A Meta-Analysis on the Association between Peptic Ulcer Disease and COVID-19 Severity
Abstract
1. Introduction
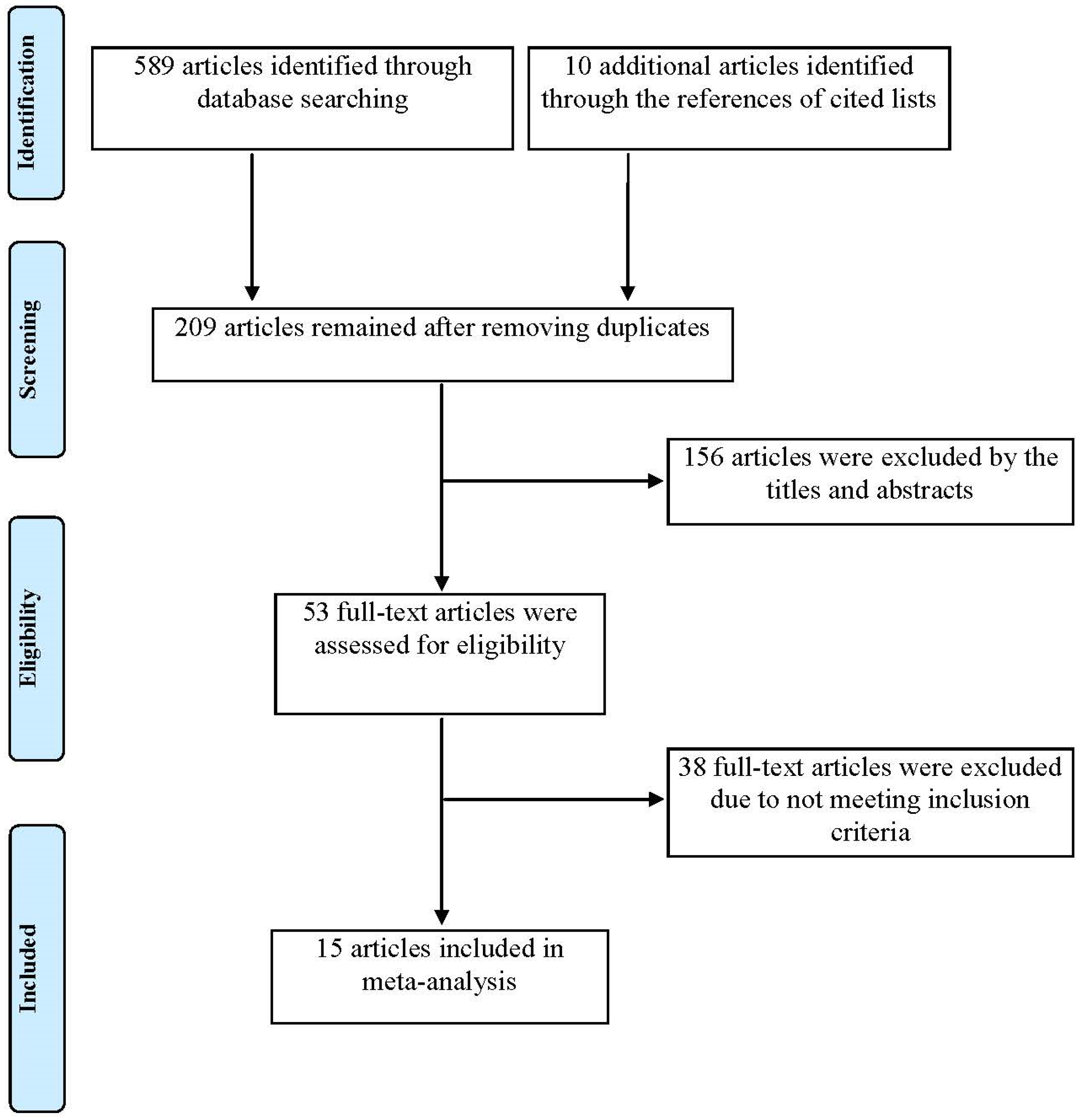
2. Materials and Methods
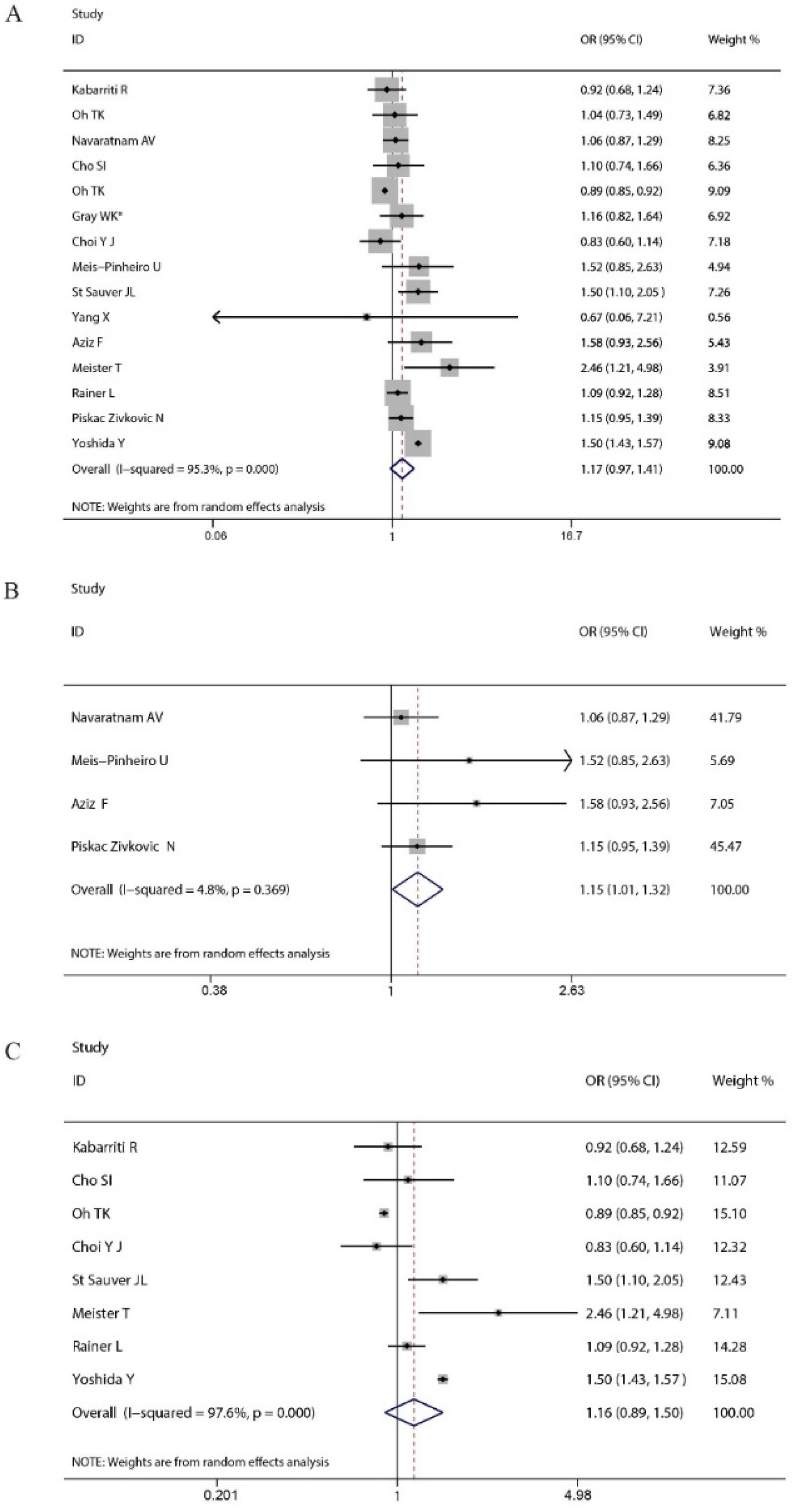
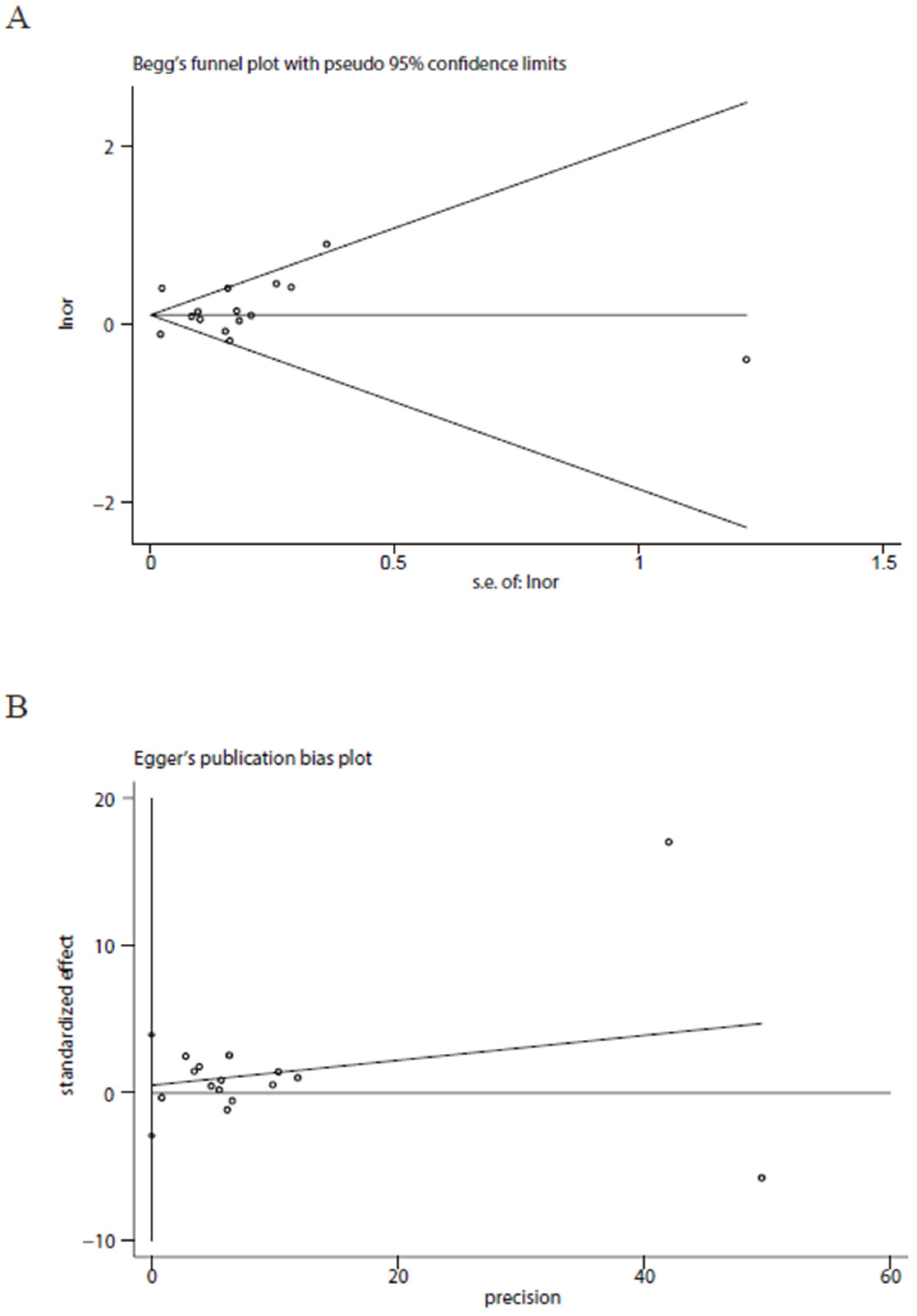
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capra, A.P.; Ardizzone, A.; Panto, G.; Paterniti, I.; Campolo, M.; Crupi, L.; Squeri, R.; Esposito, E. The Prognostic Value of Pentraxin-3 in COVID-19 Patients: A Systematic Review and Meta-Analysis of Mortality Incidence. Int. J. Mol. Sci. 2023, 24, 3537. [Google Scholar] [CrossRef]
- Li, S.; Hu, M.; Zhang, R.; Xu, J.; Hou, H.; Wang, Y.; Yang, H. The association between myocardial infarction and COVID-19 related mortality: A meta-analysis based on adjusted effect estimates. Am. J. Emerg. Med. 2022, 57, 227–229. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, L.; Hu, M.; Zhang, R.; Hao, Y.; Wang, Y.; Yang, H. Significant association between anemia and higher risk for COVID-19 mortality: A meta-analysis of adjusted effect estimates. Am. J. Emerg. Med. 2022, 58, 281–285. [Google Scholar] [CrossRef]
- Zhang, R.; Hao, Y.; Wang, Y.; Yang, H. Significant association between ischemic heart disease and elevated risk for COVID-19 mortality: A meta-analysis. Am. J. Emerg. Med. 2022, 55, 95–97. [Google Scholar] [CrossRef]
- Liang, X.; Shi, L.; Wang, Y.; Xiao, W.; Duan, G.; Yang, H.; Wang, Y. The association of hypertension with the severity and mortality of COVID-19 patients: Evidence based on adjusted effect estimates. J. Infect. 2020, 81, e44–e47. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, H.; Xu, J.; Wang, Y.; Yang, H. The association between obesity and ICU admission among COVID-19 patients: A meta-analysis of adjusted risk estimates. Am. J. Emerg. Med. 2022, 56, 318–320. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Yang, H. Cirrhosis is an independent predictor for COVID-19 mortality: A meta-analysis of confounding cofactors-controlled data. J. Hepatol. 2022, 78, e28–e31. [Google Scholar] [CrossRef]
- Bajci, M.P.; Lendak, D.F.; Ristic, M.; Drljaca, M.M.; Brkic, S.; Turkulov, V.; Petrovic, V. COVID-19 Breakthrough Infections among Patients Aged >/=65 Years in Serbia: Morbidity and Mortality Overview. Vaccines 2022, 10, 1818. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Duan, G.; Yang, H. NAFLD was independently associated with severe COVID-19 among younger patients rather than older patients: A meta-analysis. J. Hepatol. 2022, 78, e136–e139. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Yantiss, R.K. The pathogenesis of coronavirus-19 disease. J. Biomed. Sci. 2022, 29, 87. [Google Scholar] [CrossRef]
- van Doorn, A.S.; Meijer, B.; Frampton, C.M.A.; Barclay, M.L.; de Boer, N.K.H. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Aliment. Pharmacol. Ther. 2020, 52, 1276–1288. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Bian, J.; Li, Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm. Sin. B 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Abbasi-Kangevari, M.; Ahmadi, N.; Fattahi, N.; Rezaei, N.; Malekpour, M.R.; Ghamari, S.H.; Moghaddam, S.S.; Azadnajafabad, S.; Esfahani, Z.; Kolahi, A.A.; et al. Quality of care of peptic ulcer disease worldwide: A systematic analysis for the global burden of disease study 1990−2019. PLoS ONE 2022, 17, e0271284. [Google Scholar] [CrossRef]
- Idris, M.; Smiley, A.; Patel, S.; Latifi, R. Risk Factors for Mortality in Emergently Admitted Patients with Acute Gastric Ulcer: An Analysis of 15,538 Patients in National Inpatient Sample, 2005−2014. Int. J. Environ. Res. Public Health 2022, 19, 16263. [Google Scholar] [CrossRef]
- Alsinnari, Y.M.; Alqarni, M.S.; Attar, M.; Bukhari, Z.M.; Almutairi, M.; Baabbad, F.M.; Hasosah, M. Risk Factors for Recurrence of Peptic Ulcer Disease: A Retrospective Study in Tertiary Care Referral Center. Cureus 2022, 14, e22001. [Google Scholar] [CrossRef]
- Hua, Q.; Zheng, D.; Yu, B.; Tan, X.; Chen, Q.; Wang, L.; Zhang, J.; Liu, Y.; Weng, H.; Cai, Y.; et al. Effectiveness of Inactivated COVID-19 Vaccines against COVID-19 Caused by the SARS-CoV-2 Delta and Omicron Variants: A Retrospective Cohort Study. Vaccines 2022, 10, 1753. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Liu, M.; Wu, Z.; Liu, Y.; Li, W.; Liu, M.; Wang, X.; Gao, B.; Luo, Y.; et al. The Effect of the COVID-19 Vaccine on Daily Cases and Deaths Based on Global Vaccine Data. Vaccines 2021, 9, 1328. [Google Scholar] [CrossRef]
- Arbel, R.; Pliskin, J. Vaccinations versus Lockdowns to Prevent COVID-19 Mortality. Vaccines 2022, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, I.; Agahi, R.; Bimbashi, A.; Aliu, M.; Raka, L.; Bajraktari, I.; Beqiri, P.; Adams, L.V. Higher COVID-19 Vaccination Rates Are Associated with Lower COVID-19 Mortality: A Global Analysis. Vaccines 2022, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Dracz, B.; Muller, V.; Takacs, I.; Hagymasi, K.; Dinya, E.; Miheller, P.; Szijarto, A.; Werling, K. Effectiveness of COVID-19 Vaccination with mRNA Vaccines for Patients with Cirrhosis in Hungary: Multicentre Matched Cohort Study. Vaccines 2022, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.M.; Remmelzwaal, S.; Blom, M.T.; van Hoek, B.; Swart, K.M.A.; Overbeek, J.A.; Burchell, G.L.; Herings, R.M.C.; Elders, P.J.M. Effectiveness of COVID-19 Vaccines in Adults with Diabetes Mellitus: A Systematic Review. Vaccines 2022, 11, 24. [Google Scholar] [CrossRef]
- Robalo, Q.; De Mot, L.; Vandromme, M.; Van Goethem, N.; Gabrio, A.; Chung, P.Y.J.; Meurisse, M.; Belgian Collaborative Group on COVID-19 Hospital Surveillance; Catteau, L.; Thijs, C.; et al. Association between COVID-19 Primary Vaccination and Severe Disease Caused by SARS-CoV-2 Delta Variant among Hospitalized Patients: A Belgian Retrospective Cohort Study. Vaccines 2022, 11, 14. [Google Scholar] [CrossRef]
- Nittayasoot, N.; Suphanchaimat, R.; Thammawijaya, P.; Jiraphongsa, C.; Siraprapasiri, T.; Ploddi, K.; Pittayawonganon, C.; Mahasirimongkol, S.; Tharmaphornpilas, P. Real-World Effectiveness of COVID-19 Vaccines against Severe Outcomes during the Period of Omicron Predominance in Thailand: A Test-Negative Nationwide Case-Control Study. Vaccines 2022, 10, 2123. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Kirui, B.; Santosa, A.; Gisslen, M.; Leach, S.; Wettermark, B.; Vanfleteren, L.; Nyberg, F. Effectiveness of COVID-19 Vaccines over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population. Vaccines 2022, 10, 2074. [Google Scholar] [CrossRef]
- Rahman, M.S.; Harun, M.G.D.; Sumon, S.A.; Mohona, T.M.; Abdullah, S.; Khan, M.N.H.; Gazi, M.I.; Islam, M.S.; Anwar, M.M.U. Hospitalization and Mortality by Vaccination Status among COVID-19 Patients Aged >/= 25 Years in Bangladesh: Results from a Multicenter Cross-Sectional Study. Vaccines 2022, 10, 1987. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Yang, H. Intensive Care during the COVID-19 Pandemic. Vaccines 2023, 11, 125. [Google Scholar] [CrossRef]
- Dodaran, M.S.; Banihashemi, S.R.; Es-Haghi, A.; Mehrabadi, M.H.F.; Nofeli, M.; Mokarram, A.R.; Mokhberalsafa, L.; Sadeghi, F.; Ranjbar, A.; Ansarifar, A.; et al. Immunogenicity and Safety of a Combined Intramuscular/Intranasal Recombinant Spike Protein COVID-19 Vaccine (RCP) in Healthy Adults Aged 18 to 55 Years Old: A Randomized, Double-Blind, Placebo-Controlled, Phase I Trial. Vaccines 2023, 11, 455. [Google Scholar] [CrossRef]
- Hasibuan, A.S.; Koesnoe, S.; Widhani, A.; Muhadi, M.; Shatri, H.; Ginanjar, E.; Yunihastuti, E.; Soewondo, P.; Aman Nasution, S.; Djauzi, S.; et al. Incidence and Associated Factors of SARS-CoV-2 Infection Post-mRNA-1273 Booster Vaccination in Health-Care Workers. Vaccines 2023, 11, 481. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhong, Q.; Ma, Z.; Liu, S.; Lan, Y.; Peng, B.; Zhang, X.; Shi, X.; Qu, J.; Wu, Z.; et al. Neutralization Effect of Sera against Delta and Omicron in Patients Recovering from COVID-19 and Inactivated Vaccine Recipients. Vaccines 2023, 11, 471. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Isakova-Sivak, I.; Stepanova, E.; Matyushenko, V.; Niskanen, S.; Mezhenskaya, D.; Bazhenova, E.; Krutikova, E.; Kotomina, T.; Prokopenko, P.; Neterebskii, B.; et al. Development of a T Cell-Based COVID-19 Vaccine Using a Live Attenuated Influenza Vaccine Viral Vector. Vaccines 2022, 10, 1142. [Google Scholar] [CrossRef]
- Resch, M.D.; Wen, K.; Mazboudi, R.; Mulhall Maasz, H.; Persaud, M.; Garvey, K.; Gallardo, L.; Gottlieb, P.; Alimova, A.; Khayat, R.; et al. Immunogenicity and Efficacy of Monovalent and Bivalent Formulations of a Virus-Like Particle Vaccine against SARS-CoV-2. Vaccines 2022, 10, 1997. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Q. Nucleic Acid Vaccines against SARS-CoV-2. Vaccines 2022, 10, 1849. [Google Scholar] [CrossRef]
- Sriphoosanaphan, S.; Suksawatamnuay, S.; Srisoonthorn, N.; Siripon, N.; Thaimai, P.; Ananchuensook, P.; Thanapirom, K.; Nonthasoot, B.; Hansasuta, P.; Komolmit, P. Immunogenicity, Immune Dynamics, and Subsequent Response to the Booster Dose of Heterologous versus Homologous Prime-Boost Regimens with Adenoviral Vector and mRNA SARS-CoV-2 Vaccine among Liver Transplant Recipients: A Prospective Study. Vaccines 2022, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.P.; Pontes, J.P.J.; Neto, D.R.B.; Borges, C.E.R.; Campos, G.R.L.; Ribeiro, H.L.S.; Amaral, W.N.D. Mortality and Associated Factors in Patients with COVID-19: Cross-Sectional Study. Vaccines 2022, 11, 71. [Google Scholar] [CrossRef]
- Han, X.; Hou, H.; Xu, J.; Ren, J.; Li, S.; Wang, Y.; Yang, H.; Wang, Y. Significant association between HIV infection and increased risk of COVID-19 mortality: A meta-analysis based on adjusted effect estimates. Clin. Exp. Med. 2022, 1–12. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1888899 (accessed on 16 March 2023). [CrossRef]
- Pagkratis, K.; Chrysikos, S.; Antonakis, E.; Pandi, A.; Kosti, C.N.; Markatis, E.; Hillas, G.; Digalaki, A.; Koukidou, S.; Chaini, E.; et al. Predictors of Mortality in Tocilizumab-Treated Severe COVID-19. Vaccines 2022, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Gangu, K.; Garg, I.; Shuja, H.; Bobba, A.; Chourasia, P.; Shekhar, R.; Sheikh, A.B. Gender and Race-Based Health Disparities in COVID-19 Outcomes among Hospitalized Patients in the United States: A Retrospective Analysis of a National Sample. Vaccines 2022, 10, 2036. [Google Scholar] [CrossRef]
- Shi, L.; Ren, J.; Wang, Y.; Feng, H.; Liu, F.; Yang, H. Comorbid Asthma Increased the Risk for COVID-19 Mortality in Asia: A Meta-Analysis. Vaccines 2022, 11, 89. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, W.; Shi, L.; Wang, Y.; Yang, H. Is Cancer an Independent Risk Factor for Fatal Outcomes of Coronavirus Disease 2019 Patients? Arch. Med. Res. 2021, 52, 755–760. [Google Scholar] [CrossRef]
- Meister, T.; Pisarev, H.; Kolde, R.; Kalda, R.; Suija, K.; Milani, L.; Karo-Astover, L.; Piirsoo, M.; Uuskula, A. Clinical characteristics and risk factors for COVID-19 infection and disease severity: A nationwide observational study in Estonia. PLoS ONE 2022, 17, e0270192. [Google Scholar] [CrossRef]
- St Sauver, J.L.; Lopes, G.S.; Rocca, W.A.; Prasad, K.; Majerus, M.R.; Limper, A.H.; Jacobson, D.J.; Fan, C.; Jacobson, R.M.; Rutten, L.J.; et al. Factors Associated With Severe COVID-19 Infection Among Persons of Different Ages Living in a Defined Midwestern US Population. Mayo Clin. Proc. 2021, 96, 2528–2539. [Google Scholar] [CrossRef] [PubMed]
- Meis-Pinheiro, U.; Lopez-Segui, F.; Walsh, S.; Ussi, A.; Santaeugenia, S.; Garcia-Navarro, J.A.; San-Jose, A.; Andreu, A.L.; Campins, M.; Almirante, B. Clinical characteristics of COVID-19 in older adults. A retrospective study in long-term nursing homes in Catalonia. PLoS ONE 2021, 16, e0255141. [Google Scholar] [CrossRef]
- Cho, S.I.; Yoon, S.; Lee, H.J. Impact of comorbidity burden on mortality in patients with COVID-19 using the Korean health insurance database. Sci. Rep. 2021, 11, 6375. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dai, Z.; Huang, C.; Chen, H.; Wang, X.; Li, X. Gastrointestinal Disease and COVID-19: A Review of Current Evidence. Dig. Dis. 2022, 40, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Aghajafari, F.; Letourneau, N.; Mahinpey, N.; Cosic, N.; Giesbrecht, G. Vitamin D Deficiency and Antenatal and Postpartum Depression: A Systematic Review. Nutrients 2018, 10, 478. [Google Scholar] [CrossRef]
- Navaratnam, A.V.; Gray, W.K.; Day, J.; Wendon, J.; Briggs, T.W.R. Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: An observational study using administrative data. Lancet Respir. Med. 2021, 9, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kabarriti, R.; Brodin, N.P.; Maron, M.I.; Guha, C.; Kalnicki, S.; Garg, M.K.; Racine, A.D. Association of Race and Ethnicity With Comorbidities and Survival Among Patients With COVID-19 at an Urban Medical Center in New York. JAMA Netw. Open 2020, 3, e2019795. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Song, I.A. Impact of coronavirus disease-2019 on chronic respiratory disease in South Korea: An NHIS COVID-19 database cohort study. BMC Pulm. Med. 2021, 21, 12. [Google Scholar] [CrossRef]
- Rainer, L.; Bachner, F.; Eglau, K.; Ostermann, H.; Siebert, U.; Zuba, M. Comorbidities and COVID-19 hospitalization, ICU admission and hospital mortality in Austria: A retrospective cohort study. Wien. Klin. Wochenschr. 2022, 134, 856–867. [Google Scholar] [CrossRef]
- Gray, W.K.; Navaratnam, A.V.; Day, J.; Wendon, J.; Briggs, T.W.R. Changes in COVID-19 in-hospital mortality in hospitalised adults in England over the first seven months of the pandemic: An observational study using administrative data. Lancet Reg. Health Eur. 2021, 5, 100104. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, J.Y.; Lee, H.S.; Suh, J.; Song, J.Y.; Byun, M.K.; Cho, J.H.; Kim, H.J.; Park, H.J. Variable effects of underlying diseases on the prognosis of patients with COVID-19. PLoS ONE 2021, 16, e0254258. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Patel, R.C.; Zhang, J.; Guo, S.; Zheng, Q.; Olex, A.L.; Olatosi, B.; Weissman, S.B.; Islam, J.Y.; et al. Associations between HIV infection and clinical spectrum of COVID-19: A population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV 2021, 8, e690–e700. [Google Scholar] [CrossRef]
- Aziz, F.; Aberer, F.; Brauer, A.; Ciardi, C.; Clodi, M.; Fasching, P.; Karolyi, M.; Kautzky-Willer, A.; Klammer, C.; Malle, O.; et al. COVID-19 In-Hospital Mortality in People with Diabetes Is Driven by Comorbidities and Age-Propensity Score-Matched Analysis of Austrian National Public Health Institute Data. Viruses 2021, 13, 2401. [Google Scholar] [CrossRef] [PubMed]
- Piskac Zivkovic, N.; Lucijanic, M.; Busic, N.; Jurin, I.; Atic, A.; Andrilovic, A.; Penovic, T.; Domic, I.; Gnjidic, J.; Demaria, M.; et al. The associations of age, sex, and comorbidities with survival of hospitalized patients with coronavirus disease 2019: Data from 4014 patients from a tertiary-center registry. Croat. Med. J. 2022, 63, 36–43. [Google Scholar] [CrossRef]
- Yoshida, Y.; Chu, S.; Fox, S.; Zu, Y.; Lovre, D.; Denson, J.L.; Miele, L.; Mauvais-Jarvis, F. Sex differences in determinants of COVID-19 severe outcomes—Findings from the National COVID Cohort Collaborative (N3C). BMC Infect. Dis. 2022, 22, 784. [Google Scholar] [CrossRef]
- Oh, T.K.; Song, I.A.; Song, K.H.; Jeon, Y.T. Comparison of All-Cause Mortality Between Individuals With COVID-19 and Propensity Score-Matched Individuals Without COVID-19 in South Korea. Open Forum. Infect. Dis. 2021, 8, ofab057. [Google Scholar] [CrossRef]
- Zhao, S.; Ding, L.; Xie, Q.; Zhang, J.; Yang, S.; Xu, W.; Yang, J.; Xu, Y.; Zheng, C. Is there an association between peptic ulcer disease and osteoporosis: A systematic review and cumulative analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 9–16. [Google Scholar] [CrossRef]
- Sunderraj, A.; Cho, C.; Cai, X.; Gupta, S.; Mehta, R.; Isakova, T.; Leaf, D.E.; Srivastava, A.; Investigators, S.-C. Modulation of the Association Between Age and Death by Risk Factor Burden in Critically Ill Patients With COVID-19. Crit. Care Explor. 2022, 4, e0755. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, J.; Liang, X.; Shi, L.; Wang, Y. Chronic liver disease independently associated with COVID-19 severity: Evidence based on adjusted effect estimates. Hepatol. Int. 2021, 15, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hao, Y.; Nan, L.; Wang, Y.; Yang, H. Peripheral artery disease independently associated with significantly higher risk for COVID-19 mortality: Evidence based on adjusted effect estimates. Vascular 2022, 17085381221111226. [Google Scholar] [CrossRef]
- Oguz, S.H.; Koca, M.; Yildiz, B.O. Aging versus youth: Endocrine aspects of vulnerability for COVID-19. Rev. Endocr. Metab. Disord. 2022, 23, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Batsiou, A.; Mantzios, P.; Piovani, D.; Tsantes, A.G.; Kopanou Taliaka, P.; Liakou, P.; Iacovidou, N.; Tsantes, A.E.; Bonovas, S.; Sokou, R. SARS-CoV-2 Infection and Outcomes in Children with Inflammatory Bowel Diseases: A Systematic Review. J. Clin. Med. 2022, 11, 7238. [Google Scholar] [CrossRef]
- Merdad, G.A.; Seadawi, L.E.; Mustafa, A.A. Peptic ulcer associated with COVID-19 in Saudi Arabia. Saudi Med. J. 2021, 42, 1036–1040. [Google Scholar] [CrossRef]
- Li, Y.; Hou, H.; Yang, H. Lack of Marked Association Between Gastrointestinal Symptoms and COVID-19 Mortality: An Updated Meta-analysis Based on Adjusted Effect Estimates. Mayo Clin. Proc. 2021, 96, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Melazzini, F.; Lenti, M.V.; Mauro, A.; De Grazia, F.; Di Sabatino, A. Peptic Ulcer Disease as a Common Cause of Bleeding in Patients with Coronavirus Disease 2019. Am. J. Gastroenterol. 2020, 115, 1139–1140. [Google Scholar] [CrossRef]
- He, L.; Zhao, W.; Zhou, W.; Pang, P.; Liao, Y.; Liu, J. An Emergency Surgery in Severe Case Infected by COVID-19 With Perforated Duodenal Bulb Ulcer. Ann. Surg. 2020, 272, e35–e37. [Google Scholar] [CrossRef]
- Deb, A.; Thongtan, T.; Costilla, V. Gastric ulcerations in COVID-19: An ominous sign? BMJ Case. Rep. 2021, 14, e244059. [Google Scholar] [CrossRef]
- Dao, H.V.; Hoang, L.B.; Le, N.N.H.; Tran, T.T.T.; Nguyen, H.M.; Dao, L.V.; Le, N.T. Changes in the Proportion of Gastrointestinal Emergency Endoscopy and Peptic Ulcer Disease During the COVID-19 Pandemic: A Local Retrospective Observational Study From Vietnam. Front. Public Health 2022, 10, 699321. [Google Scholar] [CrossRef]
- Jian, C.; Zhou, Z.; Yang, C.; Zhao, N.; Bao, H.; Han, S.; Chen, J.; Shu, X. Increasing rate of hospitalization for severe peptic ulcer in digestive disease emergencies after the pandemic. Medicine 2022, 101, e31716. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Jarvis, C.I.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Karim, F.; Cele, S.; Reedoy, K.; San, J.E.; Lustig, G.; Tegally, H.; Rosenberg, Y.; Bernstein, M.; Jule, Z.; et al. Omicron infection enhances Delta antibody immunity in vaccinated persons. Nature 2022, 607, 356–359. [Google Scholar] [CrossRef]
- Perico, L.L.; Emilio-Silva, M.T.; Ohara, R.; Rodrigues, V.P.; Bueno, G.; Barbosa-Filho, J.M.; Rocha, L.; Batista, L.M.; Hiruma-Lima, C.A. Systematic Analysis of Monoterpenes: Advances and Challenges in the Treatment of Peptic Ulcer Diseases. Biomolecules 2020, 10, 265. [Google Scholar] [CrossRef]
- Kamada, T.; Satoh, K.; Itoh, T.; Ito, M.; Iwamoto, J.; Okimoto, T.; Kanno, T.; Sugimoto, M.; Chiba, T.; Nomura, S.; et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J. Gastroenterol. 2021, 56, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Hardgrave, H.; Wells, A.; Nigh, J.; Klutts, G.; Krinock, D.; Osborn, T.; Bhusal, S.; Rude, M.K.; Burdine, L.; Giorgakis, E. COVID-19 Mortality in Vaccinated vs. Unvaccinated Liver & Kidney Transplant Recipients: A Single-Center United States Propensity Score Matching Study on Historical Data. Vaccines 2022, 10, 1921. [Google Scholar] [PubMed]
- Gentile, I.; Scotto, R.; Schiano Moriello, N.; Pinchera, B.; Villari, R.; Trucillo, E.; Ametrano, L.; Fusco, L.; Castaldo, G.; Buonomo, A.R.; et al. Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study. Vaccines 2022, 10, 1731. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.Y.; Wu, Y.; Yau, V.; Yin, H.X.; Lowe, S.; Bentley, R.; Ahmed, M.A.; Zhao, W.; Sun, C. SARS-CoV-2 Variants, Current Vaccines and Therapeutic Implications for COVID-19. Vaccines 2022, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Korishettar, G.; Chikkahonnaiah, P.; Tulimilli, S.V.; Dallavalasa, S.; Byrappa, S.H.; Madhunapantula, S.V.; Veeranna, R.P. Assessment of Clinical Profile and Treatment Outcome in Vaccinated and Unvaccinated SARS-CoV-2 Infected Patients. Vaccines 2022, 10, 1125. [Google Scholar] [CrossRef]
| Author | Country | Data Collection Time (Year/Month/Day) | Sample Size | Male (%) | Age (Years) | Study Design | Adjusted-Effect (95% CI) | Setting | Adjusted Risk Factors | Score # |
|---|---|---|---|---|---|---|---|---|---|---|
| Kabarriti R [54] | USA | 2020/03/14~2020/04/27 | 5902 | 46.9 | 57.5 | Retrospective cohort study | 0.92 (0.68–1.24) | Hospitalized COVID-19 patients | Age, sex, socioeconomic status, ethnicity/race, body mass index, hypertension, cardiovascular disease, diabetes mellitus, cancer, liver disease, dementia, chronic pulmonary disease, hemiplegia or paraplegia, kidney disease and human immunodeficiency virus/acquired immune deficiency syndrome | 5 |
| Oh TK [55] | Korea | 2020/01/01~2020/06/26 | 7780 | NA | NA | Cohort study | 1.04 (0.73–1.49) | Hospitalized COVID-19 patients | Hypertension, diabetes mellitus without chronic complication, diabetes mellitus with chronic complication, peripheral vascular disease, renal disease, rheumatic disease, dementia, hemiplegia or paraplegia, moderate or severe liver disease, mild liver disease, cerebrovascular disease, congestive heart failure, myocardial infarction, malignancy, metastatic solid tumor and human immunodeficiency virus/acquired immune deficiency syndrome | 8 |
| Navaratnam AV [53] | UK | 2020/03/01~2020/05/31 | 91,541 | 55.4 | 72.58 | Retrospective cohort study | 1.056 (0.865–1.289) | Hospitalized COVID-19 patients | Sex, deprivation score, ethnicity, date of discharge and Charlson comorbidity index items (peripheral vascular disease, congestive heart failure, acute myocardial infarction, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease or rheumatic disease, mild liver disease, moderate or severe liver disease, diabetes mellitus without chronic complications, diabetes mellitus with chronic complications, paraplegia and hemiplegia, renal disease, primary cancer, metastatic carcinoma and obesity) | 5 |
| Cho SI [47] | Korea | 2020/05/15 | 7590 | 40.8 | 46.0 ± 19.6 | Retrospective cohort study | 1.10 (0.74–1.66) | All COVID-19 patients | Age and sex | 6 |
| Oh TK [63] | Korea | 2020/01/01~2020/08/27 | 7713 | 39.5 | 48.4 | Retrospective cohort study | 0.89 (0.85–0.92) | All COVID-19 patients | Age, sex, annual income level in 2020, residence in 2010, underlying disability, Charlson comorbidity index, hypertension, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, diabetes mellitus without chronic complications, diabetes mellitus with chronic complications, renal disease, hemiplegia or paraplegia, rheumatic disease, mild liver disease, moderate to severe liver disease, chronic pulmonary disease, any cancer, metastatic solid tumor and human immunodeficiency virus/acquired immune deficiency syndrome | 8 |
| Gray WK * [57] | UK | 2020/03/01~2020/09/30 | 101,632 | NA | NA | Retrospective cohort study | 1.16 (0.82–1.64) | Hospitalized COVID-19 patients | Age band, sex, deprivation quintile, ethnicity and Charlson comorbidity index items (congestive heart failure, peripheral vascular disease, acute myocardial infarction, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease/rheumatic disease, mild liver disease, moderate or severe liver disease, diabetes mellitus without chronic complications, diabetes mellitus with chronic complications, paraplegia and hemiplegia, renal disease, obesity, primary cancer and metastatic carcinoma) | 5 |
| Choi YJ [58] | Korea | 2020/05/15 | 7590 | 40.8 | 46.61 | Retrospective cohort study | 0.831 (0.604–1.143) | All COVID-19 patients | Age, sex and prevalence of underlying disease (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, mild liver disease, diabetes mellitus without chronic complications, diabetes mellitus with chronic complications, paralysis (hemiplegia or paraplegia), renal disease, malignancy, moderate or severe liver disease, metastatic solid tumor and acquired immune deficiency syndrome) | 4 |
| Meis-Pinheiro U [46] | Spain | 2020/03/01~2020/05/31 | 2092 | 26.77 | 86.7 ± 7.06 | Retrospective cohort study | 1.52 (0.85–2.63) | All COVID-19 patients | Age, pneumonia, fever, dyspnea, stupor, refusal to oral intake, diarrhea, mucous secretion, dry cough, eczema, asthenia, muscle pains, nasal congestion, sore throat, vomiting, lip blisters, confusion, insomnia, dementia, hepatopathy, cardiovascular disease, cerebrovascular disease, diabetes mellitus without organic involvement, diabetes mellitus with organic involvement, chronic obstructive pulmonary disease, chronic kidney disease, connective tissue disease, cancer without metastases, cancer with metastases and hematological tumor | 4 |
| St Sauver JL [45] | USA | 2020/03/01~2020/09/30 | 9928 | 47.9 | 35.8 | Retrospective cohort study | 1.50 (1.10–2.05) | All COVID-19 patients | Age (continuous variable), sex, race, ethnicity, body mass index category and smoking status | 4 |
| Yang X [59] | USA | 2020/01/01~2021/05/08 | 1544 | NA | NA | Cohort study | 0.67 (0.06–7.21) | All COVID-19 patients | Social demographics (age, sex, race, and ethnicity), lifestyle factors (body mass index, and smoking status), comorbidities (hemiplegia or paraplegia, dementia, liver disease, myocardial infarction, congestive heart failure, chronic pulmonary disease, cancer, diabetes mellitus, stroke, peripheral vascular disease, rheumatologic disease, and renal disease) and month of COVID-19 diagnosis | 8 |
| Aziz F [60] | Austria | 2020/03~2021/03 | 40,602 | 52.4 | 72.95 | Retrospective cohort study | 1.58 (0.93–2.56) | Hospitalized COVID-19 patients | Sex, age, intensive care unit admission, myocardial infarction, cardiac arrhythmias, valvular heart disease, hypertension, congestive heart failure, peripheral vascular disease, stroke, chronic obstructive pulmonary disease, dementia, liver disease, other neurological disorders, renal disease, hypothyroidism, fluid and electrolyte disorders, deficiency anemia, depression, and Charlson comorbidity index | 5 |
| Meister T [44] | Estonia | 2020/02/26~2021/02/28 | 66,295 | 55.99 | 44.1 ± 20.6 | Retrospective cohort study | 2.46 (1.21–4.98) | All COVID-19 patients | Sociodemographic characteristics (gender, and age), pre-COVID-19 comorbidity (Charlson index score, acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, chronic kidney disease, acquired immune deficiency syndrome, diabetes mellitus, cancer, liver disease, obesity, sleep apnea, hyperlipidemia, hypertension) and influenza and vaccination within 2 years before COVID-19 (Flu vaccine during last 2 years and influenza during last 2 years) | 6 |
| Rainer L [56] | Austria | 2020/02~2021/12 | 3,604,812 | 45.5 | 41.75 | Retrospective cohort study | 1.09 (0.92–1.28) | All COVID-19 patients | Age group, sex, and healthcare region | 7 |
| Piskac Zivkovic N [61] | Croatia | 2020/03~2021/03 | 4014 | 56.20 | 74 (64–82) | Retrospective cohort study | 1.15 (0.95–1.39) | Hospitalized COVID-19 patients | Age and sex | 4 |
| Yoshida Y [62] | USA | 2020/01/01~2021/12/31 | 574,391 | 46.6 | 52.3 ± 18.5 | Retrospective cohort study | 1.50 (1.43–1.57) | All COVID-19 patients | Age, race, ethnicity, visit type, and any medication use | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, J.; Shi, L.; Yang, H.; Wang, Y. A Meta-Analysis on the Association between Peptic Ulcer Disease and COVID-19 Severity. Vaccines 2023, 11, 1087. https://doi.org/10.3390/vaccines11061087
Wang Y, Xu J, Shi L, Yang H, Wang Y. A Meta-Analysis on the Association between Peptic Ulcer Disease and COVID-19 Severity. Vaccines. 2023; 11(6):1087. https://doi.org/10.3390/vaccines11061087
Chicago/Turabian StyleWang, Ying, Jie Xu, Liqin Shi, Haiyan Yang, and Yadong Wang. 2023. "A Meta-Analysis on the Association between Peptic Ulcer Disease and COVID-19 Severity" Vaccines 11, no. 6: 1087. https://doi.org/10.3390/vaccines11061087
APA StyleWang, Y., Xu, J., Shi, L., Yang, H., & Wang, Y. (2023). A Meta-Analysis on the Association between Peptic Ulcer Disease and COVID-19 Severity. Vaccines, 11(6), 1087. https://doi.org/10.3390/vaccines11061087






