics [13]. During the coronavirus 2019 (COVID-19) pandemic, the healthcare crisis caused by A. baumannii, particularly the carbapenem-resistant strains, reached its peak in ICUs [14,15]. Therefore, developing novel vaccines and drug targets remains imperative to combat the relentless emergence of drug resistance in A. baumannii against currently available antibacterial drugs [16,17]. Developing novel vaccines and therapeutics is a time-consuming process that often spans several years [18,19]. Before advancing to clinical trials, vaccine candidates must undergo testing in experimental animals to assess reactogenicity and immune response [20]. Furthermore, it is essential to evaluate the pharmacological and toxicological properties of the targeted vaccine candidates to ensure their safety and efficacy [21]. This review investigates the spread of multidrug-resistant A. baumannii infections in healthcare settings in Saudi Arabia and explores potential measures to prevent their transmission.
2. A. baumannii’s Mechanisms of Antibiotic Resistance
Healthcare facilities are currently grappling with an outbreak of multidrug-resistant A. baumannii [13,22]. Surveillance investigations worldwide have reported a significant increase in antimicrobial resistance by A. baumannii across diverse regions, including South East Asia [23], the Arabian Peninsula [24], and other parts of the world [22]. This has posed substantial healthcare and financial challenges in Saudi Arabia, where numerous cases of nosocomial multidrug-resistant A. baumannii have been documented [13].
A. baumannii, like other Gram-negative bacteria, possesses various defense mechanisms that enable it to evade the bactericidal and bacteriostatic effects of antimicrobial agents. These mechanisms include efflux pumps, which expel antibiotics from the cell through efflux mechanisms, and modifications to outer-membrane proteins (OMPs) that reduce the permeability of porins [25]. Additionally, A. baumannii has been found to produce metallo-β-lactamase enzymes such as imipenemase (IMP), Verona integron-mediated metallo-β-lactamase (VIM), New Delhi metallo β-lactamase (NDM), and Seoul imipenemase (SIM). These enzymes can degrade antibiotics, rendering them ineffective [26]. However, the most crucial resistance mechanism employed by A. baumannii is the production of β-lactamases, particularly enzymes that hydrolyze carbapenems [14], penicillins [27], cephalosporins [27], and monobactams [14].
Studies conducted in Africa have revealed that the emergence of class D β-lactamases (OXA-23, -24, -51, and -58) and New Delhi metallo-β-lactamase (NDM-1) represents a significant mechanism of A. baumannii resistance. The presence of the blaOXA-23-like gene has been documented in several African countries, indicating its prevalence in these regions [28,29,30,31]. Furthermore, reports have highlighted the isolation of A. baumannii strains carrying Guiana extended-spectrum (GES) types, including GES-11, in Tunisia [32]. In addition to these mechanisms, other factors have contributed to multidrug resistance in this bacterium, such as the expression of efflux pumps (TetA, TetB, and AdeABC) [33] and the loss of or reduction in OMPs. In Saudi Arabia, El-Mahdy et al., (2017) conducted a study on carbapenem-resistant A. calcoaceticus-baumannii complex isolates from the eastern district. The findings revealed that these isolates carried the genes for carbapenem-resistant blaOXA-23-like and ISAba1 phage, and nine of the isolates also possessed the blaADC gene [34].
3. The Epidemiology and Risk Factors Associated with A. baumannii
There have been numerous instances in which A. baumannii infections are transmitted from one medical facility to another due to the movement of infected patients. For instance, the Vietnam extended-spectrum-β-lactamase (VEB-1)-producing A. baumannii clone was responsible for spreading the VEB-1 strain across 55 hospitals in northern and southeastern France. Furthermore, European clonal types I and II have been observed circulating in healthcare settings in Italy, with evidence suggesting the transfer of this strain from healthcare settings in the Mediterranean area to those in southwestern Germany [35,36,37]. Initial reports from hospitals in New York also associated outbreaks involving the OXA-48 β-lactamase produced by A. baumannii with outbreaks detected within the hospitals. The movement of patients and healthcare staff between healthcare providers has facilitated the spread of multidrug-resistant A. baumannii [38].
In Saudi Arabia, several investigations have been conducted to identify potential risk factors for A. baumannii infections [13,39,40,41,42]. Several studies have indicated that A. baumannii endemic strains may spread to tracheal secretions in patients undergoing mechanical ventilation [43]. Patients aged 60 or older and those receiving prolonged oxygen therapy are at a higher risk of developing ventilator-associated pneumonia caused by A. baumanni [41,44]. The colonization of the intestinal tract by A. baumannii in hospitalized patients is a potential risk factor for antibiotic resistance and outbreaks of serious infections [45]. Additionally, patients with chronic conditions that compromise their immune systems, such as diabetes, malignancy, kidney disease, and persistent lung disease, are particularly susceptible [39,46,47]. An earlier study showed that out of 72 patients infected with A. baumannii, 36% had underlying illnesses, and 11% were diabetics [48].
4. The Impact of Acinetobacter baumannii among Saudi Healthcare Facilities
Saudi Arabia is geographically divided into 13 administrative regions (Figure 1), each with its own political authority. With a combined population of over 36 million as of 2023, the country accommodates millions of Muslims from around the globe during the annual Hajj pilgrimage in the cities of Makkah and Madinah. This massive congregation poses a significant risk for the transmission of infectious diseases. Consequently, Saudi Arabia serves as a central hub for the international dissemination of multidrug-resistant (MDR) strains [49,50,51].
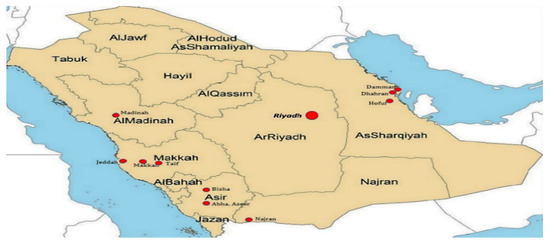
Figure 1.
The Saudi Arabian map showing administrative regions and hospitals (highlighted in red).
According to the Saudi Ministry of Health, Acinetobacter infections are among the leading causes of healthcare expenditure [52]. The negative impact on outcomes and high mortality rates associated with Acinetobacter infections are due to inappropriate therapies and limited therapeutic options, especially in ICU patients [53,54]. Saudi Arabia has observed an increase in carbapenem-resistant A. baumannii over the past decade compared to the 1990s [24]. A previous study reported that between 2005 and 2009, 26.5% of ventila-tor-associated pneumonia cases in Riyadh, Saudi Arabia, were attributed to Acinetobacter species [52]. In another investigation [55], the incidence of multidrug-resistant A. baumannii was 92.1%, the highest percentage recorded to date. A study published in 2015 unveiled the high resistance of A. baumannii infections in the El-Qassim region of Saudi Arabia to multiple antibiotics [56]. Similarly, an investigation conducted in Jeddah found that multidrug-resistant A. baumannii was present in 55% of patients in 2010 and 67% of patients in 2013 [42]. Table 1 provides a comprehensive overview of the incidence of multidrug-resistant A. baumannii over the past 15 years, with each investigation indicating a progressively higher prevalence. According to Table 1, A. baumannii displayed a variable degree of resistance, up to 100%, against carbapenems, penicillins, and cephalosporins during surveys conducted between 2004 and 2015 throughout Saudi Arabia.

Table 1.
An evaluation of the resistance rates of A. baumannii to β-lactam antibiotics was carried out in hospitals across Saudi Arabia (the numbers in the table represent the percentage of A. baumannii resistant to antibiotics).
5. Controlling Multidrug-Resistant Acinetobacter baumannii Outbreaks in Healthcare Settings
There have been numerous instances worldwide where multidrug-resistant A. baumannii has been successfully controlled through the implementation of various methods [73]. The concept of a source control program encompasses antibiotic stewardship, hand hygiene, adherence to contact precautions, education, thorough environmental cleaning, and vaccination strategies [74]. To effectively manage multidrug-resistant A. baumannii, it is imperative to gather microbial susceptibility profile data from specific regions or hospitals and establish rapid and cost-effective testing methods [75]. Several measures need to be taken to address this issue, including providing healthcare professionals with training on conducting laboratory tests and enhancing research skills among caregivers, particularly those working in the ICU [76,77].
Despite colistin being one of the few remaining effective treatments for multidrug-resistant A. baumannii, its efficacy has been compromised due to the degradation of lipopolysaccharide (LPS) and the emergence of lipid A target modifications [78]. Consequently, there is an urgent need for novel therapeutic approaches to tackle these challenges. Different organizations have recommended various strategies as the primary treatment for multidrug-resistant A. baumannii infections [79,80,81]. In line with Shields et al.’s recommendation, a combination of high-dose ampicillin–sulbactam with cefiderocol, polymyxin B, or tigecycline is recommended as the prescribed regimen in 2023 [82].
Alternative therapies, such as phage therapy, are increasingly being used to treat multidrug-resistant A. baumannii infections [83,84]. While phage therapy offers several advantages, certain aspects still require further clarification. These include the long-term viability of formulation and manufacturing scale, the potential resurgence of bacteriophage resistance, and the overall impact on gut microbiota [85]. In the future, virulent phages may emerge as potent bactericidal agents, potentially replacing antibiotics; however, the rapid emergence of phage resistance significantly impedes their widespread and continuous utilization [86].
6. Vaccine Development against Acinetobacter baumannii
A. baumannii vaccine is currently unavailable despite several vaccine candidates being tested [87]. Vaccines targeting A. baumannii are crucial for safeguarding individuals with weakened immune systems and patients with underlying medical conditions [88]. In the aftermath of McConnell et al.’s 2013 report [89], many laboratories are working on vaccines targeted to specific populations. Given the similarity among clinical isolates of A. baumannii, vaccines targeting conserved antigens may offer effective protection [90]. In the past decade, several multivalent vaccines have been considered, including live-attenuated strains, ghost bacteria, outer-membrane vesicles, and DNA (Table 2). Extensive studies have been conducted on the types and characteristics of potential vaccine candidates for A. baumannii. In this section of the review article, our objective is to assess each of these platforms, describe the experiments conducted with rodents to evaluate their ability to elicit immune responses, and assess their protective efficacy.

Table 2.
Types and characteristics of potential vaccine candidates for Acinetobacter baumannii.
6.1. Live-Attenuated Vaccines
Live-attenuated vaccines are produced by modifying bacteria to reduce their harmfulness, allowing the immune system to combat the infectious agent without causing illness [90]. Since live-attenuated strains display antigens similar to those found in fully pathogenic strains, they provide multiple targets for both immunoglobulin and cellular immune responses [97]. However, a drawback of live-attenuated vaccines is their potential to regain their harmful properties through horizontal gene transmission or spontaneous mutation [98]. Furthermore, it remains unclear whether live-attenuated vaccinations can be administered to individuals with weakened immune systems, who are most susceptible to A. baumannii infection. Recently, a live-attenuated vaccine against A. baumannii was developed based on a D-glutamate strain [98]. By modifying the murI1 and murI2 genes, a significantly less virulent strain of A. baumannii was created, which still elicited both antibody- and cell-mediated immune responses and improved survival in immunized animals exposed to multiple pathogenic strains of A. baumannii [98]. Additionally, the immunity induced by an A. baumannii strain lacking thioredoxin (∆trxA) was investigated [99]. Thioredoxins are vital in regulating redox balance and oxidative stress in bacteria. It was observed that a clinical isolate of A. baumannii lacking thioredoxin did not cause illness in mice vaccinated with the ∆trxA strain [99,100].
6.2. Outer-Membrane Vesicles and Complex-Based Vaccines
Virulent Gram-negative bacteria release outer-membrane vesicles (OMVs), which are non-infectious particles with diameters ranging from 10 to 300 nanometers [90,101]. A. baumannii is one of the bacteria that produce OMVs [102]. These spherical structures are typically composed of phospholipids, protein molecules, DNA, and LPS [103]. Alaniz et al. [104] suggest that OMVs are involved in delivering virulence factors to host cells, thereby initiating the infection process. McBroom and Kuehn [105] found that OMV production increases when stress levels are high and severe environments are present, such as during an infection. While the exact mechanism of OMV formation is yet to be determined, Deatherage and colleagues suggested that OMVs are generated when the density of outer-membrane–peptidoglycan connections is temporarily reduced. Furthermore, the researchers discovered that OMPs in OMVs contain specific domains that can interact with peptidoglycan and influence their development [106]. The effectiveness of OMV-based vaccines has been demonstrated in various studies conducted in animal models, with a variety of microorganisms, including Escherichia coli [107,108], Bordetella pertussis [109], and Burkholderia pseudomallei [110,111].
When considering a vaccination based on OMVs, it is important to remember that LPS plays a significant role in immunogenicity, as well as the potential side effects that result from the excessive presence of endotoxin. Significant progress has been made in addressing some of these issues by isolating OMVs from the A. baumannii strain IB010 [93]. It has been reported that this strain cannot produce LPS due to a mutation in the lpxD gene [93]. A study investigating the protective effects of LPS-A. baumannii outer-membrane complexes (OMCs) utilized the same strain. Mice vaccinated with the LPS-deficient OMCs showed little to no toxicity, similar to that of the LPS-deficient OMVs. On day 7 of the same experiment, mice vaccinated with LPS-deficient OMCs had a 60% survival rate, while mice vaccinated with LPS-deficient OMCs supplemented with exogenous LPS had a 95% survival rate [112]. A noticeable difference was observed between the two groups compared to the untreated control group, in which no survivors were found [112]. These investigations suggest that the LPS antigen contained within an OMV-based vaccination plays a crucial role in protecting against infection, despite the potential absence of LPS, which could help prevent excessive inflammation levels.
6.3. Nucleic-Acid-Based Vaccines
The concept of nucleic acid vaccines has gained considerable attention as a potential platform for vaccinations in recent years. However, very few studies have been conducted on nucleic acid vaccines intended to protect against A. baumannii [113]. Recent studies have demonstrated that DNA vaccines carrying the OmpA gene from A. baumannii can protect mice in animal pneumonia models from potentially fatal bacterial infections [114,115]. Researchers have found that immunization with a plasmid-vector-mediated vaccine (pVAX1) carrying the OmpA and proteoglycan-associated lipoprotein (PAL) genes of A. baumannii is effective against a lethal pulmonary challenge with four heterologous strains, resulting in 50% survival rates. Additionally, a decrease in inflammatory cytokines and the infiltration of inflammatory cells were observed in the bronchoalveolar lavage (BAL) [114], along with a strong humoral response, mixed cellular responses from T-helper cells (Th1/Th2/Th17), and a reduction in microbial burdens. Another vaccine utilizing the alternative plasmid-encoded OmpA expressed through the eukaryotic expression vector pBudCE4.1 protected 16% of mice from a lung infection for up to 15 days following a challenge. This vaccine also showed modest responses in Interleukin (IL-2, IL-4, and IL-12) and interferons (IFNs) [116]. These DNA vaccination strategies are still in their early stages but show promise as potentially safe and affordable approaches for administering multivalent vaccines.
6.4. Bacterial-Ghost-Based Vaccines
Bacterial ghosts (BGs) are initially living bacteria that undergo a process in which all their cytosolic components are removed, leaving only their outer membranes intact. Due to their non-living nature, BGs possess several inherent advantages, including the inability to revert to a pathogenic phenotype when utilized [90]. BGs can also serve as self-adjuvants and can be engineered to contain specific proteins, DNA, medications, or other small molecules to enhance their efficacy. However, while BGs maintain their original outer membrane, an excessive amount of natural LPS may lead to increased inflammation [97]. In an experiment, Sprague Dawley rats were vaccinated with A. baumannii BGs (strain Ali190) via oral, subcutaneous, intramuscular, or intraperitoneal administration before being infected with 108 CFU of the homologous A. baumannii strain [96]. A. baumannii BGs proved highly effective in protecting immunized rats against A. baumannii infection in all administration modes, except for oral vaccination, which resulted in a 67% survival rate [96]. However, despite the favorable outcomes of this initial study, it is crucial to investigate whether A. baumannii BGs can confer immunity against a wide range of A. baumannii strains.
7. Lessons Learned and Future Perspectives
Despite efforts made, the development of vaccines against A. baumannii has fallen behind that of nosocomial pathogens such as Clostridium difficile, Pseudomonas aeruginosa, and Staphylococcus aureus [95]. No vaccine against A. baumannii has undergone clinical trials, indicating the challenges associated with creating a safe and effective vaccine against this pathogen.
7.1. Technology of Modern Vaccines
Currently, research on anti-A. baumannii vaccine is still in its early stages. Most studies have focused on traditional methods and platforms, such as subunit and killed vaccines. However, there are ongoing explorations of novel antigen transporters and enhanced delivery mechanisms, including nucleic acid vaccines, virus vectors, conjugated carriers, and co-delivery [21,113,117]. It is worth noting that mRNA and DNA vaccine technologies have gained significant recognition due to the competition surrounding COVID-19 vaccines. Therefore, we can anticipate the increasing importance of nucleic-acid-based vaccines in treating A. baumannii and other infections in the coming years [113]. Additionally, proteomic approaches and reverse vaccination offer the benefit of utilizing bioinformatics to systematically evaluate and select potential immunogens for vaccine candidates in silico [113]. This method may be employed in the future to identify antigens and design vaccines that protect against A. baumannii infections.
7.2. The Long-Term Effects of Cellular Immunity
A promising vaccine candidate against A. baumannii should demonstrate its protective effects in preclinical trials by improving the survival rates of immunized animals compared to non-immunized ones, reducing bacterial burdens within organs, and suppressing the levels of inflammatory markers in the serum [113]. While vaccination and infection have been extensively researched, less attention has been given to studying cell-mediated immunity following vaccination. To formulate effective vaccines against A. baumannii, researchers need to align their assessment procedures, including animal models, intervals between vaccinations and challenges, challenge strains, doses, and administration methods. This harmonization will enable more accurate comparisons of experimental vaccines developed in different laboratories [95].
7.3. Cross-Protective Multivalent Vaccines
Vaccine candidates have been evaluated not only against homologous strains but also against challenges posed by other clinical isolates to demonstrate a broad level of protection. However, the degree of risk reduction varied significantly depending on the specific strain, vaccination protocol, and/or challenge dose [113]. Consequently, it is necessary to further assess the protective properties of these vaccines, especially when dealing with highly infectious strains at extremely high doses. Developing a vaccine that offers broad or diverse protection is challenging due to strain-dependent variation in selected antigens such as OmpA [118,119]. The complete ATCC 179878 genome revealed the presence of 28 putative alien islands, suggesting the potential for repeated infections and the difficulty of developing a vaccine [120]. A. baumannii has a high propensity for acquiring foreign DNA, leading to the emergence of novel genetic variants. Therefore, it is crucial to select conserved antigens and design vaccines with a wide range of components to provide adequate protection.
8. Limitations
This review has limitations, such as focusing solely on the prevalence of multidrug-resistant A. baumannii in Saudi Arabia without addressing other countries. To provide a more comprehensive view, further studies should be conducted in other countries to compare the data. This will enable us to better understand the global distribution of multidrug-resistant A. baumannii and the strategies needed to control its spread. Additionally, we did not highlight any of the virulence factors associated with A. baumannii infection, which should be addressed. Identifying these virulence factors is essential for understanding how this bacterium causes infections and developing strategies for their prevention and treatment. Moreover, this review did not discuss the potential risks and safety issues associated with developing a vaccine against multidrug-resistant A. baumannii, nor did it provide any information regarding the costs or potential barriers associated with its development and implementation.
9. Conclusions
The review shows that multidrug-resistant A. baumannii infections are prevalent in Saudi Arabia and that combination therapies are a key strategy for overcoming drug resistance. In particular, antibiotic stewardship is essential for preventing the emergence of drug-resistant strains, and multivalent vaccines composed of outer-membrane vesicles (OMVs), bacterial ghosts (BGs), or multiple subunit vaccines hold promise in reducing these infections. The development of vaccines has undergone significant changes due to advancements in technology, particularly in the wake of COVID-19 vaccine development.
Author Contributions
Conceptualization, A.E., E.M., I.M., Y.M., A.A.A., O.A.A., K.S.A., N.A.A., S.A.A. (Sulaiman Abdulaziz Anagreyyah), A.A. (Anwar Alzahrani), A.M.A., F.A., A.A.-O. and A.A. (Adil Abalkhail); supervision, A.E.; project administration, A.E.; validation, A.E., E.M., I.M., Y.M., A.A.A., O.A.A., K.S.A., N.A.A., S.A.A. (Sulaiman Abdulaziz Anagreyyah), A.A. (Anwar Alzahrani), A.M.A., F.A., M.A.A., S.A.A. (Suha Abdulaziz Anjiria), A.A.-O. and A.A. (Adil Abalkhail); visualization, A.E., E.M., I.M., Y.M., A.A.A., O.A.A., K.S.A., N.A.A., S.A.A. (Sulaiman Abdulaziz Anagreyyah), A.A. (Anwar Alzahrani), A.M.A., F.A., M.A.A., S.A.A. (Suha Abdulaziz Anjiria), A.A.-O. and A.A. (Adil Abalkhail); writing—original draft and writing, A.E., E.M., I.M., Y.M., A.A.A., O.A.A., K.S.A., N.A.A., S.A.A. (Sulaiman Abdulaziz Anagreyyah), A.A. (Anwar Alzahrani), A.M.A., F.A., M.A.A., S.A.A. (Suha Abdulaziz Anjiria), A.A.-O. and A.A. (Adil Abalkhail); review and editing, A.E., E.M., I.M., Y.M., A.A.A., O.A.A., K.S.A., N.A.A., S.A.A. (Sulaiman Abdulaziz Anagreyyah), A.A. (Anwar Alzahrani), A.M.A., F.A., M.A.A., S.A.A. (Suha Abdulaziz Anjiria), A.A.-O. and A.A. (Adil Abalkhail). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nguyen, M.; Joshi, S. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: A scientific review. J. Appl. Microbiol. 2021, 131, 2715–2738. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter spp. in food and drinking water–A review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef]
- Katic, V.; Todorovic, J.; Nagorni, A.; Nagorni, I.; Micev, M. A Case of Kaposi’s Sarcoma of the Rectum in a Homosexual Male with HIV–AIDS. Infect. Dis. Ther. 2017, 5, 2332–0877. [Google Scholar]
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Biderman, P.; Bugaevsky, Y.; Ben-Zvi, H.; Bishara, J.; Goldberg, E. Multidrug-resistant Acinetobacter baumannii infections in lung transplant patients in the cardiothoracic intensive care unit. Clin. Transplant. 2015, 29, 756–762. [Google Scholar] [CrossRef]
- Agyepong, N.; Fordjour, F.; Owusu-Ofori, A. Multidrug Resistant Acinetobacter baumannii in Healthcare Settings in Africa. Front. Trop. Dis. 2023, 4, 15. [Google Scholar] [CrossRef]
- Brotfain, E.; Borer, A.; Koyfman, L.; Saidel-Odes, L.; Frenkel, A.; Gruenbaum, S.E.; Rosenzweig, V.; Zlotnik, A.; Klein, M. Multidrug resistance Acinetobacter bacteremia secondary to ventilator-associated pneumonia: Risk factors and outcome. J. Intensive Care Med. 2017, 32, 528–534. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Gutiérrez-Pizarraya, A.; Díaz-Martín, A.; Cisneros-Herreros, J.M.; Cano, M.E.; Gato, E.; de Alegría, C.R.; Fernández-Cuenca, F.; Vila, J.; Martínez-Martínez, L. Acinetobacter baumannii in critically ill patients: Molecular epidemiology, clinical features and predictors of mortality. Enferm. Infecc. Y Microbiol. Clin. 2016, 34, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Visca, P.; Seifert, H.; Towner, K.J. Acinetobacter infection–an emerging threat to human health. IUBMB Life 2011, 63, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Marzouk, E.; Moussa, I.M.; Dawoud, T.M.; Mubarak, A.S.; Al-Sarar, D.; Alsubki, R.A.; Alhaji, J.H.; Hamada, M.; Abalkhail, A. Acinetobacter baumannii as a community foodborne pathogen: Peptide mass fingerprinting analysis, genotypic of biofilm formation and phenotypic pattern of antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E. Prevalence of Acinetobacter baumannii in Saudi Arabia: Risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 1. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Vuichard Gysin, D.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.-R.; Filipovic, M. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control 2022, 11, 12. [Google Scholar] [PubMed]
- Suranadi, I.W.; Fatmawati, N.; Aryabiantara, I.W.; Sinardja, C.D.; Saputra, D.J.; Senapathi, T.; Widnyana, I.; Nada, I.; Astuti, M.; Kumara, V. Acinetobacter baumannii is an opportunistic pathogen as an MDRO in ICU. Bali. J. Anesth. 2019, 3, 150–153. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, X.; Xu, C.-F.; Bilya, S.R.; Xu, W. Mechanical ventilation-associated pneumonia caused by Acinetobacter baumannii in Northeast China region: Analysis of genotype and drug resistance of bacteria and patients’ clinical features over 7 years. Antimicrob. Resist. Infect. Control 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Dema, B.; Reyes-Sandoval, A. COVID-19 vaccines: Breaking record times to first-in-human Trials. npj Vaccines 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.; Hanison, J.; Nirmalan, N. “Omics”-informed drug and biomarker discovery: Opportunities, challenges and future perspectives. Proteomes 2016, 4, 28. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Bonanni, P.; Tantawichien, T.; Zepp, F. Vaccine development. Perspect. Vaccinol. 2011, 1, 115–150. [Google Scholar] [CrossRef]
- Tan, Y.C.; Lahiri, C. Promising Acinetobacter baumannii vaccine candidates and drug targets in recent years. Front. Immunol. 2022, 13, 900509. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, X.; Dowzicky, M.J. Antimicrobial susceptibility among gram-negative isolates collected from intensive care units in North America, Europe, the Asia-Pacific Rim, Latin America, the Middle East, and Africa between 2004 and 2009 as part of the Tigecycline Evaluation and Surveillance Trial. Clin. Ther. 2012, 34, 124–137. [Google Scholar]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Balkhy, H.H.; Walsh, T.R.; Paterson, D.L. β-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin. Microbiol. Rev. 2013, 26, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Cornaglia, G.; Giamarellou, H.; Rossolini, G.M. Metallo-β-lactamases: A last frontier for β-lactams? Lancet Infect. Dis. 2011, 11, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Berrazeg, M.; Fagade, O.E.; Adelowo, O.O.; Alli, J.A.; Rolain, J.M. Emergence of multidrug-resistant Acinetobacter baumannii producing OXA-23 carbapenemase, Nigeria. Int. J. Infect. Dis. 2013, 17, e469–e470. [Google Scholar] [CrossRef]
- Bakour, S.; Olaitan, A.O.; Ammari, H.; Touati, A.; Saoudi, S.; Saoudi, K.; Rolain, J.-M. Emergence of colistin-and carbapenem-resistant Acinetobacter baumannii ST2 clinical isolate in Algeria: First case report. Microb. Drug Resist. 2015, 21, 279–285. [Google Scholar] [CrossRef]
- Mugnier, P.D.; Poirel, L.; Naas, T.; Nordmann, P. Worldwide dissemination of the blaOXA-23 Carbapenemase gene of Acinetobacter baumannii. Emerg. Infect. Dis. 2010, 16, 35. [Google Scholar] [CrossRef]
- Nogbou, N.-D.; Phofa, D.T.; Nchabeleng, M.; Musyoki, A.M. Investigating multi-drug resistant Acinetobacter baumannii isolates at a tertiary hospital in Pretoria, South Africa. Indian J. Med. Microbiol. 2021, 39, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Chihi, H.; Bonnin, R.; Bourouis, A.; Mahrouki, S.; Besbes, S.; Moussa, M.B.; Belhadj, O.; Naas, T. GES-11-producing Acinetobacter baumannii clinical isolates from Tunisian hospitals: Long-term dissemination of GES-type carbapenemases in North Africa. J. Glob. Antimicrob. Resist. 2016, 5, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Pannek, S.; Higgins, P.G.; Steinke, P.; Jonas, D.; Akova, M.; Bohnert, J.A.; Seifert, H.; Kern, W.V. Multidrug efflux inhibition in Acinetobacter baumannii: Comparison between 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-β-naphthylamide. J. Antimicrob. Chemother. 2006, 57, 970–974. [Google Scholar] [CrossRef]
- El-Mahdy, T.S.; Al-Agamy, M.H.; Al-Qahtani, A.A.; Shibl, A.M. Detection of bla OXA-23-like and bla NDM-1 in Acinetobacter baumannii from the Eastern Region, Saudi Arabia. Microb. Drug Resist. 2017, 23, 115–121. [Google Scholar] [CrossRef]
- Higgins, P.G.; Dammhayn, C.; Hackel, M.; Seifert, H. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 233–238. [Google Scholar] [CrossRef]
- D’arezzo, S.; Capone, A.; Petrosillo, N.; Visca, P. Epidemic multidrug-resistant Acinetobacter baumannii related to European clonal types I and II in Rome (Italy). Clin. Microbiol. Infect. 2009, 15, 347–357. [Google Scholar] [CrossRef]
- Naas, T.; Coignard, B.; Carbonne, A.; Blanckaert, K.; Bajolet, O.; Bernet, C.; Verdeil, X.; Astagneau, P.; Desenclos, J.; Nordmann, P. French Nosocomial Infection Early Warning Investigation and Surveillance Network. VEB-1 Extended-spectrum beta-lactamase-producing Acinetobacter baumannii, France. Emerg. Infect. Dis. 2006, 12, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Alsan, M.; Klompas, M. Acinetobacter baumannii: An emerging and important pathogen. J. Clin. Outcomes Manag. JCOM 2010, 17, 363. [Google Scholar]
- Al-Anazi, K.A.; Al-Jasser, A.M. Infections caused by Acinetobacter baumannii in recipients of hematopoietic stem cell transplantation. Front. Oncol. 2014, 4, 186. [Google Scholar] [CrossRef]
- Al-Obeid, S.; Jabri, L.; Al-Agamy, M.; Al-Omari, A.; Shibl, A. Epidemiology of extensive drug resistant Acinetobacter baumannii (XDRAB) at Security Forces Hospital (SFH) in Kingdom of Saudi Arabia (KSA). J. Chemother. 2015, 27, 156–162. [Google Scholar] [CrossRef]
- Al Bshabshe, A.; Joseph, M.R.; Al Hussein, A.; Haimour, W.; Hamid, M.E. Multidrug resistance Acinetobacter species at the intensive care unit, Aseer Central Hospital, Saudi Arabia: A one year analysis. Asian Pac. J. Trop. Med. 2016, 9, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Al Mobarak, M.F.; Matbuli, R.M.; Meir, H.; Al Gehani, N.; El Toukhy, A.A.M.; Al Qureshey, K.F.; Mutwalli, A.H.; Abdulaziz, A.M.; Hadhoud, A. Antimicrobial Resistance Patterns among Acinetobacter baumannii Isolated from King Abdulaziz Hospital, Jeddah, Saudi Arabia: Four-Year Surveillance Study (2010–2013). Egypt. J. Med. Microbiol. 2014, 38, 1–8. [Google Scholar] [CrossRef]
- Mah, M.W.; Memish, Z.A.; Cunningham, G.; Bannatyne, R.M. Outbreak of Acinetobacter baumannii in an intensive care unit associated with tracheostomy. Am. J. Infect. Control 2001, 29, 284–288. [Google Scholar] [CrossRef] [PubMed]
- El-Saed, A.; Balkhy, H.H.; Al-Dorzi, H.M.; Khan, R.; Rishu, A.H.; Arabi, Y.M. Acinetobacter is the most common pathogen associated with late-onset and recurrent ventilator-associated pneumonia in an adult intensive care unit in Saudi Arabia. Int. J. Infect. Dis. 2013, 17, e696–e701. [Google Scholar] [CrossRef]
- Aljindan, R.; Bukharie, H.; Alomar, A.; Abdalhamid, B. Prevalence of digestive tract colonization of carbapenem-resistant Acinetobacter baumannii in hospitals in Saudi Arabia. J. Med. Microbiol. 2015, 64, 400–406. [Google Scholar] [CrossRef]
- Al-Gethamy, M.M.; Faidah, H.S.; Adetunji, H.A.; Haseeb, A.; Ashgar, S.S.; Mohanned, T.K.; Mohammed, A.-H.; Khurram, M.; Hassali, M.A. Risk factors associated with multi-drug-resistant Acinetobacter baumannii nosocomial infections at a tertiary care hospital in Makkah, Saudi Arabia-a matched case–control study. J. Int. Med. Res. 2017, 45, 1181–1189. [Google Scholar] [CrossRef]
- Nurain, A.M.; Bilal, N.E.; Ibrahim, M.E. The frequency and antimicrobial resistance patterns of nosocomial pathogens recovered from cancer patients and hospital environments. Asian Pac. J. Trop. Biomed. 2015, 5, 1055–1059. [Google Scholar] [CrossRef]
- Khan, M.A.; Mahomed, M.F.; Ashshi, A.M.; Faiz, A. Drug resistance patterns of Acinetobacter baumannii in Makkah, Saudi Arabia. Pak. J. Med. Res. 2012, 51, 127–131. [Google Scholar]
- Abbott, I.; Cerqueira, G.M.; Bhuiyan, S.; Peleg, A.Y. Carbapenem resistance in Acinetobacter baumannii: Laboratory challenges, mechanistic insights and therapeutic strategies. Expert Rev. Anti-Infect. Ther. 2013, 11, 395–409. [Google Scholar] [CrossRef]
- Abdalhamid, B.; Hassan, H.; Itbaileh, A.; Shorman, M. Characterization of carbapenem-resistant Acinetobacter baumannii clinical isolates in a tertiary care hospital in Saudi Arabia. New Microbiol. 2014, 37, 65–73. [Google Scholar]
- Raees, F.; Harun, A.; Ahmed, A.; Deris, Z.Z. Antibiotic resistance, genotype and clinical significance of Acinetobacter baumannii in Saudi Arabia. Bangladesh J. Med. Sci. 2022, 21, 233–242. [Google Scholar] [CrossRef]
- Kharaba, A.; Hussein, M.A.A.; Al-Hameed, F.M.; Mandourah, Y.; Almekhlafi, G.A.; Algethamy, H.; Hamdan, A.; Azem, M.A.; Fatani, J.; al Beshabshe, A. Acinetobacter baumannii in Saudi Arabia: The new growing threat. Saudi Crit. Care J. 2019, 3, 54. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Vena, A.; Graziano, E.; Russo, A.; Peghin, M. Risk stratification and treatment of ICU-acquired pneumonia caused by multidrug-resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr. Opin. Crit. Care 2018, 24, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Giuliano, S.; Ceccarelli, G.; Alessandri, F.; Giordano, A.; Brunetti, G.; Venditti, M. Comparison of septic shock due to multidrug-resistant Acinetobacter baumannii or Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit patients. Antimicrob. Agents Chemother. 2018, 62, e02562-17. [Google Scholar] [CrossRef]
- Aly, M.; Tayeb, H.; Al Johani, S.; Alyamani, E.; Aldughaishem, F.; Alabdulkarim, I.; Balkhy, H. Genetic diversity of OXA-51-like genes among multidrug-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1223–1228. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Ahamed, F.; Al-Omari, A.S. Antibiotic susceptibility patterns of some clinical isolates from al-Rass general hospital. Int. J. Biosci. 2015, 6, 47–54. [Google Scholar]
- Saeed, N.K.; Kambal, A.M.; El-Khizzi, N.A. Antimicrobial-resistant bacteria in a general intensive care unit in Saudi Arabia. Saudi Med. J. 2010, 31, 1341–1349. [Google Scholar]
- Al-Agamy, M.H.; Jeannot, K.; El-Mahdy, T.S.; Shibl, A.M.; Kattan, W.; Plésiat, P.; Courvalin, P. First detection of GES-5 carbapenemase-producing Acinetobacter baumannii isolate. Microb. Drug Resist. 2017, 23, 556–562. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Shibl, A.M.; Ali, M.S.; Khubnani, H.; Radwan, H.H.; Livermore, D.M. Distribution of β-lactamases in carbapenem-non-susceptible Acinetobacter baumannii in Riyadh, Saudi Arabia. J. Glob. Antimicrob. Resist. 2014, 2, 17–21. [Google Scholar] [CrossRef]
- Aly, M.; Alsoud, N.A.; Elrobh, M.; Al Johani, S.; Balkhy, H. High prevalence of the PER-1 gene among carbapenem-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1759–1766. [Google Scholar] [CrossRef]
- Al-Otaibi, F.E.; Bukhari, E.E.; Badr, M.; Alrabiaa, A.A. Prevalence and risk factors of Gram-negative bacilli causing blood stream infection in patients with malignancy. Saudi Med. J. 2016, 37, 979. [Google Scholar] [CrossRef] [PubMed]
- Said, K.B.; Al-Jarbou, A.N.; Alrouji, M.; Al-Harbi, H.O. Surveillance of antimicrobial resistance among clinical isolates recovered from a tertiary care hospital in Al Qassim, Saudi Arabia. Int. J. Health Sci. 2014, 8, 3. [Google Scholar] [CrossRef]
- Asghar, A.H.; Ashshi, A.M.; Azhar, E.I.; Bukhari, S.Z.; Zafar, T.A.; Momenah, A.M. Profile of bacterial pneumonia during Hajj. Indian J. Med. Res. 2011, 133, 510. [Google Scholar]
- Asghar, A.H.; Faidah, H.S. Frequency and antimicrobial susceptibility of gram-negative bacteria isolated from 2 hospitals in Makkah, Saudi Arabia. Saudi Med. J. 2009, 30, 1017–1023. [Google Scholar] [PubMed]
- Haseeb, A.; Faidah, H.S.; Bakhsh, A.R.; Al Malki, W.H.; Elrggal, M.E.; Saleem, F.; ur Rahman, S.; Khan, T.M.; Hassali, M.A. Antimicrobial resistance among pilgrims: A retrospective study from two hospitals in Makkah, Saudi Arabia. Int. J. Infect. Dis. 2016, 47, 92–94. [Google Scholar] [CrossRef]
- El-Ageery, S.; Al-Hazmi, S. Microbiological and molecular detection of VIM-1 metallo beta lactamase-producing Acinetobacter baumannii. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 965–970. [Google Scholar]
- Eltahawy, A.; Khalaf, R. Antibiotic resistance among gram-negative non-fermentative bacteria at a teaching hospital in Saudi Arabia. J. Chemother. 2001, 13, 260–264. [Google Scholar] [CrossRef]
- Dhabaan, G.N.; AbuBakar, S.; Cerqueira, G.M.; Al-Haroni, M.; Pang, S.P.; Hassan, H. Imipenem treatment induces expression of important genes and phenotypes in a resistant Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 2016, 60, 1370–1376. [Google Scholar] [CrossRef]
- Abdalhamid, B.; Elhadi, N.; Alabdulqader, N.; Alsamman, K.; Aljindan, R. Rates of gastrointestinal tract colonization of carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa in hospitals in Saudi Arabia. New Microb. New Infect. 2016, 10, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, N.M.; Osman, A.A.; Haimour, W.O.; Sarhan, M.; Mohammed, M.N.; Zyad, E.M.; Al-Ghtani, A.M. Antimicrobial susceptibility pattern in nosocomial infections caused by Acinetobacter species in Asir Region, Saudi Arabia. Pak. J. Biol. Sci. PJBS 2013, 16, 275–280. [Google Scholar] [CrossRef]
- Elabd, F.M.; Al-Ayed, M.S.; Asaad, A.M.; Alsareii, S.A.; Qureshi, M.A.; Musa, H.A.-A. Molecular characterization of oxacillinases among carbapenem-resistant Acinetobacter baumannii nosocomial isolates in a Saudi hospital. J. Infect. Public Health 2015, 8, 242–247. [Google Scholar] [CrossRef]
- Asaad, A.M.; Al-Ayed, M.S.Z.; Qureshi, M.A. Emergence of unusual nonfermenting gram-negative nosocomial pathogens in a Saudi hospital. Jpn. J. Infect. Dis. 2013, 66, 507–511. [Google Scholar] [CrossRef]
- Teerawattanapong, N.; Kengkla, K.; Dilokthornsakul, P.; Saokaew, S.; Apisarnthanarak, A.; Chaiyakunapruk, N. Prevention and control of multidrug-resistant gram-negative bacteria in adult intensive care units: A systematic review and network meta-analysis. Clin. Infect. Dis. 2017, 64, S51–S60. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Dimopoulos, G.; Poulakou, G.; Akova, M.; Cisneros, J.M.; De Waele, J.; Petrosillo, N.; Seifert, H.; Timsit, J.F.; Vila, J. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 2015, 41, 2057–2075. [Google Scholar] [CrossRef]
- Yamamoto, N.; Hamaguchi, S.; Akeda, Y.; Santanirand, P.; Kerdsin, A.; Seki, M.; Ishii, Y.; Paveenkittiporn, W.; Bonomo, R.A.; Oishi, K. Clinical specimen-direct LAMP: A useful tool for the surveillance of bla oxa-23-positive carbapenem-resistant Acinetobacter baumannii. PLoS ONE 2015, 10, e0133204. [Google Scholar] [CrossRef] [PubMed]
- Ulu-Kilic, A.; Ahmed, S.; Alp, E.; Doğanay, M. Challenge of intensive care unit-acquired infections and Acinetobacter baumannii in developing countries. OA Crit. Care 2013, 1, 2. [Google Scholar] [CrossRef]
- Yusuf, I.; Adam, R.U. Adopting Chennai declaration strategies in the prevention and control of the spread of multidrug-resistant hospital-acquired bacterial infections in Nigeria: A call to action. J. Glob. Antimicrob. Resist. 2014, 3, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Papathanakos, G.; Andrianopoulos, I.; Papathanasiou, A.; Priavali, E.; Koulenti, D.; Koulouras, V. Colistin-resistant Acinetobacter baumannii bacteremia: A serious threat for critically ill patients. Microorganisms 2020, 8, 287. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase–Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; De Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Abdul-Mutakabbir, J.C.; Griffith, N.C.; Shields, R.K.; Tverdek, F.P.; Escobar, Z.K. Contemporary perspective on the treatment of Acinetobacter baumannii infections: Insights from the Society of Infectious Diseases Pharmacists. Infect. Dis. Ther. 2021, 10, 2177–2202. [Google Scholar] [CrossRef]
- Shields, R.K.; Paterson, D.L.; Tamma, P.D. Navigating Available Treatment Options for Carbapenem-Resistant Acinetobacter baumannii-calcoaceticus Complex Infections. Clin. Infect. Dis. 2023, 76, S179–S193. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Peng, N.; Liang, Y.; Li, K.; Li, Y. Phage therapy: Consider the past, embrace the future. Appl. Sci. 2020, 10, 7654. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, Y.; Galgano, S.; Houdijk, J.; Xie, W.; Jin, Y.; Lin, J.; Song, W.; Fu, Y.; Li, X. Recent Progress in Phage Therapy to Modulate Multidrug-Resistant Acinetobacter baumannii, including in Human and Poultry. Antibiotics 2022, 11, 1406. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Li, X.; Tan, D.; Cong, C.; Xu, Y. Efficacy of a phage cocktail in controlling phage resistance development in multidrug resistant Acinetobacter baumannii. Virus Res. 2019, 272, 197734. [Google Scholar] [CrossRef]
- Singh, R.; Capalash, N.; Sharma, P. Vaccine development to control the rising scourge of antibiotic-resistant Acinetobacter baumannii: A systematic review. Biotech 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Sharndama, H.C.; Anyaegbunam, Z.K.G.; Anekpo, C.C.; Amadi, B.C.; Morumda, D.; Doowuese, Y.; Ihezuo, U.J.; Chukwukelu, J.U.; Okeke, O.P. Vaccine development for bacterial pathogens: Advances, challenges and prospects. Trop. Med. Int. Health 2023, 28, 275–299. [Google Scholar] [CrossRef]
- McConnell, M.J.; Actis, L.; Pachón, J. Acinetobacter baumannii: Human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef]
- Gellings, P.S.; Wilkins, A.A.; Morici, L.A. Recent advances in the pursuit of an effective Acinetobacter baumannii vaccine. Pathogens 2020, 9, 1066. [Google Scholar] [CrossRef]
- García-Quintanilla, M.; Pulido, M.R.; Pachon, J.; McConnell, M.J. Immunization with lipopolysaccharide-deficient whole cells provides protective immunity in an experimental mouse model of Acinetobacter baumannii infection. PLoS ONE 2014, 9, e114410. [Google Scholar] [CrossRef]
- KuoLee, R.; Harris, G.; Yan, H.; Xu, H.H.; Conlan, W.J.; Patel, G.B.; Chen, W. Intranasal immunization protects against Acinetobacter baumannii-associated pneumonia in mice. Vaccine 2015, 33, 260–267. [Google Scholar] [CrossRef]
- Pulido, M.R.; García-Quintanilla, M.; Pachón, J.; McConnell, M.J. A lipopolysaccharide-free outer membrane vesicle vaccine protects against Acinetobacter baumannii infection. Vaccine 2020, 38, 719–724. [Google Scholar] [CrossRef]
- Luo, G.; Lin, L.; Ibrahim, A.S.; Baquir, B.; Pantapalangkoor, P.; Bonomo, R.A.; Doi, Y.; Adams, M.D.; Russo, T.A.; Spellberg, B. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS ONE 2012, 7, e29446. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Current advances and challenges in the development of Acinetobacter vaccines. Hum. Vaccines Immunother. 2015, 11, 2495–2500. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.; Batah, A.; Ghazy, A.; Hussein, A.; Amara, A. A new strain of Acinetobacter baumannii and characterization of its ghost as a candidate vaccine. J. Infect. Public Health 2019, 12, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, C.A.; Walcher, P.; Mayr, U.B.; Stiedl, T.; Binder, M.; McGrath, J.; Lubitz, W. Bacterial ghosts–biological particles as delivery systems for antigens, nucleic acids and drugs. Curr. Opin. Biotechnol. 2004, 15, 530–537. [Google Scholar] [CrossRef]
- Cabral, M.P.; García, P.; Beceiro, A.; Rumbo, C.; Pérez, A.; Moscoso, M.; Bou, G. Design of live attenuated bacterial vaccines based on D-glutamate auxotrophy. Nat. Commun. 2017, 8, 15480. [Google Scholar] [CrossRef]
- Ainsworth, S.; Ketter, P.M.; Yu, J.-J.; Grimm, R.C.; May, H.C.; Cap, A.P.; Chambers, J.P.; Guentzel, M.N.; Arulanandam, B.P. Vaccination with a live attenuated Acinetobacter baumannii deficient in thioredoxin provides protection against systemic Acinetobacter infection. Vaccine 2017, 35, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Klug, G. Thioredoxins in bacteria: Functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften 2006, 93, 259–266. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Q.; Li, W.; Chen, Y.; Shu, C.; Li, Q.; Zhou, J.; Ye, C.; Bai, H.; Sun, W. Anti-outer membrane vesicle antibodies increase antibiotic sensitivity of pan-drug-resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1379. [Google Scholar] [CrossRef]
- Jin, J.S.; Kwon, S.-O.; Moon, D.C.; Gurung, M.; Lee, J.H.; Kim, S.I.; Lee, J.C. Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles. PLoS ONE 2011, 6, e17027. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 2007, 179, 7692–7701. [Google Scholar] [CrossRef]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef]
- Deatherage, B.L.; Lara, J.C.; Bergsbaken, T.; Barrett, S.L.R.; Lara, S.; Cookson, B.T. Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 2009, 72, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Hong, B.S.; Park, K.-S.; Yoon, Y.J.; Choi, S.J.; Lee, W.H.; Roh, T.-Y.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J. Immunol. 2013, 190, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, K.; Kong, Q.; Liu, Q. Immunization with outer membrane vesicles of avian pathogenic Escherichia coli O78 induces protective immunity in chickens. Vet. Microbiol. 2019, 236, 108367. [Google Scholar] [CrossRef]
- Gasperini, G.; Biagini, M.; Arato, V.; Gianfaldoni, C.; Vadi, A.; Norais, N.; Bensi, G.; Delany, I.; Pizza, M.; Arico, B. Outer membrane vesicles (OMV)-based and proteomics-driven antigen selection identifies novel factors contributing to Bordetella pertussis adhesion to epithelial cells. Mol. Cell. Proteom. 2018, 17, 205–215. [Google Scholar] [CrossRef]
- Nieves, W.; Petersen, H.; Judy, B.M.; Blumentritt, C.A.; Russell-Lodrigue, K.; Roy, C.J.; Torres, A.G.; Morici, L.A. A Burkholderia upseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin. Vaccine Immunol. 2014, 21, 747–754. [Google Scholar] [CrossRef]
- Baker, S.M.; Davitt, C.J.; Motyka, N.; Kikendall, N.L.; Russell-Lodrigue, K.; Roy, C.J.; Morici, L.A. A Burkholderia pseudomallei outer membrane vesicle vaccine provides cross protection against inhalational glanders in mice and non-human primates. Vaccines 2017, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Pulido, M.R.; García-Quintanilla, M.; Pachón, J.; McConnell, M.J. Immunization with lipopolysaccharide-free outer membrane complexes protects against Acinetobacter baumannii infection. Vaccine 2018, 36, 4153–4156. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; McClean, S. Mapping global prevalence of Acinetobacter baumannii and recent vaccine development to tackle it. Vaccines 2021, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Q.; Yang, H.Y.; Guo, S.J.; Xie, Y.E. MF59 adjuvant enhances the immunogenicity and protective immunity of the OmpK/Omp22 fusion protein from Acineterbacter baumannii through intratracheal inoculation in mice. Scand. J. Immunol. 2019, 90, e12769. [Google Scholar] [CrossRef]
- García-Patiño, M.G.; García-Contreras, R.; Licona-Limón, P. The immune response against Acinetobacter baumannii, an emerging pathogen in nosocomial infections. Front. Immunol. 2017, 8, 441. [Google Scholar] [CrossRef]
- Lei, L.; Yang, F.; Zou, J.; Jing, H.; Zhang, J.; Xu, W.; Zou, Q.; Zhang, J.; Wang, X. DNA vaccine encoding OmpA and Pal from Acinetobacter baumannii efficiently protects mice against pulmonary infection. Mol. Biol. Rep. 2019, 46, 5397–5408. [Google Scholar] [CrossRef]
- Lau, Y.T.; Tan, H.S. Acinetobacter baumannii subunit vaccines: Recent progress and challenges. Crit. Rev. Microbiol. 2023, 2023, 1–16. [Google Scholar] [CrossRef]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef]
- Hounsome, J.D.; Baillie, S.; Noofeli, M.; Riboldi-Tunnicliffe, A.; Burchmore, R.J.; Isaacs, N.W.; Davies, R.L. Outer membrane protein A of bovine and ovine isolates of Mannheimia haemolytica is surface exposed and contains host species-specific epitopes. Infect. Immun. 2011, 79, 4332–4341. [Google Scholar] [CrossRef]
- Smith, M.G.; Gianoulis, T.A.; Pukatzki, S.; Mekalanos, J.J.; Ornston, L.N.; Gerstein, M.; Snyder, M. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 2007, 21, 601–614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).