A Phase 1 Two-Arm, Randomized, Double-Blind, Active-Controlled Study of Live, Oral Plasmid-Derived Adenovirus Type 4 and Type 7 Vaccines in Seronegative Adults
Abstract
1. Introduction
2. Results
2.1. Vaccine Safety
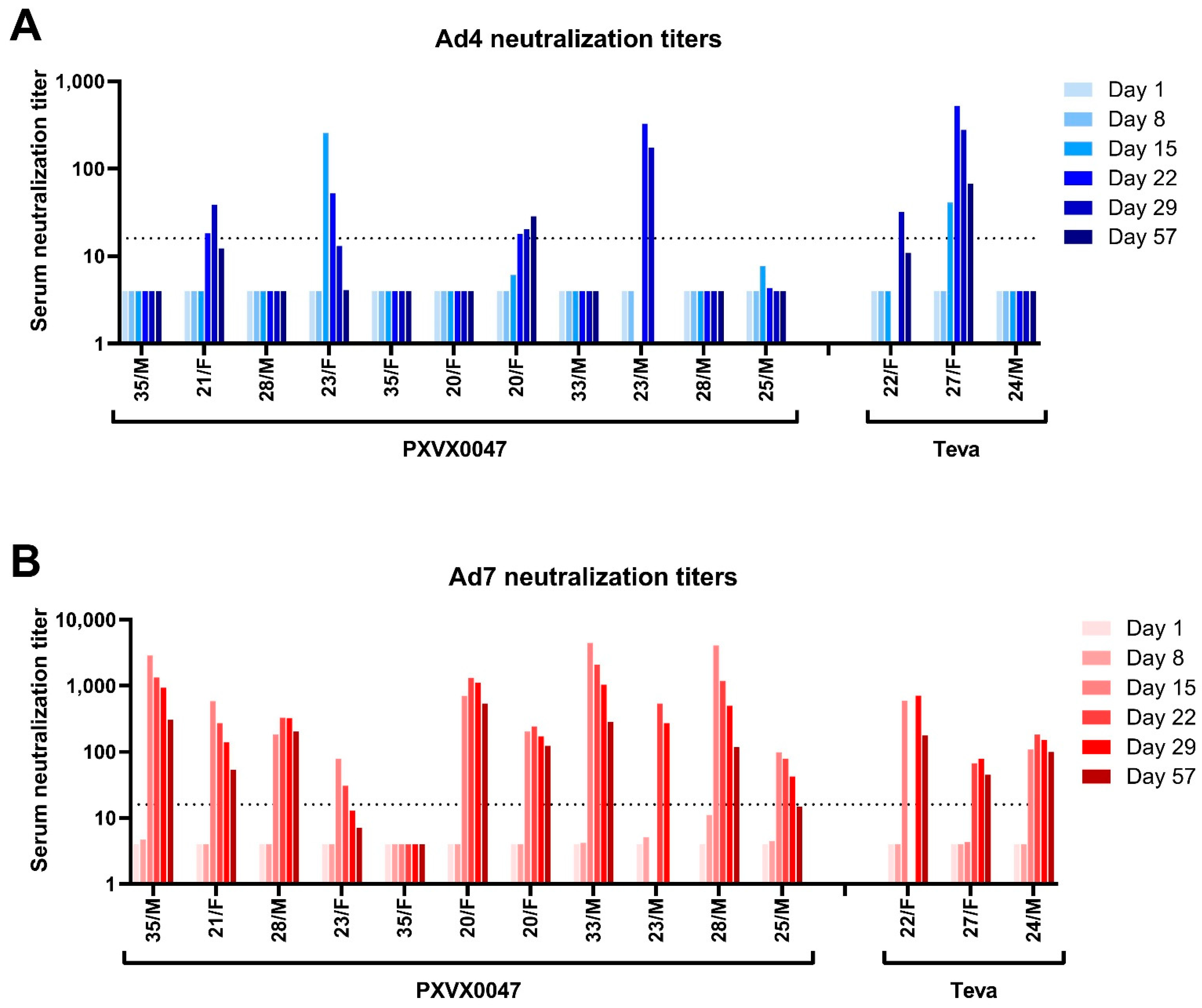
2.2. Immunogenicity
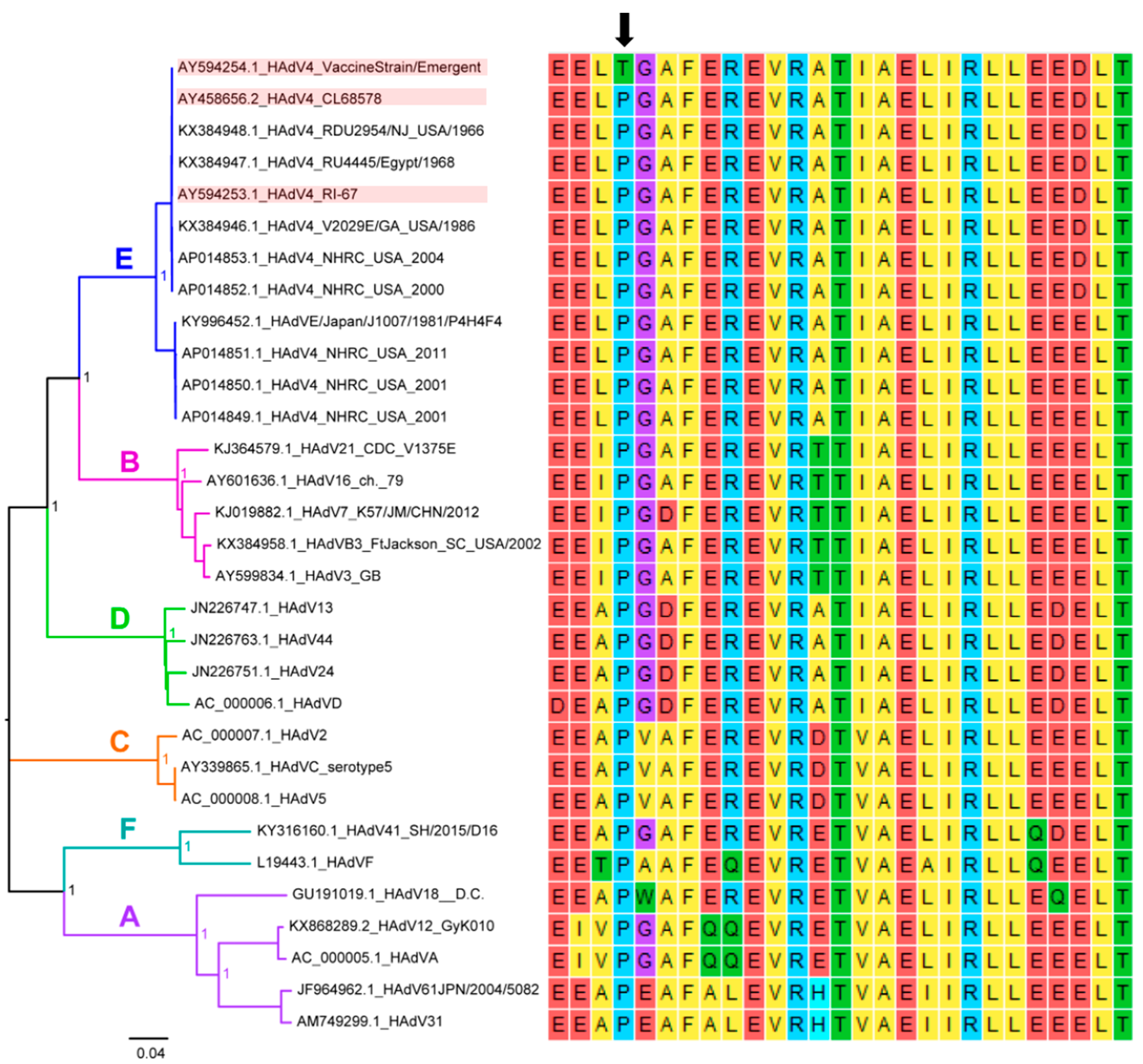
2.3. Identification of a Mutation in the Ad4 Vaccine Strains at a Highly Conserved Amino Acid Position
3. Discussion
4. Methods
4.1. Test Vaccine
4.2. Reference Vaccine
4.3. Luciferase-Based Neutralization Assay
4.4. Conventional Colorimetric-Based Neutralization Assay
4.5. Genetic Comparison of Ad4 Component of PXVX0047
4.6. Selection of Study Population
4.7. Study Design
4.8. Randomization
4.9. Safety Analysis
4.10. Specimen Collection and Immunogenicity Analysis
4.11. Clinical Study Ethics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoke, C.H., Jr.; Snyder, C.E., Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine 2013, 31, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Chanock, R.M.; Ludwig, W.; Heubner, R.J.; Cate, T.R.; Chu, L.W. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA 1996, 195, 445–452. [Google Scholar] [CrossRef]
- Top, F.H., Jr.; Dudding, B.A.; Russell, P.K.; Buescher, E.L. Control of respiratory disease in recruits with types 4 and 7 adenovirus vaccines. Am. J. Epidemiol. 1971, 94, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Top, F.H., Jr.; Grossman, R.A.; Bartelloni, P.J.; Segal, H.E.; Dudding, B.A.; Russell, P.K.; Buescher, E.L. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J. Infect. Dis. 1971, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.C.; Goswami, P.R.; Malasig, M.D.; Hawksworth, A.W.; Trump, D.H.; Ryan, M.A.; Schnurr, D.P. Adult adenovirus infections: Loss of orphaned vaccines precipitates military respiratory disease epidemics. For the Adenovirus Surveillance Group. Clin. Infect. Dis. 2000, 31, 663–670. [Google Scholar] [CrossRef] [PubMed]
- McNeill, K.M.; Ridgely Benton, F.; Monteith, S.C.; Tuchscherer, M.A.; Gaydos, J.C. Epidemic spread of adenovirus type 4-associated acute respiratory disease between U.S. Army installations. Emerg. Infect. Dis. 2000, 6, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Kolavic-Gray, S.A.; Binn, L.N.; Sanchez, J.L.; Cersovsky, S.B.; Polyak, C.S.; Mitchell-Raymundo, F.; Asher, L.V.; Vaughn, D.W.; Feighner, B.H.; Innis, B.L. Large epidemic of adenovirus type 4 infection among military trainees: Epidemiological, clinical, and laboratory studies. Clin. Infect. Dis. 2002, 35, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Kuschner, R.A.; Russell, K.L.; Abuja, M.; Bauer, K.M.; Faix, D.J.; Hait, H.; Henrick, J.; Jacobs, M.; Liss, A.; Lynch, J.A.; et al. A phase 3, randomized, double-blind, placebo-controlled study of the safety and efficacy of the live, oral adenovirus type 4 and type 7 vaccine, in U.S. military recruits. Vaccine 2013, 31, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.; Longfield, J.; Kuschner, R.; Straight, T.; Binn, L.; Seriwatana, J.; Reitstetter, R.; Froh, I.B.; Craft, D.; McNabb, K.; et al. A double-blind, placebo-controlled study of the safety and immunogenicity of live, oral type 4 and type 7 adenovirus vaccines in adults. Vaccine 2008, 26, 2890–2898. [Google Scholar] [CrossRef] [PubMed]
- Package Insert—Adenovirus Type 4 and Type 7 Vaccine, Live, Oral. Food Drug. Adm. Available online: https://www.fda.gov/media/80211/download (accessed on 19 March 2019).
- Hoke, C.H., Jr.; Hawksworth, A.; Snyder, C.E., Jr. Initial assessment of impact of adenovirus type 4 and type 7 vaccine on febrile respiratory illness and virus transmission in military basic trainees, March 2012. MSMR 2012, 19, 2–4. [Google Scholar] [PubMed]
- Choudhry, A.; Mathena, J.; Albano, J.D.; Yacovone, M.; Collins, L. Safety evaluation of adenovirus type 4 and type 7 vaccine live, oral in military recruits. Vaccine 2016, 34, 4558–4564. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, A.; Su, J.; Carlisle, S.; Tibbetts, C.; Seto, D. Genomic and bioinformatics analysis of HAdV-7, a human adenovirus of species B1 that causes acute respiratory disease: Implications for vector development in human gene therapy. Virology 2005, 332, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Houng, H.S.; Clavio, S.; Graham, K.; Kuschner, R.; Sun, W.; Russell, K.L.; Binn, L.N. Emergence of a new human adenovirus type 4 (Ad4) genotype: Identification of a novel inverted terminal repeated (ITR) sequence from majority of Ad4 isolates from US military recruits. J. Clin. Virol. 2006, 35, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.D.; Adhikari, A.; Yang, Y.; Kuschner, R.A.; Karasavvas, N.; Binn, L.N.; Walls, S.D.; Graf, P.C.F.; Myers, C.A.; Jarman, R.G.; et al. Live Oral Adenovirus Type 4 and Type 7 Vaccine Induces Durable Antibody Response. Vaccines 2020, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Beaty, S.; Wallace, E.; Li, Y.; Sanborn, M.; Yang, Y.; Adhikari, A.; Shabram, P.; Warfield, K.; Karasavvas, N.; et al. Differential replication of a unique single amino acid mutation 2 in the Adenovirus-4 component of the live oral Adenovirus 3 type 4 and type 7 vaccine. Vaccines 2023, submitted.
- Sprangers, M.C.; Lakhai, W.; Koudstaal, W.; Verhoeven, M.; Koel, B.F.; Vogels, R.; Goudsmit, J.; Havenga, M.J.; Kostense, S. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: Addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 2003, 41, 5046–5052. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A.; Coull, B. Approximate Is Better than "Exact" for Interval Estimation of Binomial Proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar] [CrossRef]
| Characteristic | PXVX0047 N = 11 | Teva Ad4/Ad7 N = 3 | All Subjects N = 14 |
|---|---|---|---|
| Age in y, median (range) | 25 (20–35) | 24 (22–27) | 24.5 (20–35) |
| Sex, n (%) | |||
| Female | 5 (45.5) | 2 (66.7) | 7 (50.0) |
| Male | 6 (54.5) | 1 (33.3) | 7 (50.0) |
| Race, n (%) | |||
| Black/African American | 6 (54.5) | 1 (33.3) | 7 (50.0) |
| White | 4 (36.4) | 2 (66.7) | 6 (42.9) |
| Multiple | 1 (9.1) | 0 | 1 (7.1) |
| Other | 0 | 0 | 0 |
| Safety Event | PXVX0047 N = 11 | Teva Ad4/Ad7 N = 3 | All Subjects N = 14 | |
|---|---|---|---|---|
| Subjects reporting at least one solicited adverse event | Mild (grade 1) | 1 (9.1%) | 0 | 1 (7.1%) |
| Moderate (grade 2) | 3 (27.3%) | 1 (33.3%) | 4 (28.6%) | |
| Severe (grade 3) | 1 (9.1%) * | 0 | 1 (7.1%) * | |
| Potentially life-threatening (grade 4) | 0 | 0 | 0 | |
| Total | 5 (45.5%) | 1 (33.3%) | 6 (42.9%) | |
| Subjects reporting at least one unsolicited adverse event | Mild (grade 1) | 5 (45.5%) | 3 (100%) | 8 (57.1%) |
| Moderate (grade 2) | 2 (18.2%) | 0 | 2 (14.3%) | |
| Severe (grade 3) | 1 (9.1%)* | 0 | 1 (7.1%) * | |
| Potentially life-threatening (grade 4) | 0 | 0 | 0 | |
| Total | 8 (72.7%) | 3 (100%) | 11 (78.6%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaty, S.; Collins, N.; Karasavvas, N.; Kuschner, R.; Hang, J.; Adhikari, A.; Maljkovic Berry, I.; Fung, C.; Walls, S.; Betancourt, E.; et al. A Phase 1 Two-Arm, Randomized, Double-Blind, Active-Controlled Study of Live, Oral Plasmid-Derived Adenovirus Type 4 and Type 7 Vaccines in Seronegative Adults. Vaccines 2023, 11, 1091. https://doi.org/10.3390/vaccines11061091
Beaty S, Collins N, Karasavvas N, Kuschner R, Hang J, Adhikari A, Maljkovic Berry I, Fung C, Walls S, Betancourt E, et al. A Phase 1 Two-Arm, Randomized, Double-Blind, Active-Controlled Study of Live, Oral Plasmid-Derived Adenovirus Type 4 and Type 7 Vaccines in Seronegative Adults. Vaccines. 2023; 11(6):1091. https://doi.org/10.3390/vaccines11061091
Chicago/Turabian StyleBeaty, Shannon, Natalie Collins, Nicos Karasavvas, Robert Kuschner, Jun Hang, Anima Adhikari, Irina Maljkovic Berry, Christian Fung, Shannon Walls, Elena Betancourt, and et al. 2023. "A Phase 1 Two-Arm, Randomized, Double-Blind, Active-Controlled Study of Live, Oral Plasmid-Derived Adenovirus Type 4 and Type 7 Vaccines in Seronegative Adults" Vaccines 11, no. 6: 1091. https://doi.org/10.3390/vaccines11061091
APA StyleBeaty, S., Collins, N., Karasavvas, N., Kuschner, R., Hang, J., Adhikari, A., Maljkovic Berry, I., Fung, C., Walls, S., Betancourt, E., Mendy, J., Lock, M., Gierman, E., Bennett, S., Shabram, P., & Warfield, K. (2023). A Phase 1 Two-Arm, Randomized, Double-Blind, Active-Controlled Study of Live, Oral Plasmid-Derived Adenovirus Type 4 and Type 7 Vaccines in Seronegative Adults. Vaccines, 11(6), 1091. https://doi.org/10.3390/vaccines11061091






