Safety and Immunogenicity of Homologous and Heterologous Adenoviral-Vectored and mRNA COVID-19 Vaccine Regimens in Radiotherapy Patients
Abstract
:1. Introduction
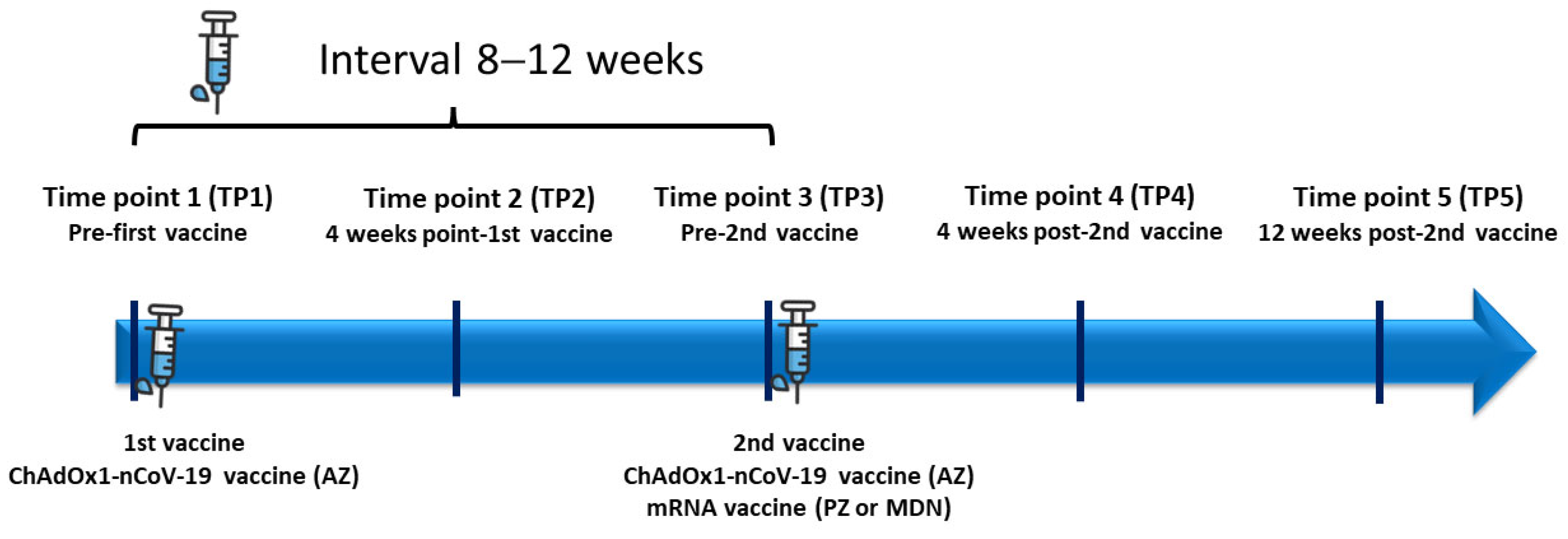
2. Materials and Methods
2.1. Study Population
2.2. Radiotherapy Patients
2.3. Healthy Controls
2.4. COVID-19 Vaccines
2.5. Laboratory Tests
2.6. Study Endpoints
2.7. Statistical Analysis
3. Results
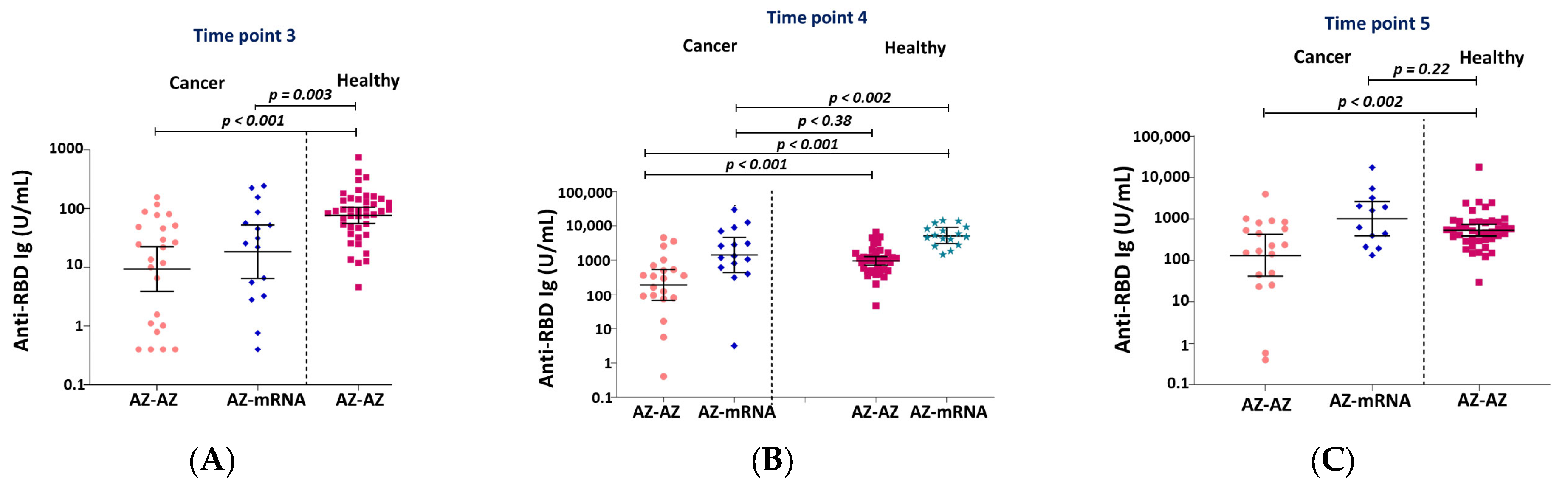
3.1. Immunogenicity and Serology after Vaccination
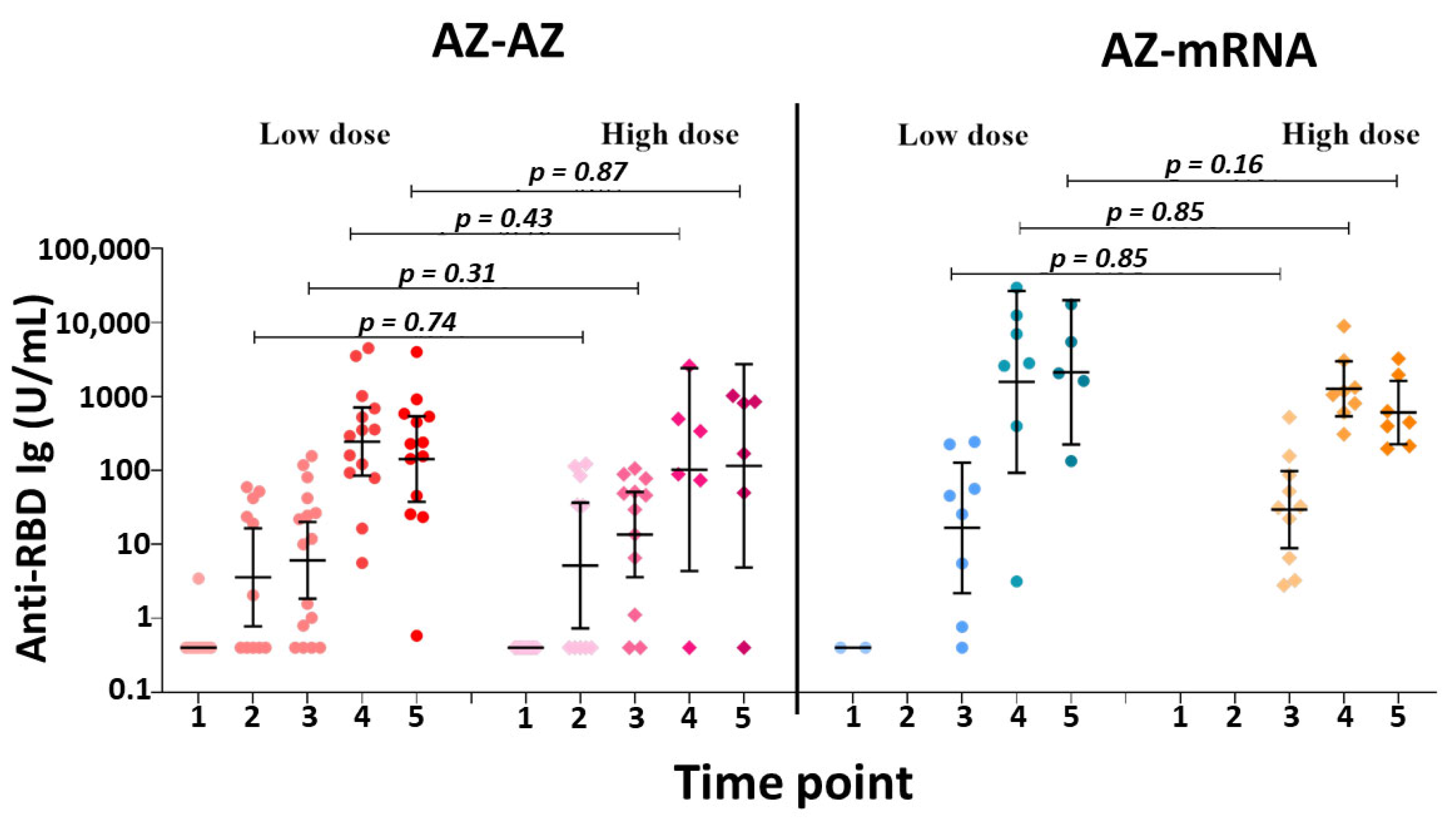
3.2. Subgroup Analysis between Low-Dose and High-Dose Radiation
3.3. Neutralization against SARS-CoV-2 Delta Strain and Omicron (BA.1) Strain
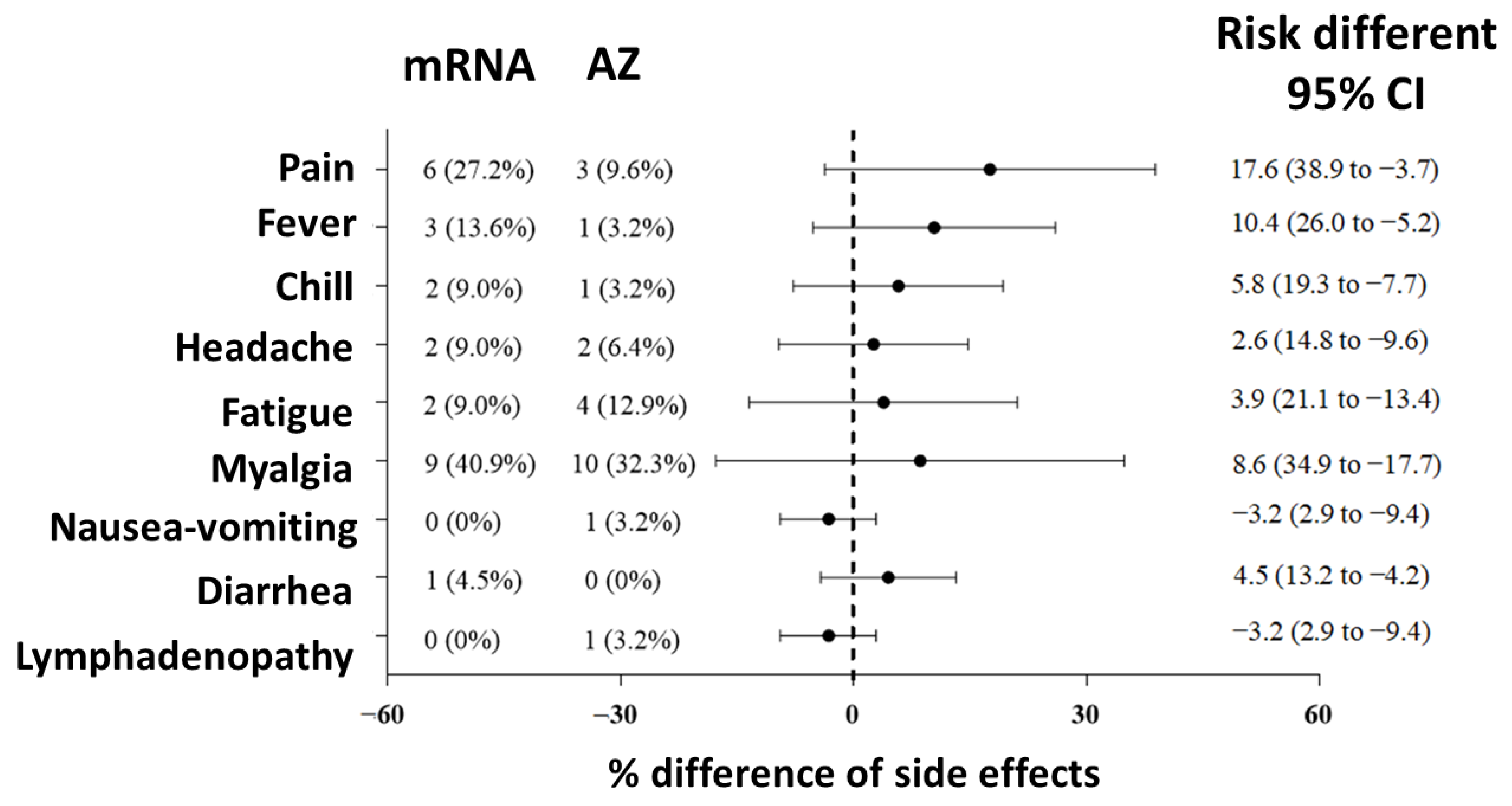
3.4. Adverse Effects after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [PubMed]
- Liu, C.; Zhao, Y.; Okwan-Duodu, D.; Basho, R.; Cui, X. COVID-19 in cancer patients: Risk, clinical features, and management. Cancer Biol. Med. 2020, 17, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Recommendations of the National Comprehensive Cancer Network® (NCCN®) COVID-19 Vaccination Advisory Committee. Available online: https://www.eviq.org.au/getmedia/6de8cf1f-54d5-4c5d-9045-f3b5ff384bbc/2021-covid-19-vaccination-guidance-v8-0.pdf.aspx (accessed on 20 June 2023).
- Cavanna, L.; Citterio, C.; Biasini, C.; Madaro, S.; Bacchetta, N.; Lis, A.; Cremona, G.; Muroni, M.; Bernuzzi, P.; Cascio, G.L.; et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: Seropositivity and safety. A prospective observational study in Italy. Eur. J. Cancer 2021, 157, 441–449. [Google Scholar] [PubMed]
- Goshen-Lago, T.; Waldhorn, I.; Holland, R.; Szwarcwort-Cohen, M.; Reiner-Benaim, A.; Shachor-Meyouhas, Y.; Hussein, K.; Fahoum, L.; Baruch, M.; Peer, A.; et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1507–1513. [Google Scholar] [PubMed]
- Oosting, S.F.; van der Veldt, A.A.M.; GeurtsvanKessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.-M.C.; Smit, E.F.; Hiltermann, T.J.N.; den Hartog, G.; Jalving, M.; et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: A prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021, 22, 1681–1691. [Google Scholar]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar]
- Teeyapun, N.; Luangdilok, S.; Pakvisal, N.; Sainamthip, P.; Mingmalairak, S.; Poovorawan, N.; Sitthideatphaiboon, P.; Parinyanitikul, N.; Sriuranpong, V.; Namkanisorn, T.; et al. Immunogenicity of ChAdOx1-nCoV-19 vaccine in solid malignancy patients by treatment regimen versus healthy controls: A prospective, multicenter observational study. EClinicalMedicine 2022, 52, 101608. [Google Scholar]
- Nelli, F.; Fabbri, A.; Onorato, A.; Giannarelli, D.; Silvestri, M.A.; Giron Berrios, J.R.; Virtuoso, A.; Marrucci, E.; Signorelli, C.; Chilelli, M.G.; et al. Effects of active cancer treatment on safety and immunogenicity of COVID-19 mRNA-BNT162b2 vaccine: Preliminary results from the prospective observational Vax-On study. Ann. Oncol. 2022, 33, 107–108. [Google Scholar]
- Bowes, C.L.; Naranbhai, V.; St Denis, K.J.; Lam, E.C.; Bertaux, B.; Keane, F.K.; Khandekar, M.J.; Balazs, A.B.; Iafrate, J.A.; Gainor, J.F.; et al. Heterogeneous immunogenicity of SARS-CoV-2 vaccines in cancer patients receiving radiotherapy. Radiother. Oncol. 2022, 166, 88–91. [Google Scholar] [CrossRef]
- Yorsaeng, R.; Suntronwong, N.; Phowatthanasathian, H.; Assawakosri, S.; Kanokudom, S.; Thongmee, T.; Vichaiwattana, P.; Auphimai, C.; Wongsrisang, L.; Srimuan, D.; et al. Immunogenicity of a third dose viral-vectored COVID-19 vaccine after receiving two-dose inactivated vaccines in healthy adults. Vaccine 2022, 40, 524–530. [Google Scholar]
- Wanlapakorn, N.; Suntronwong, N.; Phowatthanasathian, H.; Yorsaeng, R.; Vichaiwattana, P.; Thongmee, T.; Auphimai, C.; Srimuan, D.; Thatsanatorn, T.; Assawakosri, S.; et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral-vectored COVID-19 vaccine regimens in healthy adults: A prospective cohort study. Hum. Vaccines Immunother. 2022, 18, 2029111. [Google Scholar]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Stein, N.; Saliba, W. Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score–Matched Analysis. Clin. Infect Dis. 2022, 76, 1067–1073. [Google Scholar]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; Morales, M.T.G.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar]
- Hammerschmidt, S.I.; Bosnjak, B.; Bernhardt, G.; Friedrichsen, M.; Ravens, I.; Dopfer-Jablonka, A.; Hoffmann, M.; Pöhlmann, S.; Behrens, G.M.N.; Förster, R. Neutralization of the SARS-CoV-2 Delta variant after heterologous and homologous BNT162b2 or ChAdOx1 nCoV-19 vaccination. Cell. Mol. Immunol. 2021, 18, 2455–2456. [Google Scholar]
- Fendler, A.; de Vries, E.G.E.; GeurtsvanKessel, C.H.; Haanen, J.B.; Wörmann, B.; Turajlic, S.; von Lilienfeld-Toal, M. COVID-19 vaccines in patients with cancer: Immunogenicity, efficacy and safety. Nat. Rev. Clin. Oncol. 2022, 19, 385–401. [Google Scholar]
- Nguyen, Y.; Flahault, A.; Chavarot, N.; Melenotte, C.; Cheminant, M.; Deschamps, P.; Carlier, N.; Lafont, E.; Thomas, M.; Flamarion, E.; et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin. Microbiol. Infect. 2022, 28, 1654.e1–1654.e4. [Google Scholar]
- Stuver, R.; Shah, G.L.; Korde, N.S.; Roeker, L.E.; Mato, A.R.; Batlevi, C.L.; Chung, D.J.; Doddi, S.; Falchi, L.; Gyurkocza, B.; et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell 2022, 40, 590–591. [Google Scholar] [CrossRef]
- Scoccianti, S.; Delli Paoli, C.; Grilli Leonulli, B.; Paoletti, L.; Alpi, P.; Caini, S.; Barca, R.; Fondelli, S.; Russo, S.; Perna, M.; et al. Acute tolerance of Moderna mRNA-1273 vaccine against COVID-19 in patients with cancer treated with radiotherapy. Lancet Oncol. 2021, 22, 1212–1214. [Google Scholar] [PubMed]
- Pakvisal, N.; Sainamthip, P.; Teeyapun, N.; Luangdilok, S.; Wanlapakorn, N.; Yorsaeng, R.; Poovorawan, Y.; Pakvisal, P.; Susiriwatananont, T.; Zungsontiporn, N.; et al. Vaccine-Related adverse events following AZD1222 (ChAdOx1-nCoV-19) Covid-19 vaccine in solid malignancy patients receiving cancer treatment, as compared to age-matched healthy controls. Hum. Vaccines Immunother. 2022, 18, 2094149. [Google Scholar]
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021, 22, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Benjamanukul, S.; Traiyan, S.; Yorsaeng, R.; Vichaiwattana, P.; Sudhinaraset, N.; Wanlapakorn, N.; Poovorawan, Y. Safety and immunogenicity of inactivated COVID-19 vaccine in health care workers. J. Med. Virol. 2022, 94, 1442–1449. [Google Scholar] [CrossRef]
- Moujaess, E.; Zeid, N.B.; Samaha, R.; Sawan, J.; Kourie, H.; Labaki, C.; Chebel, R.; Chahine, G.; El Karak, F.; Nasr, F.; et al. Perceptions of the COVID-19 vaccine among patients with cancer: A single-institution survey. Future Oncol. 2021, 17, 4071–4079. [Google Scholar]
- Forster, M.; Wuerstlein, R.; Koenig, A.; Amann, N.; Beyer, S.; Kaltofen, T.; Degenhardt, T.; Burges, A.; Trillsch, F.; Mahner, S.; et al. COVID-19 vaccination in patients with breast cancer and gynecological malignancies: A German perspective. Breast 2021, 60, 214–222. [Google Scholar]
- Chan, W.-L.; Ho, Y.-H.T.; Wong, C.K.-H.; Choi, H.C.-W.; Lam, K.-O.; Yuen, K.-K.; Kwong, D.; Hung, I. Acceptance of COVID-19 Vaccination in Cancer Patients in Hong Kong: Approaches to Improve the Vaccination Rate. Vaccines 2021, 9, 792. [Google Scholar] [CrossRef]
- Suntronwong, N.; Assawakosri, S.; Kanokudom, S.; Yorsaeng, R.; Auphimai, C.; Thongmee, T.; Vichaiwattana, P.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. Strong Correlations between the Binding Antibodies against Wild-Type and Neutralizing Antibodies against Omicron BA.1 and BA.2 Variants of SARS-CoV-2 in Individuals Following Booster (Third-Dose) Vaccination. Diagnostics 2022, 12, 1781. [Google Scholar]

| N (%) | ||
|---|---|---|
| Sex | Male | 19 (35.8) |
| Female | 34 (64.2) | |
| Age | Mean ± SD (years) | 56 ± 13.6 |
| Primary Tumor | CNS | 5 (9.4) |
| Head and neck | 17 (32.1) | |
| Thorax (breast, lung) | 19 (35.8) | |
| Abdomen | 11 (20.7) | |
| Bone | 1 (1.9) | |
| Stage | I-II | 13 (24.5) |
| III-IV | 40 (75.5) | |
| Systemic treatment | Chemotherapy | 24 (45.3) |
| Steroids | Yes | 9 (17) |
| Smoking | Current smoker | 7 (13.2) |
| Previous smoker | 4 (7.5) | |
| Never smoke | 42 (79.2) |
| N (%) | |
|---|---|
| Aim of treatment | |
-Curative aim | 39 (73.6) |
-Palliative aim | 14 (26.4) |
| Number of fraction (fractionation) (median, IQR) | 25 (10–30) |
| Dose in Gy (median, IQR) | 45 (40–63) |
| Technique | |
-3D-CRT | 17 (32.1) |
-IMRT/VMAT | 31 (58.5) |
-SRS/SRT/SBRT | 4 (7.5) |
-Proton therapy | 1 (1.9) |
| Studies | Vaccine | Number of Cancer Patients (Active Treatment/All) | Seropositivity | Antibody Titers at 4 Weeks Post-Vaccination | ||
|---|---|---|---|---|---|---|
| After the First Dose | After the Second Dose | After the First Dose | After the Second Dose | |||
| Teeyapan | AZ-AZ | 385/385 | 60.8% | 93.6% | 3.4 U/mL | 224.5 U/mL |
| Nelli | PZ-PZ | 285/366 | 52% | 91.2% | 62 AU/mL | 1530 AU/mL |
| Cavanna | PZ-PZ | 219/257 | NA | 76% | NA | 118 AU/mL |
| Yasin | PZ-PZ | 426/776 309 CMT 117 Targeted/IO | NA | 85.2% 78.6% 84.6% | NA | NA |
| Bowes | PZ-PZ MDN-MDN JJ | */33 | NA | NA | NA | 263 U/mL |
| Ours | AZ-AZ AZ-PZ | 53 | 54.2% | 95% 100% | 4.3 U/mL | 188.4 U/Ml 1400.8 U/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prayongrat, A.; Noppaving, P.; Chobarporn, T.; Sudhinaraset, N.; Teeyapun, N.; Pakvisal, N.; Jantarabenjakul, W.; Sophonphan, J.; Lertbutsayanukul, C.; Poovorawan, Y. Safety and Immunogenicity of Homologous and Heterologous Adenoviral-Vectored and mRNA COVID-19 Vaccine Regimens in Radiotherapy Patients. Vaccines 2023, 11, 1135. https://doi.org/10.3390/vaccines11071135
Prayongrat A, Noppaving P, Chobarporn T, Sudhinaraset N, Teeyapun N, Pakvisal N, Jantarabenjakul W, Sophonphan J, Lertbutsayanukul C, Poovorawan Y. Safety and Immunogenicity of Homologous and Heterologous Adenoviral-Vectored and mRNA COVID-19 Vaccine Regimens in Radiotherapy Patients. Vaccines. 2023; 11(7):1135. https://doi.org/10.3390/vaccines11071135
Chicago/Turabian StylePrayongrat, Anussara, Patjaya Noppaving, Thitiporn Chobarporn, Natthinee Sudhinaraset, Nattaya Teeyapun, Nussara Pakvisal, Watsamon Jantarabenjakul, Jiratchaya Sophonphan, Chawalit Lertbutsayanukul, and Yong Poovorawan. 2023. "Safety and Immunogenicity of Homologous and Heterologous Adenoviral-Vectored and mRNA COVID-19 Vaccine Regimens in Radiotherapy Patients" Vaccines 11, no. 7: 1135. https://doi.org/10.3390/vaccines11071135
APA StylePrayongrat, A., Noppaving, P., Chobarporn, T., Sudhinaraset, N., Teeyapun, N., Pakvisal, N., Jantarabenjakul, W., Sophonphan, J., Lertbutsayanukul, C., & Poovorawan, Y. (2023). Safety and Immunogenicity of Homologous and Heterologous Adenoviral-Vectored and mRNA COVID-19 Vaccine Regimens in Radiotherapy Patients. Vaccines, 11(7), 1135. https://doi.org/10.3390/vaccines11071135






