Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion
Abstract
1. Introduction
| aTIV (Fluad, Seqirus Inc.) | aQIV (Fluad, Seqirus Inc.) | HD-TIV (Fluzone, Sanofi) | HD-QIV (Fluzone, Sanofi) | QIVr (Flublok, Sanofi) | |
|---|---|---|---|---|---|
| Composition | MF59®-adjuvanted trivalent influenza vaccine | MF59®-adjuvanted quadrivalent influenza vaccine | High-dose trivalent influenza vaccine | High-dose quadrivalent influenza vaccine | Recombinant quadrivalent influenza vaccine |
| Approvals in select countries | |||||
| Argentina | 2021 Adults ≥ 65 years of age [22] | NA | 2010 Adults 18–59 years of age [36] | NA | NA |
| Canada | 2011 Adults ≥ 65 years of age [37] | NA | 2010 adults 18–59 years of age [36] 2020 adults ≥ 65 years of age [38] | 2021 Adults ≥ 65 years of age [38] | 2021 Adults ≥ 18 years of age [39] |
| United States | 2015 Adults ≥ 65 years of age [40] | 2020 Adults ≥ 65 years of age [41] | 2009 adults ≥ 65 years of age [38] 2011 adults 18–64 years of age [42] | 2019 Adults ≥ 65 years of age [42] | 2013 Adults ≥ 18 years of age [43] |
| United Kingdom | 2017 Adults ≥ 65 years of age [44] | 2021 Adults ≥ 65 years of age [45] | 2019 Adults ≥ 65 years of age [46] | 2021 Adults ≥ 60 years of age [47] | 2022 Adults ≥ 18 years of age [45] |
| European Union | 2017 Adults ≥ 65 years of age [48] | 2020 Adults ≥ 65 years of age [15] | 2009 Adults 18–59 years of age [36] | 2021 Adults ≥ 60 years of age [47] | 2020 Adults ≥ 18 years of age [49] |
2. Methods
2.1. Targeted Literature Search
- (“Adjuvanted quadrivalent influenza vaccine” OR “Fluad” OR “aIIV4” OR “aQIV”) AND (“economic” OR “cost” OR “cost effectiveness” OR “cost utility” OR “budget impact”)
- (“Adjuvanted trivalent influenza vaccine” OR “Fluad” OR “aIIV3” OR “aTIV”) AND (“economic” OR “cost” OR “cost effectiveness” OR “cost utility” OR “budget impact”)
- (“High dose quadrivalent influenza vaccine” OR “IIV4 HD” OR “QIV HD” OR “Fluzone HD”) AND (“economic” OR “cost” OR “cost effectiveness” OR “cost utility” OR “budget impact”)
- (“High dose trivalent influenza vaccine” OR “IIV3 HD” OR “TIV HD” OR “Fluzone HD”) AND (“economic” OR “cost” OR “cost effectiveness” OR “cost utility” OR “budget impact”)
- (“Quadrivalent recombinant influenza vaccine” OR “QIVr” OR “Flublok”) AND (“economic” OR “cost” OR “cost-effectiveness” OR “cost-utility” OR “budget impact”).
2.2. Supplemental Searches
2.3. Included Studies
3. Cost-Effectiveness Studies with Enhanced Influenza Vaccines
3.1. Comparison between CEA for Enhanced and Standard Vaccines
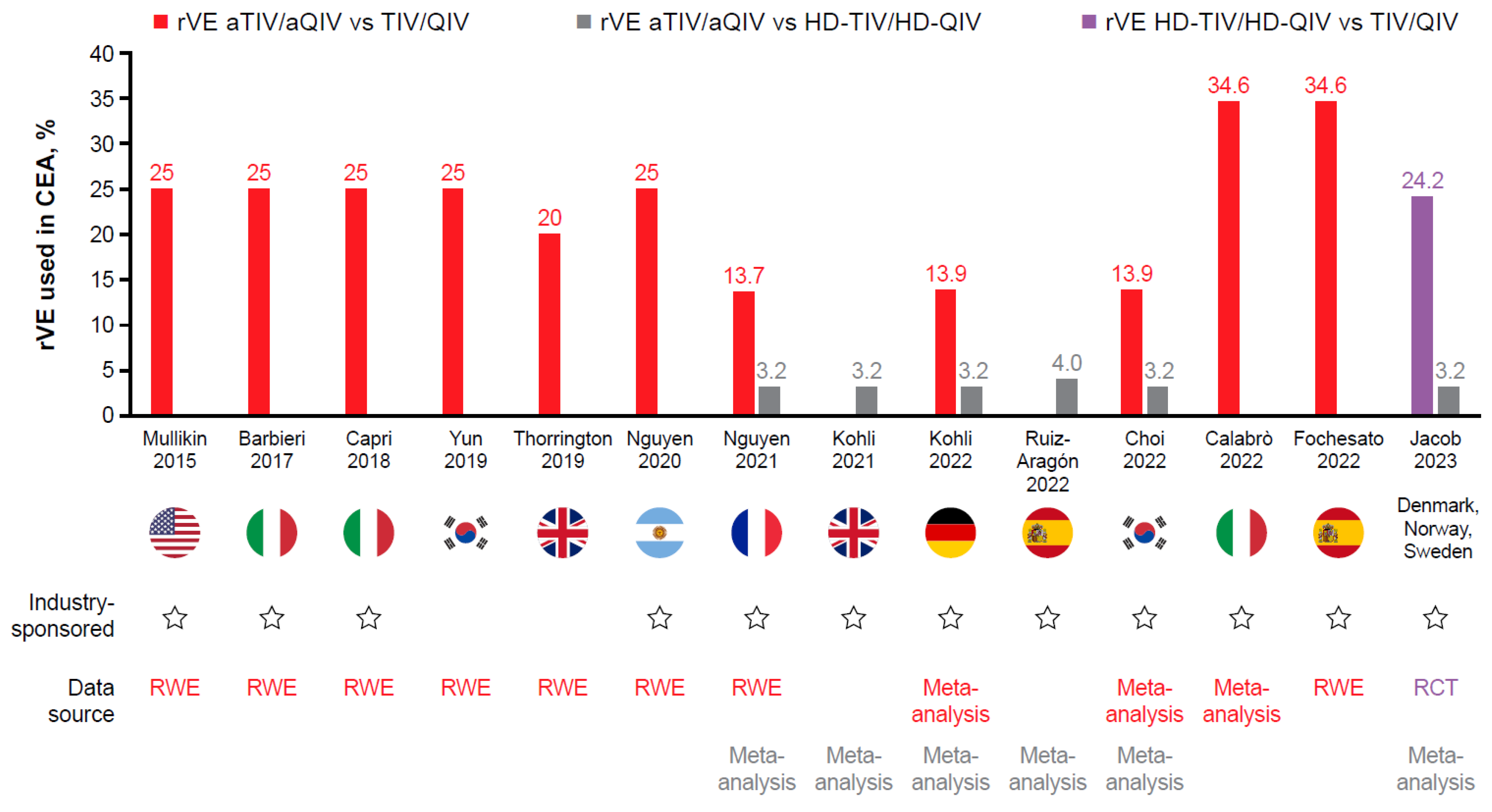
| Author Year | Country | Strategy | Model Type | Perspective | Time Horizon | Selected Costs | Year, Currency | rVE * | Discounting | Uncertainty Analysis | Findings | Author Conclusion | Industry Sponsor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) Adjuvanted vaccines vs. TIV/QIV | |||||||||||||
| Lee BY, et al., 2009 [82] | USA | aTIV vs. TIV | Decision analytic computer simulation model | Societal, third-party payer | 1 influenza season | TIV $15.75 (price obtained from Red Book) aTIV varied $0–100 to that of TIV Hospitalization Death Complications Medical visits Lost productivity | 2007, US$ | aTIV potency 50% (ability to overcome immunosenescence; origin of estimate undisclosed) | NR | Univariate, multi- dimensional, PSA | aTIV vs. TIV could prevent: 496,533 influenza cases 171,981 hospitalizations 70,429 deaths Save society $824 million if aTIV cost the same as TIV (dominant), and continue to be cost-saving if aTIV cost $30 more than TIV | Introducing aTIV to older adults could save significant morbidity, mortality, and costs. aTIV remained a dominant strategy in several scenarios | No |
| Fisman DN and Tuite AR 2011 [83] | Canada | aTIV vs. TIV | Age-structured compartmental model | NR | 10 years | TIV CAN$7.55 aTIV CAN$11.59 (from literature; type of price undisclosed) Influenza infection Hospitalization ICU admission ED visit GP visit Death | 2009, CAN$ | VE aTIV 40% VE TIV 20% (multiple RWE sources used for model calibration, including meta-analysis by Jefferson [84]) | Costs 5% QALYs lost 5% | One-way, Multivariate | aTIV cost more vs. TIV, but cost was offset by fewer influenza cases and decreased healthcare resource use from CAN$501.76 million to CAN$473.50 million ICER $2111/QALY | aTIV in adults ≥ 65 years of age was highly cost-effective vs. TIV | Yes (Novartis) |
| Mullikin M, et al., 2015 [51] | USA | aTIV vs. TIV and QIV | Compartmental, dynamic epidemiologic module (SIR model) and tree-structured outcomes model | NR | 1 year | TIV $9.45 aTIV $13.65 QIV $13.65 (price assumed, or from CDC) Hospitalization Death Complications Medical visits Comedication Lost productivity Administration | NA, US$ | rVE aTIV vs. TIV 25% any strain (from prospective, observational study [85]) | Costs 3% Life-years and QALYs lost 3% | Univariate, PSA | aTIV vs. TIV in persons ≥ 65 years of age: ICER $9980–28,800/QALY aTIV vs. QIV in persons ≥ 65 years of age: dominant | aTIV in adults ≥ 65 years of age may enable clinical and economic benefit vs. QIV and TIV | Yes (Novartis) |
| Ruiz-Aragón J, et al., 2015 [86] | Spain | aTIV vs. TIV | Scenario-based budget impact analysis † | NR | NR | TIV €3.75 aTIV €4.30 (weighted average of the prices extracted from the contract of tender for the 2012–2013 campaign of the Andalusian Service of Health) Medical consultation Hospitalization Comedication | NA, Euro € | rVE, NR | NR | Univariate | 113,189 influenza cases were avoided €79.99 million was saved, leading to a budget impact of €76.13 million saved | Adding aTIV to those > 64 years of age would provide significant savings for the health system (article in Spanish) | No |
| Barbieri M and Capri S 2017 [52] | Italy | aTIV vs. TIV. QIV, ID-TIV, no vaccination | Decision tree model | NR | NR | aTIV €6.99 TIV €5.35 ID-TIV €6.99 QIV €11.08 (ex-factory prices) Hospitalization Medical visits Death Complications | NR, Euro € | rVE aTIV vs. TIV 25% (from prospective, observational study [85]) ID-TIV vs. TIV: 16.5% (from modeled data [87]) VE TIV 58% (from meta-analysis [84]) | NR | Univariate, PSA | aTIV vs. TIV ICER €4527/QALY aTIV dominated ID-TIV aTIV dominated QIV aTIV vs. no vaccination ICER €10,750/QALY | aTIV should be the vaccine of choice for older adults ≥ 65 years of age in Italy and is cost-effective vs. TIV and no vaccination (article in Italian) | No |
| Pérez-Rubio A and Eiros JM 2018 [88] | Spain | aTIV vs. TIV | Scenario-based budget impact analysis † | NR | NR | TIV €2.90 aTIV €4.30 (public data) Medical consultation Comedication | NA, Euro € | rVE, not available | NR | Univariate | Budgetary impact of replacing TIV with aTIV was €6.97 million, suggesting a potential saving of €82 million Cost–benefit ratio of 12.83 | Replacing TIV with aTIV in those ≥ 65 years of age would increase the efficiency of the vaccination programs in Spain and its autonomous communities (article in Spanish) | Seqirus acknowledged |
| Capri S, et al., 2018 [53] | Italy | aTIV vs. TIV, ID-TIV, QIV | Decision tree model | Italian NHS | 1 year | TIV €5.35 aTIV €6.99 ID-TIV €6.99 QIV €11.08 (ex-factory price; public data) Medical consultation Comedication Complications | 2017, Euro € | VE TIV 58% (from meta-analysis [84]) rVE aTIV vs. TIV 25% (from prospective, observational study [85]) ID-TIV vs. TIV: 16.5% (from modeled data [87]) rVE QIV vs. TIV 3.8% (estimated) | Costs 0% Loss of QALYs discounted | One-way, DSA, PSA | aTIV vs. TIV ICER €4527/QALY aTIV dominated ID-TIV and QIV | aTIV should be preferred for Italians ≥ 65 years of age | Yes (Seqirus) |
| Yun JW, et al., 2019 [54] | South Korea | aTIV vs. TIV QIV vs. TIV | Static lifetime Markov model Analyzed across three age groups (65–74, 75–84, and ≥85 years of age) | Societal | Lifetime | TIV $7.47 QIV $8.59 aTIV $8.59 (purchase price of NIP or assumed) Administration Hospitalization Medical visits Death Complications | 2016, US$ | VE aTIV 60.30% (calculated from prospective, observational study [85]) VE TIV 48.24%, VE QIV 57–58% (calculated from several meta-analyses [84,89,90]) | Costs 3% Outcomes 3% | One-way, PSA | Compared with TIV, aTIV reduced: cases by 1,812,395 and complications by 89,747 aTIV was highly cost-saving and dominated TIV QIV vs. TIV ICER $17,699/QALY | aTIV and QIV were more cost-effective than TIV for those ≥ 65 years of age | No |
| Thorrington D, et al., 2019 [55] | England | aTIV vs. TIV | Dynamic SEIR-type transmission model with economic framework in adults ≥ 65 and ≥75 years of age | Healthcare provider | 14 seasons used in model | £11.75 aTIV £9.05 TIV (list price including VAT) GP consultation Hospitalization | NR, GBP£ | rVE aTIV vs. TIV 20% (assumption, designed to be more conservative than community-based case–control study [91]) | Costs adjusted for inflation | DSA, PSA | Compared with TIV, aTIV reduced: GP consultations by 18,913, hospitalizations by 1152, and deaths by 380 aTIV vs. TIV ICER £469/QALY | Compared with TIV, aTIV reduced healthcare use and was more cost-effective in persons ≥ 65 years of age Persons ≥ 75 years of age may receive the greatest benefit from aTIV given the lack of efficacy of TIV in this age group | No |
| Nguyen VH, et al., 2020 [56] | Argentina | aTIV vs. TIV | Decision tree model | Payer | 1 year | TIV $4.73 (public price) aTIV $7.00 (list price) Hospitalization Outpatient care Administration Consultation Drug/antivirals | NR, US$ | rVE aTIV vs. TIV 25% (from prospective, observational study [85]) | Costs 0% Outcomes 0% | Univariate DSA, PSA | Compared with TIV, switching to aTIV could reduce: cases by 20,930, GP visits by 15,120, hospitalizations by 530, deaths by 170, and life years lost by 1640 Gain 1310 QALYs aTIV vs. TIV ICER $2660.59/QALY | aTIV yielded substantial health benefits and cost savings vs. TIV in older adults. rVE and influenza attack rate were most influential in DSA. | Yes (Seqirus) |
| Nguyen VH, et al., 2021 [57] | France | aQIV vs. QIVe aQIV vs. HD-QIV | Static decision tree model | Payer | NR | QIV €11.11 aQIV €26.00 HD-QIV €26.00 (assumption) Healthcare visit In/outpatient complications Hospitalization Mortality | NR, Euro € | rVE aTIV vs. QIV 13.7% (95% CI 3.1, 24.2) * rVE aTIV vs. HD-TIV 3.2% (−2.5, 8.9) * rVE aTIV vs. TIV 13.9% (4.2, 23.5) * (from meta-analysis [92]) | NR | DSA | Replacing QIVe with aQIV over a 3-year period could prevent: 56,028 influenza cases, 13,449 medical care visits, 30,815 outpatient complications, 3902 inpatient complications, and 745 influenza-associated deaths Budget savings were driven by avoidance of medical care visits costs (€470 K); outpatient complication costs (€788 K) and inpatient complication costs (€23.2 M). | aQIV for the older adult population would be clinically favorable, with a small incremental cost impact | Yes (Seqirus) |
| Angerami R, et al., 2021 [93] | Brazil | aTIV vs. TIVe | Static decision tree model based on epidemiology and demography across 10 seasons | Societal, payer | 1 year | TIVe R$15.12 aTIV R$27.65 (list prices with or without adjustment) Medical visit Hospitalization Absenteeism Death | NR, Brazilian Reais R$ | rVE assumed from Italian multi-season analysis (value not stated) | NR | PSA | Compared with TIVe, aTIV reduced: cases by 300,035, outpatient visits by 90,589, hospitalizations by 23,100, and deaths by 4931 QALYs increased by 49,457 aTIV vs. TIVe ICER R$6253/QALY (payer perspective) | aTIV was highly cost-effective compared with TIVe | Yes (Seqirus) |
| Kohli M, et al., 2022 [58] | Germany | aQIV vs. QIVe aQIV vs. HD-QIV | SEIR compartmental transmission model | Societal, Statutory health insurance | 10 seasons from 2010–2019 | QIVe €12.56 aQIV €19.21 HD-QIV €40.55 (reimbursement price per dose) Hospitalization Death In/outpatient visits Medication Sickness benefit Lost working time | NA, Euro € | aQIV vs. QIVe 13.9% (4.2, 23.5) * aQIV vs. HD-QIV 3.2% (−2.5, 8.9) * (from meta-analysis [92]) VE QIVe 62%, 24%, and 79% against A/H1N1, A/H3N2, and B types (assumptions, related to meta-analysis [94] and systematic review [90]) | Costs 3% QALYs 3% | DSA, PSA | aQIV and HD-QIV reduced the number of influenza cases, hospitalizations, and deaths in the German population vs. QIVe. aQIV dominated HD-QIV because it was slightly more effective in the base case (rVE = 3.2%), and was less costly to implement | aQIV may be cost-effective compared with QIVe at current prices aQIV and HD-QIV had similar clinical effectiveness, but aQIV is less costly than HD-QIV. CE of aQIV was most sensitive to changes in VE and rate of hospitalization due to influenza | Yes (Seqirus) |
| Choi MJ, et al., 2022 [59] | South Korea | aQIV vs. QIV aQIV vs. HD-QIV | Static, 1-year decision tree model Analyzed across three age groups (65–74, 75–84, and ≥85 years of age) | Healthcare system | 1 year | Hospitalization Death Complications Influenza cases Vaccine price NR | NR | aQIV vs. QIVe 13.9% (4.2, 23.5) * aQIV vs. HD-QIV 3.2% (−2.5, 8.9) * (from meta-analysis [92]) VE QIV 62%, 24%, and 63% vs. A(H1N1), A(H3N2), and B, respectively (from meta-analysis [94]) | NR | DSA, PSA | Compared with QIV, aQIV reduced: cases by 35,390, complications by 1602, hospitalizations by 709, and deaths by 145 Compared with HD-QIV, aQIV reduced: cases by 7247, complications by 328, hospitalizations by 145, and deaths by 30 | Replacing QIV with aQIV is predicted to reduce disease burden in South Korean adults ≥ 65 years of age Benefits of aQIV and HD-QIV are predicted to be similar due to comparable VE CE estimates were most influenced by changes to rVE | Yes (Seqirus) |
| Calabrò GE, et al., 2022 [60] | Italy | aQIV vs. QIVe | SEIR dynamic transmission model | Societal, health system payer | Nine seasons | Infection Hospitalization Death Medical visits Complications Vaccine price NR | 2020, Euro € | rVE aTIV vs. TIVe or QIVe 34.6% (2.0, 66.0) LCI* (estimated based on data from meta-analysis [in Italian]) | Indirect costs 3% QALYs 3% Costs inflated to 2020 | DSA, PSA | Across all age categories, aQIV could avoid 363 hospitalizations and 195 deaths vs. QIVe—of these, 93% of avoided hospitalizations and 98% of avoided deaths would be recorded in those > 65 years of age aQIV vs. QIVe ICER: €14,441/QALY | aQIV in individuals ≥ 65 years of age is cost-effective | Yes (Seqirus) |
| Fochesato A, et al., 2022 [61] | Spain | aQIV vs. QIVe | SEIR dynamic transmission model | Societal, public payer | Cost time horizon = one season Effect time horizon = lifetime | aQIV €13.00 QIVe €9.50 (per dose, unspecified) Disease management Hospitalization Medical visits Vaccines Loss of productivity Death | 2021, Euro € | rVE aTIV vs. TIVe or QIVe 34.6% (2.0, 66.0) LCI* (estimated based on data from meta-analysis [in Italian]) rVE aQIV vs. QIVe 13.9% (4.2, 23.5) * (from meta-analysis [92]) VE QIVe 62%, 24%, and 52.1% vs. A(H1N1), A(H3N2), and B, respectively (taken from secondary sources [in Italian] including [95] | Costs 3% QALY 3% | DSA, PSA | aQIV vs. QIVe with rVE 34.6% reduced: cases by 43,664, hospitalizations by 1111, and deaths by 569 aQIV vs. QIVe with rVE 13.9% reduced: cases by 19,104, hospitalizations by 486, and deaths by 252 ICER €2240/QALY for rVE 34.6% ICER €6694/QALY for rVE 13.9% (payer perspective) | Replacing QIVe with aQIV when vaccinating adults ≥ 65 years of age in Spain is a cost-effective strategy in high and moderate rVE scenarios | Yes (Seqirus) |
| Jacob J, et al., 2023 [62] | Denmark, Norway, Sweden | aQIV vs. QIV | Static decision tree model | Healthcare payer, societal | NR | QIV €9.10–11.00 aQIV 170–189% that of QIV (prices from IQVIA or assumption) Hospitalization GP visit Outpatient visit Comedication Lost productivity Death Complications Influenza cases | 2022, Euro € | VE QIV 62%, 24%, and 63% vs. A(H1N1), A(H3N2), and B, respectively (from meta-analysis [94]) rVE HD-QIV to QIV 24.2% * from FIM12 RCT [50] | 3–4% outcomes and costs | DSA, PSA | Across Denmark, Norway, and Sweden in one influenza season, aQIV vs. QIV could prevent: 18,772 symptomatic influenza infections, 925 hospitalizations, and 161 deaths aQIV vs. QIV ICER €10,170/QALY in Denmark ICER €12,515/QALY in Norway ICER €9894/QALY in Sweden | Introducing aQIV to those ≥ 65 years of age may reduce influenza disease and economic burden in Denmark, Norway, and Sweden | Yes (Seqirus) |
| (B) High-dose vaccines vs. TIV/QIV | |||||||||||||
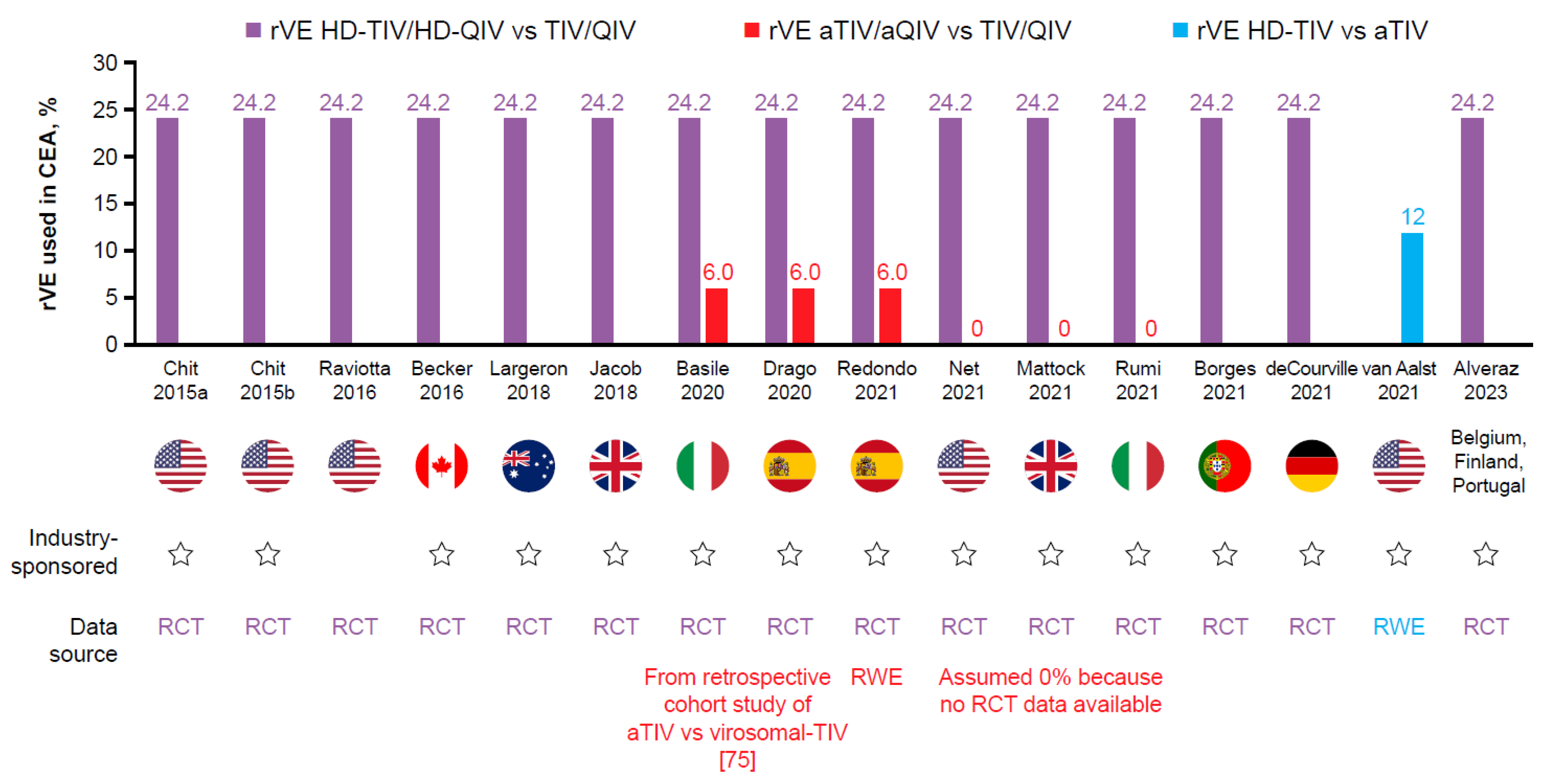
| Chit A, et al., 2015a [65] | USA | HD-TIV vs. TIV | CEA, person-level study | Societal Third-party payer | Cost = one influenza season Effect = lifetime | HD-TIV $31.82 TIV $12.04 (unit costs) Hospitalization Deaths Medical visits Prescription medication Study vaccine Lost work force | NR, USD$ | rVE HD-TIV vs. TIV 24.2% from FIM12 RCT [50] | NR | PSA | Societal and Medicare perspectives: HD-TIV dominated TIV Mean per-participant medical costs were lower with HD-TIV ($1376.72) than TIV ($1492.64)Hospital admissions contributed 95% of the total healthcare-payer cost and 87% of the total societal costs | HD-TIV is less costly and more effective vs. TIV, driven by a reduction in the number of hospital admissions PSA showed HD-TIV 93% likely to be cost-saving | Yes (Sanofi) |
| Chit A, et al., 2015b [66] | USA | HD-TIV vs. TIV HD-TIV vs. QIV | Economic model evaluating three health states: symptomatic influenza, influenza-associated hospitalizations, and influenza-associated deaths | Societal, Third-party payer | Cost time horizon = one influenza season Effect time horizon = lifetime | HD-TIV $32.82 TIV $12.39 QIV $19.41 (CMS costs per dose) Symptomatic influenza Hospitalization Medical visits Comedication Work loss Co-payments | NR, USD$ | rVE HD-TIV vs. TIV 24.24% (9.69, 36.52) symptomatic influenza from FIM12 RCT [50] VE TIV 49% (33.00, 62.00) symptomatic influenza (from meta-analysis [96]) VE QIV 50.68% (34.13, 64.13) symptomatic influenza (estimated based on multiple sources including from meta-analysis [96]) | Costs 0% Outcomes 3% | DSA, PSA | Compared with TIV, HD-TIV could avoid 195,958 cases of influenza, 22,567 influenza-related hospitalizations, and 5423 influenza-related deaths Compared with QIV, HD-TIV could avoid 169,257 cases of influenza, 21,222 hospitalizations, and 5212 deaths Societal: HD-TIV vs. TIV ICER $5299/QALY HD-TIV dominated QIV Third-party payer: HD-TIV vs. TIV ICER $10,350/QALY HD-TIV vs. QIV ICER $4365 | HD-TIV is expected to be cost-effective vs. TIV and QIV. 60–71% probability HD-TIV is at least cost-effective compared with TIV. 70–81% probability HD-TIV is at least cost-effective compared with QIV | Yes (Sanofi) |
| Cheng X and Roïz J 2015 [97] | Canada | HD-TIV vs. TIV | Analytical decision model | Healthcare, societal | NR | Comedication Long-term impact of influenza infections Vaccine price NR | NR, CAN$ | NR | Costs NR Outcomes NR | DSA, PSA | HD-TIV vs. TIV ICER CAN$3763/QALY healthcare perspective ICER CAN$190/QALY societal perspective HD-TIV dominated TIV when long-term care costs were considered | HD-TIV may reduce influenza-associated morbidity and mortality, and is cost-effective in the studied population vs. TIV | No |
| Becker D, et al., 2016 [67] | Canada | HD-TIV vs. TIV | CEA, person-level study | Societal Public health payer | Cost time horizon = one influenza season Effect time horizon = lifetime | HD-TIV: $31.82 TIV: $5.82 (CMS price schedule and manufacturer) ER visits Hospitalization Medical visits Comedication Lost work force | 2014, CAN$ | rVE HD-TIV vs. TIV 24.2% (9.7, 36.5) LCI from FIM12 RCT [50] | Costs 0% Outcomes 5% | PSA | HD-TIV dominated TIV from public payer and societal perspective Per-participant total societal costs were were lower with HD-TIV (CAN$814) than TIV (CAN$874). 91% of healthcare payer costs and 76% of the total societal costs were due to hospital admissions | HD-TIV is expected to be a less costly and more effective vs. TIV driven by a reduction in hospitalizations PSA indicated HD-TIV is 89% likely to be cost-saving | Yes (Sanofi) |
| Raviotta J, et al., 2016 [68] | USA | HD-TIV vs. QIV | Markov state transition model | Societal | Cost time horizon = one influenza season Effect time horizon = lifetime | HD-TIV: $31.20 TIV: $10.69 QIV $16.15 (CMS price schedule and medical literature) Hospitalization Influenza illness Death Outpatient Medication Vaccine Productivity loss | 2014 USD$ | VE all vaccines 39% (from modeled US data [98]) rVE HD-TIV vs. TIV: 24.2% * from FIM12 RCT [50] | Costs 0% Outcomes 3% | One-way, PSA | HD-TIV vs. QIV ICER $31,214/QALY. Despite a substantially higher per-dose cost ($21.51 more), HD-TIV is an economically favorable strategy in for US adults ≥ 65 years of age Secondary analysis: aTIV was not favored vs. TIV if rVE was < 15% but was favored if rVE aTIV vs. TIV ≥ 32%. If rVE was equivalent to that of HD-TIV (i.e., 24.2%), it would be favored if it cost less than HD-TIV | HD-TIV for adults ≥ 65 years of age is likely to be favored from economic and public health standpoints. Results were sensitive to yearly influenza attack rates, virus variability, and VE | No |
| Crépey P, et al. 2018 [99] | England and Wales | HD-TIV vs. TIV | Dynamic compartmental transmission model | NR | Cost time horizon = 8 years Effect time horizon = 8 years | Hospitalization Influenza cases GP consultations Death Vaccine price NR | NR, GBP£ | rVE from FIM12 RCT [50] (specific value NR in abstract) | Costs NR Outcomes NR | PSA | In an average season, HD-TIV rather than TIV could prevent: 8500 GP consultations, 800 influenza-related hospitalizations, and 600 deaths HD-TIV economically justifiable prices of £27.00 and £36.80 per dose for ICER thresholds of £20,000/QALY and £30,000/QALY, respectively; higher prices were justifiable when accounting for the vaccine impact on cardiorespiratory events | Vaccination of adults ≥ 65 years of age with HD-TIV in the UK is likely to be a highly cost-effective vs. TIV. This benefit is driven by a reduction in influenza-related hospitalizations | Yes (Sanofi) |
| Jacob J, et al., 2018 [69] | England and Wales | HD-TIV vs. TIV | Age-structured decision tree model | Public healthcare payer | 1 year, with longer time horizon for QALYs | Hospitalization Influenza cases GP consultations Death Vaccine list price | 2017, GBP£ | rVE HD-TIV vs. TIV 24.2% from FIM12 RCT [50] | Costs 0% Outcomes 3.5% | DSA | In an average season, HD-TIV rather than TIV could prevent: 75,000 cases of confirmed influenza, 19,000 influenza-related hospitalizations, and 4000 deaths Using thresholds of £20,000/QALY and £30,000/QALY, HD-TIV was estimated to be cost-effective at £23.75 and £30.70 per dose, respectively | HD-TIV resulted in significant benefits across adults ≥ 65 years of age and has the potential to be cost-effective vs. TIV. Results were most sensitive to the rVE of HD-TIV vs. TIV against hospitalizations | Yes (Sanofi) |
| Largeron N, et al., 2018 [70] | Australia | HD-TIV vs. QIV | Static decision tree model | Payer | Cost time horizon = 1 year Effect time horizon = 1 year | QIV AUS$9 Hospitalizations Medical visits Healthcare costs Deaths | 2018, AUS$ | rVE HD-TIV vs. TIV 24.2% * from FIM12 RCT [50] VE TIV 58.4% VE QIV 59.8% (based on prior CEA [100]) | Costs 5% Outcomes 5% | DSA | In an average season, HD-TIV rather than QIV could prevent: 11,364 confirmed influenza cases, 17,576 cardiorespiratory-related hospitalizations, and 446 influenza-related deaths | HD-TIV vs. QIV in elderly adults ≥ 65 years of age is cost-effective at prices up to AUS$92/dose. HD-TIV becomes cost-saving if the price/dose does not exceed AUS$58 | Yes (Sanofi) |
| Shireman T, et al., 2019 [101] | USA | HD-TIV vs. TIV | Cost–benefit analysis, person-level study | Payer (Medicare) | Cost time horizon = one influenza season Effect time horizon = one influenza season | HD-TIV $31.82 TIV $12.04 (CMS price schedule) Medical visits Hospitalization Home/hospice care Medications Vaccine price NR Skilled nursing facility Outpatient rehab | NR, USD$ | NR | NR | Down-weighting top 1% of outliers | The $20 incremental cost of HD-TIV to TIV offset adjusted expenditures for a net benefit of $526 per nursing home resident and a financial return on investment of 27:1 | HD-TIV reduced hospitalizations and resulted in lower Medicare expenditures. The magnitude of the estimated savings overwhelmed the incremental cost of HD-TIV vs. TIV | Yes (Sanofi) |
| Basile M, et al., 2020 [71] | Italy | HD-QIV vs. QIV | Static decision tree model | Healthcare system | 1 year Deaths: life-year | Influenza cases Hospitalizations GP consultation ED visits Comedications Deaths Ex-factory vaccine price | NR, € Euro | rVE HD-QIV to QIV 24.2% * from FIM12 RCT [50] | Outcomes 3% | DSA | HD-QIV generated an excess 18,052 life years saved and 17,100 QALYs vs. QIV, saving €21.0 million to the healthcare system HD-QIV dominated QIV | HD-QIV could reduce the public health burden of influenza-related complications, and be cost-saving or cost-effective vs. QIV | Yes (Sanofi) |
| Borges M, et al., 2021 [72] | Portugal | HD-QIV vs. QIV | Decision tree model | NR | 1 year | Influenza cases GP visits ER visits Hospitalizations Deaths Vaccine price NR | NR, € Euro | rVE HD-QIV to QIV 24.2% * from FIM12 RCT [50] | NR | DSA | HD-QIV reduced influenza cases by 12% and influenza-related deaths by 12%. HD-QIV reduced GP appointments by 1229 and ER visits by 532. Influenza-related hospitalizations were reduced by 10%. Respiratory hospitalizations were decreased by 14% and cardiorespiratory hospitalizations by 11%. | Switching to HD-QIV would contribute to reaching public health objectives, reducing excess mortality and the consumption of healthcare resources | Yes (Sanofi) |
| de Courville C, et al., 2021 [73] | Belgium | HD-QIV vs. QIV | Static decision tree model | Payer | 1 year Deaths: life-year | QIV €16.46 HD-QIV €43.04 (NIHDI official prices) Influenza cases GP visits ER visits Hospitalizations Deaths | NR, € Euro | rVE HD-QIV to QIV 24.2% * from FIM12 RCT [50] VE QIV: 50% (based on RCT [102]) | Outcomes 1.5% | DSA, PSAF | HD-QIV vs. QIV ICER €1397/QALY. HD-QIV was cost-effective considering a WTP threshold of €35,000/QALY | Key drivers of model outcomes were efficacy against influenza-associated hospitalization for HD-QIV vs. QIV, acquisition costs, the cost of influenza-related hospitalization and hospitalization rates | Yes (Sanofi) |
| Zeevat F, et al., 2023 [103] | Netherlands | HD-QIV vs. QIV | NR | NR | One season | Hospitalizations (all, respiratory, and CV) Complications Vaccine price NR | NR | NR | NR | NR | HD-QIV usage rather than QIV could have averted 220 hospitalizations, avoiding an expenditure of €1,219,779. Expenditure of €841,531 (i.e., 69% of the total costs) is attributable to avoidance of CV hospitalizations. | Switching from QIV to HD-QIV comes with cost savings. Benefits come from avoided CV-related hospital admissions | No |
| Alvarez P, et al., 2023 [74] | Belgium, Finland, Portugal | HD-QIV vs. QIV | Decision tree model | Payer, NHS | 1 year Deaths: life-year | Comedication Influenza cases GP visits ER visits Hospitalization Vaccine price NR | NR | rVE HD-QIV to QIV 24.2% * from FIM12 RCT [50] | Costs 0% Outcomes 1.5 to 4% | DSA, PSA | HD-QIV resulted in improved health outcomes (visits, hospitalizations, and deaths) vs. QIV HD-QIV vs. QIV ICER €1397/QALY Belgium ICER €9581/QALY Finland ICER €15,267/QALY Portugal | HD-QIV would contribute to a significant improvement in the prevention of influenza health outcomes while being cost-effective | Yes (Sanofi) |
3.2. Comparison between Enhanced Vaccines in CEA
| Author Year | Country | Strategy | Model Type | Perspective | Time Horizon | Selected Costs | Year, Currency | rVE * | Discounting | Uncertainty Analysis | Findings | Author Conclusion | Industry Sponsor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) Adjuvanted vaccines vs. high-dose vaccines | |||||||||||||
| Nguyen VH, et al., 2021 [57] | France | aQIV vs. QIVe aQIV vs. HD-QIV | Static decision tree model | Payer | NR | QIV €11.11 aQIV €26.00 HD-QIV €26.00 (origin not specified) Healthcare visit In/outpatient complications Hospitalization Mortality | NR, Euro € | rVE aTIV vs. QIV 13.7% (95% CI 3.1, 24.2) * rVE aTIV vs. HD-TIV 3.2% (−2.5, 8.9) * rVE aTIV vs. TIV 13.9% (4.2, 23.5) * (from meta-analysis [92]) | NR | DSA | Replacing QIVe with aQIV over a 3-year period can prevent: 56,028 influenza cases, 13,449 medical care visits, 30,815 outpatient complications, 3902 inpatient complications, and 745 influenza-associated deaths Budget savings were driven by avoidance of medical care visits costs (€470 K); outpatient complication costs (€788 K) and inpatient complication costs (€23.2 M) | aQIV for the older adult population would be clinically favorable, with a small incremental cost impact (Data for aQIV vs. HD-QIV not presented) | Yes (Seqirus) |
| Kohli MA, et al., 2021 [63] | UK | aQIV vs. HD-QIV | SEIR compartmental transmission model | Societal, National Healthcare Service | 10 seasons | aQIV £11.88 HD-QIV £20.00 (list price) Hospitalization Vaccine Death Medical visits Complications | NR, GBP£ | rVE aQIV vs. HD-QIV 3.2% (−2.5, 8.9) * (from meta-analysis [92]) | Costs 3.5% Outcomes 3.5% | Scenario analyses | For ICER to fall below £20,000/QALY, unit price of HD-QIV should be less than £12.94, £10.44, or £7.67 for rVEs of −2.5%, 3.2%, and 8.9%, respectively aQIV is cost-saving vs. HD-QIV priced at the existing list price of HD-TIV | As the effectiveness of the vaccines was not statistically significantly different, the differences between the vaccines in clinical cases and influenza treatment costs are minimal | Yes (Seqirus) |
| Kohli M, et al., 2022 [58] | Germany | aQIV vs. QIVe aQIV vs. HD-QIV | SEIR compartmental model calibrated to German population | Societal, Statutory Health insurance | 10 seasons from 2010–2019 | QIVe €12.56 aQIV €19.21 HD-QIV €40.55 (reimbursed prices) Hospitalization Death In/outpatient visits Medication Sickness benefit Lost working time | NR, Euro € | aQIV vs. QIVe 13.9% (4.2, 23.5) * aQIV vs. HD-QIV 3.2% (−2.5, 8.9) * (from meta-analysis [92]) VE QIVe 62%, 24% and 79% against A/H1N1, A/H3N2 and B types (assumptions, related to meta-analysis [94] and systematic review [90]) | Costs 3% Outcomes 3% | DSA, PSA | Both enhanced vaccines reduced the number of influenza cases, hospitalizations, and deaths in the German population compared with QIVe aQIV dominated HD-QIV because it was considered marginally more effective in the base case (rVE = 3.2%), and less costly to implement | aQIV may be cost-effective compared with QIVe at current prices. aQIV and HD-QIV had similar clinical effectiveness, but aQIV is less costly than HD-QIV The CE of aQIV was most sensitive to changes in VE and rate of hospitalization due to influenza | Yes (Seqirus) |
| Ruiz-Aragón J, et al., 2022 [64] | Spain | aQIV vs. HD-QIV | Static decision tree model Calibrated to the Spanish population | Societal, direct medical payer | Cost: three seasons Effect: lifetime | aQIV €23.00 HD-QIV €32.00 (list price) Hospitalization Death Medical visits Comedication Productivity loss | NR, Euro € | rVE aTIV vs. HD-TIV 4.0% (−0.05, 8.4) * (from meta-analysis published in own paper [64]) | Costs 3% Outcomes 3% | DSA, PSA | Compared with HD-QIV, aQIV reduced: cases by 5405, primary care visits by 760, ER visits by 171, hospitalizations by 442, and deaths by 26 aQIV dominated HD-QIV, as it is less expensive and more effective from both the societal and direct medical payer perspectives | aQIV is a cost-effective vs. HD-QIV for older Spanish adults Vaccine costs are the most influential parameters in the model, followed by vaccine coverage | Yes (Seqirus) |
| Choi MJ, et al., 2022 [59] | South Korea | aQIV vs. QIV aQIV vs. HD-QIV | Static decision tree Analyzed across three age groups (65–74, 75–84, and ≥85 years of age) | Healthcare system | 1 year | Hospitalization Death Complications Influenza cases Vaccine price | NR | aQIV vs. QIVe 13.9% (4.2, 23.5) * aQIV vs. HD-QIV 3.2% (−2.5, 8.9) * (from meta-analysis [92]) VE QIV 62%, 24%, and 63% vs. A(H1N1), A(H3N2), and B, respectively (from meta-analysis [94]) | NR | DSA, PSA | Compared with QIV, aQIV reduced: cases by 35,390, complications by 1602, hospitalizations by 709, and deaths by 145 Compared with HD-QIV, aQIV reduced: cases by 7247, complications by 328, hospitalizations by 145, and deaths by 30 | Replacing QIV with aQIV is predicted to reduce disease burden in the South Korean ≥ 65 years of age group Benefits of aQIV and HD-QIV are predicted to be similar due to comparable VE rVE was the most important factor influencing CE | Yes (Seqirus) |
| Jacob J, et al., 2023 [62] | Denmark, Norway, Sweden | aQIV vs. HD-QIV | Static decision tree model | Healthcare payer, societal | NR | QIV €9.10–11.00 aQIV 170–189% that of QIV HD-QIV €25 (public sources; assumption) Hospitalization GP visit Outpatient visit Comedication Lost productivity Death Complications Influenza cases | 2022, Euro € | aQIV vs. HD-QIV 3.2% (−2.5, 8.9) * (from meta-analysis [92]) rVE HD-QIV to QIV 24.2% * from FIM12 RCT [50] | 3–4% outcomes and costs | DSA, PSA | Across Denmark, Norway, and Sweden, aQIV vs. QIV could prevent a combined total of 18,772 symptomatic influenza infections, 925 hospitalizations, and 161 deaths in one influenza season across the three countries aQIV cost-saving vs. HD-QIV. As aQIV and HD-QIV were assumed to have comparable VE, the health benefits in favor of aQIV were marginal | Introducing aQIV to those ≥ 65 years of age may reduce the influenza disease and economic burden in Denmark, Norway, and Sweden | Yes (Seqirus) |
| (B) High-dose vaccines vs. adjuvanted vaccines | |||||||||||||
| Skinner L, et al., 2019 [106] | England and Wales | HD-TIV vs. aTIV | Static decision tree model | Public healthcare payer | 1 year | Hospitalization Influenza complications GP consultations Death Vaccine list price | NR, GBP£ | NR | Costs 0% Outcomes 3.5% | NR | HD-TIV vs. aTIV ICER £2154–8757/QALY for influenza/pneumonia hospitalizations analysis HD-TIV vs. aTIV ICER £2800 for respiratory hospitalizations analysis | HD-TIV is cost-effective vs. aTIV, driven by reduction in hospitalizations | Yes (Sanofi) |
| Basile M, et al., 2020 [71] | Italy | HD-QIV vs. aTIV | Decision tree model | Healthcare system | 1 year Deaths: life-year | Influenza cases Hospitalizations GP consultation ED visits Comedications Deaths Ex-factory vaccine price | NR, Euro € | rVE HD-QIV to QIV 24.2% * from FIM12 RCT [50] rVE aTIV vs. TIV: 6.0% influenza cases (from retrospective cohort study of aTIV vs. virosomal-TIV [75]). No rVE sensitivity analysis stated. | Outcomes 3% | DSA | HD-QIV generated an excess 18,173 life years saved and 16,438 QALYs vs. aTIV HD-QIV vs. aTIV ICER €11,138/QALY | Vaccination with HD-QIV in those ≥ 65 years of age could be cost-effective vs. aTIV considering hospitalizations conditional on influenza cases | Yes (Sanofi) |
| Gibbons I, et al., 2020 [107] | England | HD-QIV vs. aTIV | Static decision tree model | Healthcare system | 1 year | Influenza cases GP consultation Hospitalizations Deaths Vaccine price NR | NR, £GBP | NR, rVE HD-QIV vs. aTIV for three distinct analyses rVE from FIM12 RCT* [50] (specific value NR in abstract) | NR | DSA | HD-QIV was cost-neutral vaccination strategy (ICER: £824/QALY) vs. aTIV regarding influenza/pneumonia events in base-case scenario When hospitalizations were considered (broader respiratory and cardiovascular hospitalizations), HD-QIV dominated aTIV | HD-QIV could reduce the annual public health burden of influenza-related complications, while being a highly cost-effective, and in some cases dominant, alternative to aTIV in England Results remained robust across three values tested for the rVE of HD-QIV versus aTIV | Yes (Sanofi) |
| Net P, et al., 2021 [76] | USA | US standard of care with and without HD-TIV | Budget impact, decision tree framework | Medicare perspective | 9 years | Influenza cases ED visits Hospitalizations Comedications Deaths Vaccine price NR | 2019, US$ | rVE HD-TIV vs. TIV 24.2% * from FIM12 RCT [50] rVE aTIV vs. TIV 0% (assumed 0% because no RCT data available). rVE varied to 4.7% aTIV vs. TIV in scenario analysis | 0% costs NR outcomes | DSA, PSA | HD-TIV estimated to potentially avert 1,333,479 influenza cases, 769,476 medical visits, 40,004 ED presentations, 520,342 cardiorespiratory hospitalizations, and 73,689 deaths Generate $4.6 billion in savings over 10 years HD-TIV cost-saving under all the scenarios | HD-TIV provided improved efficacy and economic outcomes. Hospitalizations and rVE of HD-TIV vs. TIV were major cost drivers | Yes (Sanofi) |
| Rumi F, et al., 2021 [77] | Italy | HD-QIV vs. aQIV | Decision tree model | Health system | 1 year | Hospitalizations GP visits ED visits Deaths Vaccine price NR | NR, Euro € | rVE HD-QIV to QIV 24.2% * from FIM12 RCT [50] rVE HD-QIV to QIV 18.2% in preventing CV hospitalization (from meta-analysis [108]) rVE aQIV vs. QIV 0% (assumed 0% because no RCT data available. Varied to 6% and 12% in scenario analysis) | NR | DSA, PSA | HD-QIV vs. aQIV ICER €7301/QALY rVE aQIV vs. QIV 0% ICER €9805/QALY rVE aQIV vs. QIV 6% ICER €14,733/QALY rVE aQIV vs. QIV 12% HD-QIV dominated aQIV, saving the healthcare system more than €53 million while improving clinical results | HD-QIV would be cost-effective when influenza hospitalizations were included, and cost-saving when the full burden of influenza is considered. DSA determined VE and rVE inputs most impactful on CE results | Yes (Sanofi) |
| Redondo E, et al., 2021 [78] | Spain | HD-QIV vs. aTIV | Decision tree model | Payer | 6 months | Influenza cases GP visits ED visits Hospitalizations Deaths Vaccine price NR | NR, Euro € | HD-TIV vs. TIV 24.2% or 24.3% * from FIM12 RCT [50] rVE aTIV vs. TIV 6.0% influenza cases and hospitalizations (from retrospective cohort study of aTIV vs. virosomal-TIV [75]). Varied to 0.0% and 6.0% in sensitivity analysis | QALY 3% | PSA, DSA | Switching from aTIV to HD-QIV would prevent: 6476 cases of influenza, 5143 visits to the GP, 1054 visits to the ED, 9193 episodes of hospitalization due to influenza or pneumonia, and 357 deaths due to influenza HD-QIV vs. aTIV ICER €24,353/QALY | HD-QIV in people > 65 years of age is an influenza-prevention strategy that is at least cost-effective, if not dominant, in Spain. | Yes (Sanofi) |
| Nguyen VH, et al., 2022 [109] | Canada | QIVe vs. 1. QIVe + aTIV 2. QIVe + HD-QIV 3. QIVc + aTIV | SEIR model | Health care system | 8 years | Hospitalization Death Medical visits Comedication Vaccine price NR | NR, Canada$ | rVE QIVc vs. QIVe when egg-adapted against A/H3N2 15.6% (7, 20) rVE HD-QIV or aTIV vs. QIVe when egg-adapted against A/H3N2 9% (7.2, 10) rVE HD-QIV or aTIV vs. QIVe when matched against A and B strains 24% (9.7, 36) (all calculated based on electronic medical records [110]) | 5% | DSA, PSA | Three scenarios were compared vs. baseline scenario of QIVe for all age groups Scenario 1 (QIVe + aTIV for adults ≥ 65 years of age) was cost-saving Scenario 2 (QIVe + HD-QIV for adults ≥ 65 years of age) was above willingness-to-pay threshold at all rVE estimates Scenario 3 (QIVc + aTIV for adults ≥ 65 years of age) was cost-effective across all three rVE estimates, with ICER CA$1300 to CA$6900 | Vaccination of individuals 6 months to 64 years of age with QIVc and ≥65 years of age with aTIV is cost-effective across varying assumptions of rVE and varying egg-adapted influenza seasons | Yes (Seqirus) |
| Mattock R, et al., 2021 [79] | England and Wales | HD-TIV vs. aTIV | Decision tree model | Healthcare payer | Cost: one season Effect: lifetime | aTIV £9.79 HD-TIV £20.00 (list prices) LCI cases that could result in a GP visit Hospital stays that could lead to premature death Vaccine price NR | 2018, GBP£ | rVE HD-TIV 24.2% or 24.3% * from FIM12 RCT [50] rVE aTIV vs. HD-TIV 0% LCI (assumed 0% because no RCT data available; varied to 6% and 12% in scenario analysis) rVE aTIV vs. HD-TIV 0% hospitalization (estimated at 0% because no RCT data available; varied to 10% and 20% in scenario analysis) | Costs 0% Outcomes 3.5% | DSA | HD-TIV cost-effective vs. aTIV for all three hospitalization effectiveness scenarios, with ICER equal to £1932, £4181, and £8767 per QALY | HD-TIV is cost-effective vs. aTIV in people ≥ 65 years of age in England and Wales DSA identified the rVE of HD-TIV on hospitalization outcomes as an important area of uncertainty | Yes (Sanofi) |
| Drago G, et al., 2020 [80] | Spain | HD-QIV vs. aTIV | Decision tree model | Healthcare system | Cost: 1 year Effect: lifetime | Influenza cases Hospitalizations GP consultation ED visits Deaths Vaccine price NR | NR, Euro € | rVE HD-TIV 24.2 * from FIM12 RCT [50] rVE aTIV vs. TIV 6.0% influenza cases (from retrospective cohort study of aTIV vs. virosomal-TIV [75]). Varied to 0% and 6% in sensitivity analysis | Outcomes 3% | DSA | Compared with aTIV, HD-QIV generated an excess 3514 life-years and 3304 QALYs, resulting in an ICER of €23,872/QALY | HD-QIV could annually reduce the public health burden of influenza-related complications and be cost-effective in influenza vs. aTIV VE against influenza cases and rVE against influenza and pneumonia hospitalizations were the most impactful parameters in DSA | Yes (Sanofi) |
| van Aalst R, et al. 2021 [81] | USA | HD-TIV vs. aTIV | PERR method | Healthcare payer | NR | HD-TIV $46.23 aTIV $48.26 (average list price) Hospitalization Vaccine price NR | NR, USD$ | rVE HD-TIV vs. aTIV 7% (2.3, 12) respiratory or CV hospitalization; 12% (3.3, 20) respiratory hospitalization (from retrospective cohort study [111]) | Costs NR Outcomes NR | PERR | Hospitalization rates for respiratory disease in HD-TIV and aTIV recipients were 187 and 212 per 10,000 persons-years, respectively. Estimated net savings of HD-TIV were $34 ($10–$62) per recipient | HD-TIV was associated with lower hospitalization costs vs. aTIV. HD-TIV remained cost-saving in all sensitivity analyses performed for hospitalizations with underlying cardiorespiratory disease | Yes (Sanofi) |
| (C) Recombinant vaccine versus other enhanced vaccines | |||||||||||||
| Drago Manchón G, et al., 2021 [104] | Spain | Switching from QIV/aQIV to QIVr | Decision tree model | Spanish National Healthcare System | 1 year | Influenza cases GP visits ER visits Hospitalizations Deaths Vaccine price NR | NR | VE QIV 50% influenza cases (based on RCT [102]) VE QIV 40% influenza hospitalizations (from meta-analysis [112]) rVE QIVr vs. QIV 30% (from RCT [113]) rVE aQIV vs. QIV 6% (from retrospective cohort study of aTIV vs. virosomal-TIV [75]) | NR | NR | Mortality, hospitalizations, GP visits, and ER services would decrease by 12%, 13%, 11%, and 12%, respectively, should the switch from QIV (and from aQIV for those ≥ 65 years of age) to QIVr be implemented | Costs, currency year, discounting, and uncertainty analyses could not be assessed | NR |
| Ruiz-Aragón J & Márquez-Peláez S 2023 [105] | Spain | QIVr vs. aQIV | Static, decision tree model | Public payer, societal | 1 year | aQIV €13 QIVr €25 (list prices) Influenza cases Hospitalizations GP consultation ED visits Deaths | 2021, Euro € | rVE QIVr vs. aTIV 10.7% (2.7, 17.9) inpatient stays (from observational study [114]) | Costs 3% Outcomes 3% | PSA, DSA | QIVr vs. aQIV ICER €101,612.41/QALY To be cost-effective, rVE of QIVr vs. aQIV would need to be 34.1% | QIVr is not cost-effective vs. aQIV for older persons living in Spain | Yes (Seqirus) |
3.3. Systematic Reviews of CEA
4. Critical Assessment of CEA Inputs and Approaches
4.1. Effectiveness Input
4.1.1. Importance of RWE for Influenza
4.1.2. Importance of RWE Meta-Analysis
4.1.3. Limitations of Currently Available Influenza RCT Evidence
4.2. Vaccine Acquisition Price
4.3. Sensitivity/Scenario Analyses
4.4. Interpretation of ICERs
5. Future Directions and Conclusions
5.1. Future Directions
5.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Putri, W.; Muscatello, D.J.; Stockwell, M.S.; Newall, A.T. Economic burden of seasonal influenza in the United States. Vaccine 2018, 36, 3960–3966. [Google Scholar] [CrossRef]
- Near, A.M.; Tse, J.; Young-Xu, Y.; Hong, D.K.; Reyes, C.M. Burden of influenza hospitalization among high-risk groups in the United States. BMC Health Serv. Res. 2022, 22, 1209. [Google Scholar] [CrossRef]
- Calabro, G.E.; D’Ambrosio, F.; Fallani, E.; Ricciardi, W. Influenza Vaccination Assessment according to a Value-Based Health Care Approach. Vaccines 2022, 10, 1675. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Blanton, L.H.; Ferdinands, J.M.; Chung, J.R.; Broder, K.R.; Talbot, H.K.; Morgan, R.L.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022–2023 Influenza Season. MMWR Recomm. Rep. 2022, 71, 1–28. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. S4), D49–D53. [Google Scholar] [CrossRef]
- Petrova, V.N.; Russell, C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 60. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Recommendations Announced for Influenza Vaccine Composition for the 2022–2023 Northern Hemisphere Influenza Season. Available online: https://www.who.int/news/item/25-02-2022-recommendations-announced-for-influenza-vaccine-composition-for-the-2022-2023-northern-hemisphere-influenza-season (accessed on 13 February 2023).
- Tenforde, M.W.; Kondor, R.J.G.; Chung, J.R.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Effect of Antigenic Drift on Influenza Vaccine Effectiveness in the United States-2019–2020. Clin. Infect. Dis. 2021, 73, e4244–e4250. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Haq, K.; McElhaney, J.E. Immunosenescence: Influenza vaccination and the elderly. Curr. Opin. Immunol. 2014, 29, 38–42. [Google Scholar] [CrossRef]
- Gartner, B.C.; Weinke, T.; Wahle, K.; Kwetkat, A.; Beier, D.; Schmidt, K.J.; Schwarz, T.F. Importance and value of adjuvanted influenza vaccine in the care of older adults from a European perspective—A systematic review of recently published literature on real-world data. Vaccine 2022, 40, 2999–3008. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Calabro, S.; Tortoli, M.; Baudner, B.C.; Pacitto, A.; Cortese, M.; O’Hagan, D.T.; De Gregorio, E.; Seubert, A.; Wack, A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011, 29, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Verma, N.; Yewdell, J.W.; Hilbert, A.K.; Castellino, F.; Lattanzi, M.; Del Giudice, G.; Rappuoli, R.; Golding, H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 2011, 3, 85ra48. [Google Scholar] [CrossRef] [PubMed]
- Seqirus. FLUAD TETRA Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/fluad-tetra-epar-product-information_en.pdf (accessed on 12 April 2021).
- Arunachalam, A.B.; Post, P.; Rudin, D. Unique features of a recombinant haemagglutinin influenza vaccine that influence vaccine performance. NPJ Vaccines 2021, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- European Observatory on Health Systems and Policies. The Organization and Delivery of Vaccination Services in the European Union. Available online: https://health.ec.europa.eu/system/files/2018-11/2018_vaccine_services_en_0.pdf (accessed on 13 February 2023).
- National Center for Immunization & Respiratory Diseases (US) Influenza Division; Advisory Committee on Immunization Practices. Influenza Vaccines for Older Adults: GRADE Summary. Available online: https://stacks.cdc.gov/view/cdc/114834 (accessed on 13 October 2022).
- Joint Committee on Vaccination and Immunisation. Advice on Influenza Vaccines for 2021/22. Available online: https://app.box.com/s/t5ockz9bb6xw6t2mrrzb144njplimfo0/file/737845224649 (accessed on 31 January 2022).
- Australia Government Department of Health and Aged Care. 2022 Seasonal Influenza Vaccines. Available online: https://www.tga.gov.au/news/media-releases/2022-seasonal-influenza-vaccines (accessed on 6 November 2022).
- Advisory Committee on Immunization Practices. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2022–2023. Available online: https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm (accessed on 13 February 2023).
- Ministerio de Salud Argentina. II Reunión Ordinaria de la Comisión Nacional de Inmunizaciones, 22 de Octubre de 2020. Available online: https://www.argentina.gob.ar/sites/default/files/2020-10-22_acta-conain.pdf (accessed on 4 May 2023).
- Ultsch, B.; Damm, O.; Beutels, P.; Bilcke, J.; Brüggenjürgen, B.; Gerber-Grote, A.; Greiner, W.; Hanquet, G.; Hutubessy, R.; Jit, M.; et al. Methods for Health Economic Evaluation of Vaccines and Immunization Decision Frameworks: A Consensus Framework from a European Vaccine Economics Community. Pharmacoeconomics 2016, 34, 227–244. [Google Scholar] [CrossRef]
- Hall, A.J. The United Kingdom Joint Committee on Vaccination and Immunisation. Vaccine 2010, 28 (Suppl. S1), A54–A57. [Google Scholar] [CrossRef]
- Jones, D.S.; Podolsky, S.H. The history and fate of the gold standard. Lancet 2015, 385, 1502–1503. [Google Scholar] [CrossRef]
- Schad, F.; Thronicke, A. Real-World Evidence-Current Developments and Perspectives. Int. J. Environ. Res. Public Health 2022, 19, 159. [Google Scholar] [CrossRef]
- Frieden, T.R. Evidence for health decision making—Beyond randomized, controlled trials. N. Engl. J. Med. 2017, 377, 465–475. [Google Scholar] [CrossRef]
- Katkade, V.B.; Sanders, K.N.; Zou, K.H. Real world data: An opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J. Multidiscip. Healthc. 2018, 11, 295–304. [Google Scholar] [CrossRef]
- Burns, L.; Roux, N.L.; Kalesnik-Orszulak, R.; Christian, J.; Hukkelhoven, M.; Rockhold, F.; O’Donnell, J. Real-world evidence for regulatory decision-making: Guidance from around the world. Clin. Ther. 2022, 44, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Bowrin, K.; Briere, J.B.; Levy, P.; Millier, A.; Clay, E.; Toumi, M. Cost-effectiveness analyses using real-world data: An overview of the literature. J. Med. Econ. 2019, 22, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Loong, D.; Pham, B.; Amiri, M.; Saunders, H.; Mishra, S.; Radhakrishnan, A.; Rodrigues, M.; Yeung, M.W.; Muller, M.P.; Straus, S.E.; et al. Systematic Review on the Cost-Effectiveness of Seasonal Influenza Vaccines in Older Adults. Value Health 2022, 25, 1439–1458. [Google Scholar] [CrossRef]
- Shields, G.E.; Elvidge, J.; Davies, L.M. A systematic review of economic evaluations of seasonal influenza vaccination for the elderly population in the European Union. BMJ Open 2017, 7, e014847. [Google Scholar] [CrossRef] [PubMed]
- Colrat, F.; Thommes, E.; Largeron, N.; Alvarez, F.P. Economic evaluation of high-dose inactivated influenza vaccine in adults aged >/=65 years: A systematic literature review. Vaccine 2021, 39 (Suppl. S1), A42–A50. [Google Scholar] [CrossRef]
- Loperto, I.; Simonetti, A.; Nardone, A.; Triassi, M. Use of adjuvanted trivalent influenza vaccine in older-age adults: A systematic review of economic evidence. Hum. Vaccin. Immunother. 2019, 15, 1035–1047. [Google Scholar] [CrossRef]
- Walker, D.G.; Wilson, R.F.; Sharma, R.; Bridges, J.; Niessen, L.; Bass, E.B.; Frick, K. AHRQ Methods for Effective Health Care. In Best Practices for Conducting Economic Evaluations in Health Care: A Systematic Review of Quality Assessment Tools; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2012. [Google Scholar]
- Bragazzi, N.L.; Orsi, A.; Ansaldi, F.; Gasparini, R.; Icardi, G. Fluzone® intra-dermal (Intanza®/Istivac® Intra-dermal): An updated overview. Hum. Vaccin. Immunother. 2016, 12, 2616–2627. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Canada Communicable Disease Report. Available online: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/publicat/ccdr-rmtc/11vol37/acs-dcc-6/assets/pdf/acs-dcc-6-eng.pdf (accessed on 27 March 2023).
- Sanofi Pasteur. FLUZONE® High-Dose. Influenza Virus Vaccine Trivalent Types A and B (Split Virion). Product Monograph. Available online: https://products.sanofi.ca/en/fluzone-hd.pdf (accessed on 8 May 2023).
- Sanofi Pasteur. SupemtekTM Quadrivalent Recombinant Influenza Vaccine. Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00059645.PDF (accessed on 8 May 2023).
- Food and Drug Administration. FLUAD® Seqirus Inc US Package Insert. Available online: https://www.fda.gov/media/94583/download (accessed on 1 June 2021).
- Seqirus. FLUAD QUADRIVALENT (Influenza Vaccine, Adjuvanted). Available online: https://www.fda.gov/media/135432/download (accessed on 2 June 2021).
- Sanofi Pasteur. Fluzone® High-Dose Quadrivalent. Available online: www.fda.gov/media/132238/download (accessed on 19 August 2021).
- Protein Sciences Corporation. Flublok Quadrivalent (Influenza Vaccine, Recombinant). Available online: https://www.fda.gov/media/123144/download (accessed on 23 March 2023).
- NHS England. Update on Use of Adjuvanted Trivalent Flu Vaccine for 2018–19 Flu Season. Available online: https://www.england.nhs.uk/wp-content/uploads/2017/12/2018-19-aTIV-vaccine.pdf (accessed on 8 May 2023).
- UK Health Security Agency. The National Influenza Immunisation Programme 2022 to 2023: Information for Healthcare Practitioners. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1105068/Flu-information-for-HCPs-2022-to-2023-20Sept22.pdf (accessed on 8 May 2023).
- UK Medicines and Healthcare products Regulatory Agency. Innovation: MHRA’s Speedy Approval of High Dose Flu Vaccine Demonstrates Flexibilities in National Applications. Available online: https://www.gov.uk/government/case-studies/innovation-mhras-speedy-approval-of-high-dose-flu-vaccine-demonstrates-flexibilities-in-national-applications (accessed on 8 May 2023).
- Sanofi Pasteur. Press Release: Sanofi to Build New Facility in Canada to Increase Global Availability of High-Dose Influenza Vaccine. Available online: https://ml-eu.globenewswire.com/Resource/Download/22550b60-1c00-4977-a2e7-7e43f2e59050 (accessed on 8 May 2023).
- Seqirus. Fluad, Suspension for Injection in Pre-Filled Syringe. Available online: https://www.medicines.org.uk/emc/product/9223/smpc (accessed on 2 June 2021).
- Sanofi Pasteur. Supemtek Quadrivalent Influenza Vaccine (Recombinant, Prepared in Cell Culture). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/supemtek (accessed on 23 March 2023).
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Mullikin, M.; Tan, L.; Jansen, J.P.; Van Ranst, M.; Farkas, N.; Petri, E. A Novel Dynamic Model for Health Economic Analysis of Influenza Vaccination in the Elderly. Infect. Dis. Ther. 2015, 4, 459–487. [Google Scholar] [CrossRef]
- Barbieri, M.; Capri, S. Analisi Di Costo-Efficacia Della Vaccinazione Antinfluenzale Nella Popolazione Anziana In Italia: Confronto Tra Vaccino Inattivato Trivalente Adiuvato Con Mf59® E Altri Tipi Di Vaccini. QIJPH 2017, 6, 69–82. [Google Scholar]
- Capri, S.; Barbieri, M.; de Waure, C.; Boccalini, S.; Panatto, D. Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly Italian population. Hum. Vaccin. Immunother. 2018, 14, 1331–1341. [Google Scholar] [CrossRef]
- Yun, J.W.; Choi, M.J.; Shin, G.S.; Lim, J.O.; Noh, J.Y.; Kim, Y.K.; Song, J.Y.; Kim, W.J.; Choi, S.E.; Cheong, H.J. Cost-effectiveness of influenza vaccine strategies for the elderly in South Korea. PLoS ONE 2019, 14, e0209643. [Google Scholar] [CrossRef]
- Thorrington, D.; van Leeuwen, E.; Ramsay, M.; Pebody, R.; Baguelin, M. Assessing optimal use of the standard dose adjuvanted trivalent seasonal influenza vaccine in the elderly. Vaccine 2019, 37, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Vizzotti, C.; Uruena, A.; Giglio, N.; Magneres, C.; Richmond, H. Cost-effectiveness of introducing an MF59-adjuvanted trivalent influenza vaccine for older adults in Argentina. Vaccine 2020, 38, 3682–3689. [Google Scholar] [CrossRef]
- Nguyen, V.H.; D’Agostino, P.; Phalippon, N.; McCracken, A.; Mould-Quevedo, J. Budget Impact Analysis of the MF59-Adjuvanted Quadrivalent Influenza Vaccine in the Older Adult French Population. In Proceedings of the 8th European Scientific Working Group on Influenza (ESWI), Online, 4–7 December 2021. [Google Scholar]
- Kohli, M.A.; Maschio, M.; Cartier, S.; Mould-Quevedo, J.; Fricke, F.U. The cost-effectiveness of vaccination of older adults with an MF59-adjuvanted quadrivalent influenza vaccine compared to other available quadrivalent vaccines in Germany. Vaccines 2022, 10, 1386. [Google Scholar] [CrossRef]
- Choi, M.J.; Yun, J.W.; Song, J.Y.; Ko, K.; Mould, J.F.; Cheong, H.J. A Comparative Analysis of Influenza-Associated Disease Burden with Different Influenza Vaccination Strategies for the Elderly Population in South Korea. Vaccines 2022, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Calabro, G.E.; Boccalini, S.; Panatto, D.; Rizzo, C.; Di Pietro, M.L.; Abreha, F.M.; Ajelli, M.; Amicizia, D.; Bechini, A.; Giacchetta, I.; et al. The New Quadrivalent Adjuvanted Influenza Vaccine for the Italian Elderly: A Health Technology Assessment. Int. J. Environ. Res. Public Health 2022, 19, 4166. [Google Scholar] [CrossRef] [PubMed]
- Fochesato, A.; Sottile, S.; Pugliese, A.; Marquez-Pelaez, S.; Toro-Diaz, H.; Gani, R.; Alvarez, P.; Ruiz-Aragon, J. An Economic Evaluation of the Adjuvanted Quadrivalent Influenza Vaccine Compared with Standard-Dose Quadrivalent Influenza Vaccine in the Spanish Older Adult Population. Vaccines 2022, 10, 1360. [Google Scholar] [CrossRef]
- Jacob, J.; Biering-Sørensen, T.; Holger Ehlers, L.; Edwards, C.H.; Mohn, K.G.-I.; Nilsson, A.; Hjelmgren, J.; Ma, W.; Sharma, Y.; Ciglia, E.; et al. Cost-Effectiveness of Vaccination of Older Adults with an MF59®-Adjuvanted Quadrivalent Influenza Vaccine Compared to Standard-Dose and High-Dose Vaccines in Denmark, Norway, and Sweden. Vaccines 2023, 11, 753. [Google Scholar] [CrossRef]
- Kohli, M.A.; Maschio, M.; Mould-Quevedo, J.F.; Drummond, M.; Weinstein, M.C. The cost-effectiveness of an adjuvanted quadrivalent influenza vaccine in the United Kingdom. Hum. Vaccin. Immunother. 2021, 17, 4603–4610. [Google Scholar] [CrossRef]
- Ruiz-Aragon, J.; Marquez-Pelaez, S.; Gani, R.; Alvarez, P.; Guerrero-Luduena, R. Cost-Effectiveness and Burden of Disease for Adjuvanted Quadrivalent Influenza Vaccines Compared to High-Dose Quadrivalent Influenza Vaccines in Elderly Patients in Spain. Vaccines 2022, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Chit, A.; Becker, D.L.; DiazGranados, C.A.; Maschio, M.; Yau, E.; Drummond, M. Cost-effectiveness of high-dose versus standard-dose inactivated influenza vaccine in adults aged 65 years and older: An economic evaluation of data from a randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1459–1466. [Google Scholar] [CrossRef]
- Chit, A.; Roiz, J.; Briquet, B.; Greenberg, D.P. Expected cost effectiveness of high-dose trivalent influenza vaccine in US seniors. Vaccine 2015, 33, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.L.; Chit, A.; DiazGranados, C.A.; Maschio, M.; Yau, E.; Drummond, M. High-dose inactivated influenza vaccine is associated with cost savings and better outcomes compared to standard-dose inactivated influenza vaccine in Canadian seniors. Hum. Vaccin. Immunother. 2016, 12, 3036–3042. [Google Scholar] [CrossRef]
- Raviotta, J.M.; Smith, K.J.; DePasse, J.; Brown, S.T.; Shim, E.; Nowalk, M.P.; Zimmerman, R.K. Cost-Effectiveness and Public Health Effect of Influenza Vaccine Strategies for U.S. Elderly Adults. J. Am. Geriatr. Soc. 2016, 64, 2126–2131. [Google Scholar] [CrossRef]
- Skinner, L.; Jacob, J.; Bianic, F.; Carroll, S.; Bricout, H.; Largeron, N.; Alvarez, F.P.; Clark-Wright, J. PIN30—An Economic Model to Estimate the Cost-Effectiveness of Trivalent Influenza Vaccine High Dose for the Elderly Population in England And Wales. Value Health 2018, 21, S225. [Google Scholar] [CrossRef]
- Largeron, N.; Manton, A.; Net, P.; Choi, S.; Becker, D.L.; Bianic, F.; Jacob, J.; Maschio, M. PIN22—An Economic Model To Estimate The Public Health Impact and Cost-Effectiveness of Vaccination of Seniors with Fluzone High Dose Influenza Vaccine in Australia. Value Health 2018, 21, S65. [Google Scholar] [CrossRef]
- Basile, M.; Rumi, F.; Cicchetti, A.; Nascimento Costa, M.; Bianic, F.; Noelle, H.; Alvarez, F.; Muzii, B. Cost-Effectiveness of Quadrivalent Influenza Vaccine High Dose Versus Standard Dose Quadrivalent Influenza Vaccine in Italy. In Proceedings of the 7th European Scientific Working Group on Influenza (ESWI), Virtual, 6–9 December 2021. [Google Scholar]
- Borges, M.; Lopes, R.; Bricout, H.; Martins, M.; de Courville, C.; Miguel, L.S. Public Health Benefit of Switching to High Dose Quadrivalent Vaccine for Influenza Seasonal Vaccination in Portuguese Elderly Population. In Proceedings of the Professional Society for Health Economics and Outcomes Research (ISPOR), Online, 17–20 May 2020. [Google Scholar]
- De Courville, C.; Chevalier, P.; Borms, M.; Bricout, H.; Petit, C.; Alvarez, F. Costs-Effectiveness of Influenza Vaccination with a High Dose Quadrivalent Vaccine of the Belgian Elderly Population. In Proceedings of the 8th European Scientific Working Group on Influenza (ESWI), Online, 4–7 December 2021. [Google Scholar]
- Alvarez, F.P.; Chevalier, P.; Borms, M.; Bricout, H.; Marques, C.; Soininen, A.; Sainio, T.; Petit, C.; de Courville, C. Cost-effectiveness of influenza vaccination with a high dose quadrivalent vaccine of the elderly population in Belgium, Finland, and Portugal. J. Med. Econ. 2023, 26, 710–719. [Google Scholar] [CrossRef]
- Puig-Barberà, J.; Natividad-Sancho, A.; Calabuig-Pérez, J.; Lluch-Rodrigo, J.A.; Pastor-Villalba, E.; Martínez-Úbeda, S.; Pérez-Vilar, S.; Díez-Domingo, J. MF59-adjuvanted and virosomal influenza vaccines for preventing influenza hospitalization in older people: Comparative effectiveness using the Valencia health care information system. Vaccine 2013, 31, 3995–4002. [Google Scholar] [CrossRef]
- Net, P.; Colrat, F.; Nascimento Costa, M.; Bianic, F.; Thommes, E.; Alvarez, F.P. Estimating public health and economic benefits along 10 years of Fluzone® High Dose in the United States. Vaccine 2021, 39 (Suppl. S1), A56–A69. [Google Scholar] [CrossRef]
- Rumi, F.; Basile, M.; Cicchetti, A.; Alvarez, F.P.; Muzii, B.; Azzi, M.V. Cost Effectiveness of High Dose Quadrivalent Influenza Vaccine (HD-QIV) Versus Adjuvanted Quadrivalent Influenza Vaccine (aQIV) in the Italian Elderly Population. In Proceedings of the 8th European Scientific Working Group on Influenza (ESWI), Online, 4–7 December 2021. [Google Scholar]
- Redondo, E.; Drago, G.; Lopez-Belmonte, J.L.; Guillen, J.M.; Bricout, H.; Alvarez, F.P.; Callejo, D.; Gil de Miguel, A. Cost-utility analysis of influenza vaccination in a population aged 65 years or older in Spain with a high-dose vaccine versus an adjuvanted vaccine. Vaccine 2021, 39, 5138–5145. [Google Scholar] [CrossRef]
- Mattock, R.; Gibbons, I.; Moss, J.; Mealing, S.; Largeron, N.; Carroll, S.; Alvarez, F.P. Cost-effectiveness of high dose versus adjuvanted trivalent influenza vaccines in England and Wales. J. Med. Econ. 2021, 24, 1261–1271. [Google Scholar] [CrossRef]
- Drago, G.R.E.; Gil de Miguel, A.; Alvarex, F.; Costa, M.; Bianic, F.; Noelle, H.; Velasco, D.; Lopez-Belmonte, J.L. Cost-Effectiveness of Quadrivalent Influenza Vaccine High Dose Versus Adjuvanted Trivalent Influenza Vaccine in Spain. In Proceedings of the ESWI Conference, Online, 6–9 December 2020. [Google Scholar]
- van Aalst, R.; Gravenstein, S.; Mor, V.; Mahmud, S.M.; Wilschut, J.; Postma, M.; Chit, A. Economic Assessment of High-Dose Versus Adjuvanted Influenza Vaccine: An Evaluation of Hospitalization Costs Based on a Cohort Study. Vaccines 2021, 9, 1065. [Google Scholar] [CrossRef]
- Lee, B.Y.; Ercius, A.K.; Smith, K.J. A predictive model of the economic effects of an influenza vaccine adjuvant for the older adult (age 65 and over) population. Vaccine 2009, 27, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.N.; Tuite, A.R. Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada. PLoS ONE 2011, 6, e27420. [Google Scholar] [CrossRef]
- Jefferson, T.; Di Pietrantonj, C.; Al-Ansary, L.A.; Ferroni, E.; Thorning, S.; Thomas, R.E. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. 2010, CD004876. [Google Scholar] [CrossRef] [PubMed]
- Mannino, S.; Villa, M.; Apolone, G.; Weiss, N.S.; Groth, N.; Aquino, I.; Boldori, L.; Caramaschi, F.; Gattinoni, A.; Malchiodi, G.; et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am. J. Epidemiol. 2012, 176, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aragón, J.; Grande Tejada, A.M.; Márquez-Peláez, S.; García-Cenoz, M. Estimación del impacto de la vacunación antigripal con adyuvante MF59 en población mayor de 64 años para el Sistema Nacional de Salud: Efectos y costes. Vacunas 2015, 16, 6–11. [Google Scholar] [CrossRef]
- Coudeville, L.; Andre, P.; Bailleux, F.; Weber, F.; Plotkin, S. A new approach to estimate vaccine efficacy based on immunogenicity data applied to influenza vaccines administered by the intradermal or intramuscular routes. Hum. Vaccin. 2010, 6, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rubio, A.; Eiros, J.M. Economic and Health impact of influenza vaccination with adjuvant MF59 in population over 64 years in Spain. Rev. Esp. Quimioter. 2018, 31, 43–52. [Google Scholar]
- Jefferson, T.; Di Pietrantonj, C.; Rivetti, A.; Bawazeer, G.A.; Al-Ansary, L.A.; Ferroni, E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2010, CD001269. [Google Scholar] [CrossRef]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef]
- Van Buynder, P.G.; Konrad, S.; Van Buynder, J.L.; Brodkin, E.; Krajden, M.; Ramler, G.; Bigham, M. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013, 31, 6122–6128. [Google Scholar] [CrossRef]
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Angerami, R.; Pires, B.; Mould-Quevedo, J.F.; Magneres, C.; Kfouri, R. Cost-Effectiveness of Introducing an Mf59-Adjuvanted Trivalent Influenza Vaccine for Older Adults in Brazil. In Proceedings of the 15th Vaccine Congress, Online, 19 April 2021. [Google Scholar]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Boccalini, S.; Pariani, E.; Calabrò, G.E.; De Waure, C.; Panatto, D.; Amicizia, D.; Lai, P.L.; Rizzo, C.; Amodio, E.; Vitale, F.; et al. Health Technology Assessment (HTA) of the introduction of influenza vaccination for Italian children with Fluenz Tetra®. J. Prev. Med. Hyg. 2021, 62, E1–E118. [Google Scholar] [CrossRef]
- Beyer, W.E.; McElhaney, J.; Smith, D.J.; Monto, A.S.; Nguyen-Van-Tam, J.S.; Osterhaus, A.D. Cochrane re-arranged: Support for policies to vaccinate elderly people against influenza. Vaccine 2013, 31, 6030–6033. [Google Scholar] [CrossRef]
- Cheng, X.; Roïz, J. PIN34—Cost-Utility analysis of three types of Influenza Vaccines (Trivalent, Trivalent High dose and quadrivalent) in adults aged 65 and Older Under Universal Influenza Immunization Program (UIIP) In Ontario, Canada. Value Health 2015, 18, A233–A234. [Google Scholar] [CrossRef]
- Molinari, N.A.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef]
- Crépey, P.; Skinner, L.; Carroll, S.; Bricout, H.; Jacob, J.; Largeron, N.; Alvarez, F.P.; Clark-Wright, J. PIN33—A Dynamic Transmission Model to Estimate the Public Health Impact and Cost-Effectiveness of Trivalent Influenza Vaccine High Dose for the Elderly Population in England and Wales. Value Health 2018, 21, S226. [Google Scholar] [CrossRef]
- Clements, K.M.; Meier, G.; McGarry, L.J.; Pruttivarasin, N.; Misurski, D.A. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum. Vaccin. Immunother. 2014, 10, 1171–1180. [Google Scholar] [CrossRef]
- Shireman, T.I.; Ogarek, J.; Gozalo, P.; Zhang, T.; Mor, V.; Davidson, H.E.; Han, L.; Taljaard, M.; Gravenstein, S. Cost Benefit of High-Dose vs. Standard-Dose Influenza Vaccine in a Long-Term Care Population During an A/H1N1-Predominant Influenza Season. J. Am. Med. Dir. Assoc. 2019, 20, 874–878. [Google Scholar] [CrossRef]
- Govaert, T.M.; Thijs, C.T.; Masurel, N.; Sprenger, M.J.; Dinant, G.J.; Knottnerus, J.A. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA 1994, 272, 1661–1665. [Google Scholar] [CrossRef]
- Zeevat, F.; Wilschut, J.C.; Boersma, C.; Postma, M.J. Reducing Hospital Capacity Needs for Seasonal Respiratory Infections: The case of switching to High-Dose influenza vaccine for Dutch older adults. Value Health 2023, 26, 461–464. [Google Scholar] [CrossRef]
- Drago Manchón, G.; López-Belmonte, J.L.; Bricout, H.; de Courville, C. Public Health Benefits of Switching into a Recombinant Quadrivalent Vaccine in the Spanish Murcia and Valencia Regions the Recommended Adult Population (18+) for Influenza Seasonal Vaccination. Proceedings of 8th European Scientific Working Group on Influenza (ESWI), Online, 4–7 December 2021. [Google Scholar]
- Ruiz-Aragón, J.; Márquez-Peláez, S. An Economic Comparison in the Elderly of Adjuvanted Quadrivalent Influenza Vaccine with Recombinant Quadrivalent Influenza Vaccine in Spain. Vaccines 2023, 11, 427. [Google Scholar] [CrossRef]
- Skinner, L.; Chit, A.; Bianic, F.; Largeron, N.; Alvarez, F.P.; Carroll, S. PIN20 Expected Cost-Effectiveness of High Dose Versus Adjuvanted Standard Dose Trivalent Influenza Vaccines in England and Wales: Assessments Using Direct and Indirect Comparative Effectiveness Data. Value Health 2019, 22, S643. [Google Scholar] [CrossRef]
- Gibbons, I.; Davidson, C.; Clark-Wright, J.; Miller, C.; Carroll, S.; Costa, M.; Bricout, H.; Alvarez, F.P. PIN60 Cost-Effectiveness of Quadrivalent Influenza Vaccine High Dose Versus Adjuvanted Standard Dose Trivalent Influenza Vaccine in England. Value Health 2020, 23, S555. [Google Scholar] [CrossRef]
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Kim, J.; Krishnan, A.; Greenberg, D.P.; Chit, A. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: A systematic review and meta-analysis. Expert Rev. Vaccines 2018, 17, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Roy, B. Modelling the Economic Impact of lnfluenza Vaccine Programs with the Cell-Based Quadrivalent Influenza Vaccine and Adjuvanted Trivalent Influenza Vaccine in Canada. Vaccines 2022, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Hilsky, Y.; Mould-Quevedo, J. The Epidemiological and Economic Impact of a Cell-Based Quadrivalent Influenza Vaccine in Adults in the US: A Dynamic Modeling Approach. Vaccines 2021, 9, 1095. [Google Scholar] [CrossRef]
- Van Aalst, R.; Gravenstein, S.; Mor, V.; Mahmud, S.M.; Wilschut, J.; Postma, M.; Chit, A. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: A retrospective cohort study. Vaccine 2020, 38, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Rondy, M.; El Omeiri, N.; Thompson, M.G.; Levêque, A.; Moren, A.; Sullivan, S.G. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. J. Infect. 2017, 75, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M.J. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Lu, M.; Kelman, J.; Lu, Y.; Lindaas, A.; Loc, J.; Pratt, D.; Wei, Y.; Chillarige, Y.; Wernecke, M.; et al. Comparative Effectiveness of Influenza Vaccines Among US Medicare Beneficiaries Ages 65 Years and Older during the 2019–2020 Season. Clin. Infect. Dis. 2021, 73, e4251–e4259. [Google Scholar] [CrossRef]
- Jayawardana, S.; Mossialos, E. How should economic evaluation be used to measure value and set priorities in health care? AMA J. Ethics 2021, 23, E613–E618. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: A Report of the ISPOR CHEERS II Good Practices Task Force. Value Health 2022, 25, 10–31. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Evaluation of Influenza Vaccine Effectiveness: A Guide to the Design and Interpretation of Observational Studies. Available online: https://apps.who.int/iris/bitstream/handle/10665/255203/9789241512121-eng.pdf (accessed on 13 February 2023).
- World Health Organization (WHO). Guidance on the Economic Evaluation of Influenza Vaccination. Available online: https://apps.who.int/iris/bitstream/handle/10665/250086/WHO-IVB-16.05-eng.pdf (accessed on 6 March 2023).
- Mori, M.; Oura, A.; Ohnishi, H.; Washio, M. Confounding in evaluating the effectiveness of influenza vaccine. Vaccine 2008, 26, 6459–6461. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Fisman, D.; Cane, D.J.; Oliver, M.; Macintyre, C.R. Adapt or die: How the pandemic made the shift from EBM to EBM+ more urgent. BMJ Evid. Based Med. 2022, 27, 253–260. [Google Scholar] [CrossRef]
- Domnich, A.; Panatto, D.; Pariani, E.; Napoli, C.; Chironna, M.; Manini, I.; Rizzo, C.; Orsi, A.; Icardi, G.; IT-BIVE-HOSP Network Study Group. Relative effectiveness of the adjuvanted vs non-adjuvanted seasonal influenza vaccines against severe laboratory-confirmed influenza among hospitalized Italian older adults. Int. J. Infect. Dis. 2022, 125, 164–169. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Wang, S.V.; Pinheiro, S.; Hua, W.; Arlett, P.; Uyama, Y.; Berlin, J.A.; Bartels, D.B.; Kahler, K.H.; Bessette, L.G.; Schneeweiss, S. STaRT-RWE: Structured template for planning and reporting on the implementation of real world evidence studies. BMJ 2021, 372, m4856. [Google Scholar] [CrossRef]
- Khambholja, K.; Gehani, M. Use of Structured Template and Reporting Tool for Real-World Evidence Template for Critical Appraisal of the Quality of Reporting of Real-World Evidence Studies: A Systematic Review. Value Health 2023, 26, 427–434. [Google Scholar] [CrossRef]
- Pelton, S.; Divino, V.; Shah, D.; Mould-Quevedo, J.; DeKoven, M.; Krishnarajah, G.; Postma, M. Evaluating the relative vaccine effectiveness of adjuvanted trivalent influenza vaccine compared to high-dose trivalent and other egg-based influenza vaccines among older adults in the US during the 2017–2018 influenza season. Vaccines 2020, 8, 446. [Google Scholar] [CrossRef]
- Postma, M.; Biundo, E.; Chicoye, A.; Devlin, N.; Mark Doherty, T.; Garcia-Ruiz, A.J.; Jaros, P.; Sheikh, S.; Toumi, M.; Wasem, J.; et al. Capturing the value of vaccination within health technology assessment and health economics: Country analysis and priority value concepts. Vaccine 2022, 40, 3999–4007. [Google Scholar] [CrossRef]
- Franklin, J.M.; Lin, K.J.; Gatto, N.M.; Rassen, J.A.; Glynn, R.J.; Schneeweiss, S. Real-World Evidence for Assessing Pharmaceutical Treatments in the Context of COVID-19. Clin. Pharmacol. Ther. 2021, 109, 816–828. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef]
- Prasad, S.; Kalafat, E.; Blakeway, H.; Townsend, R.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Ladhani, S.; et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat. Commun. 2022, 13, 2414. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. Weekly National Influenza and COVID-19 Surveillance Report Week 2 Report (up to Week 1 Data), 12 January 2023. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1129894/Weekly_Flu_and_COVID-19_report_w2.pdf (accessed on 11 January 2023).
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; de Waure, C. Comparative effectiveness of adjuvanted versus high-dose seasonal influenza vaccines for older adults: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022, 122, 855–863. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Systematic Review of the Efficacy, Effectiveness and Safety of Newer and Enhanced Seasonal Influenza Vaccines for the Prevention of Laboratory-Confirmed Influenza in Individuals Aged 18 Years and Over. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-vaccines-systematic-review-efficacy.pdf (accessed on 6 November 2022).
- Wilkinson, K.; Wei, Y.; Szwajcer, A.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M.; Mahmud, S.M. Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine 2017, 35, 2775–2780. [Google Scholar] [CrossRef]
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Samson, S.I.; Greenberg, D.P.; Chit, A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: An updated systematic review and meta-analysis. Vaccine 2021, 39 (Suppl. S1), A24–A35. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.; Chaimani, A.; Li, T. Network meta-analysis: An introduction for clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef]
- Chaimani, A.; Caldwell, D.M.; Li, T.; Higgins, J.P.T.; Salanti, G. Chapter 11: Undertaking network meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 30 May 2023).
- Rotshild, V.; Hirsh-Raccah, B.; Miskin, I.; Muszkat, M.; Matok, I. Comparing the clinical efficacy of COVID-19 vaccines: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 22777. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.; Carlos-Bolumbu, M.; Diallo, M.H.; Makinson, A.; Galtier, F. Efficacy of approved vaccines to prevent COVID-19: A systematic review and network meta-analysis of reconstructed individual patient data from randomized trials. Z. Gesundh. Wiss. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, S.; Lytras, T.; Gianola, S.; Gonzalez-Lorenzo, M.; Castellini, G.; Galli, C.; Cereda, D.; Bonovas, S.; Pariani, E.; Moja, L. Comparative efficacy and safety of vaccines to prevent seasonal influenza: A systematic review and network meta-analysis. EClinicalMedicine 2022, 46, 101331. [Google Scholar] [CrossRef]
- Ferrara, P.; Mantovani, L.G. The importance of real-world evidence in understanding influenza vaccine effectiveness. Farmeconomia. Health Econ. Ther. Pathw. 2022, 23, 29–32. [Google Scholar] [CrossRef]
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef]
- Greenhalgh, T. Will COVID-19 be evidence-based medicine’s nemesis? PLoS Med. 2020, 17, e1003266. [Google Scholar] [CrossRef]
- O’Reilly, D.J.; Blackhouse, G.; Burns, S.; Bowen, J.M.; Burke, N.; Mehltretter, J.; Waite, N.M.; Houle, S.K. Economic analysis of pharmacist-administered influenza vaccines in Ontario, Canada. Clinicoecon. Outcomes Res. 2018, 10, 655–663. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Seasonal Influenza Vaccines Pricing. Available online: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/VaccinesPricing (accessed on 8 May 2023).
- Mauskopf, J.; Standaert, B.; Connolly, M.P.; Culyer, A.J.; Garrison, L.P.; Hutubessy, R.; Jit, M.; Pitman, R.; Revill, P.; Severens, J.L. Economic Analysis of Vaccination Programs: An ISPOR Good Practices for Outcomes Research Task Force Report. Value Health 2018, 21, 1133–1149. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Rebaza, A.; Dela Cruz, C.S. When “B” becomes “A”: The emerging threat of influenza B virus. Eur. Respir. J. 2019, 54, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Huber, V.C. The Unexpected Impact of Vaccines on Secondary Bacterial Infections Following Influenza. Viral Immunol. 2018, 31, 159–173. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postma, M.; Fisman, D.; Giglio, N.; Márquez-Peláez, S.; Nguyen, V.H.; Pugliese, A.; Ruiz-Aragón, J.; Urueña, A.; Mould-Quevedo, J. Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion. Vaccines 2023, 11, 1089. https://doi.org/10.3390/vaccines11061089
Postma M, Fisman D, Giglio N, Márquez-Peláez S, Nguyen VH, Pugliese A, Ruiz-Aragón J, Urueña A, Mould-Quevedo J. Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion. Vaccines. 2023; 11(6):1089. https://doi.org/10.3390/vaccines11061089
Chicago/Turabian StylePostma, Maarten, David Fisman, Norberto Giglio, Sergio Márquez-Peláez, Van Hung Nguyen, Andrea Pugliese, Jesús Ruiz-Aragón, Analia Urueña, and Joaquin Mould-Quevedo. 2023. "Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion" Vaccines 11, no. 6: 1089. https://doi.org/10.3390/vaccines11061089
APA StylePostma, M., Fisman, D., Giglio, N., Márquez-Peláez, S., Nguyen, V. H., Pugliese, A., Ruiz-Aragón, J., Urueña, A., & Mould-Quevedo, J. (2023). Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion. Vaccines, 11(6), 1089. https://doi.org/10.3390/vaccines11061089






