Identification by Reverse Vaccinology of Three Virulence Factors in Burkholderia cenocepacia That May Represent Ideal Vaccine Antigens
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatic Analysis
2.2. Bacterial Strains and Plasmids
2.3. General Molecular Biology Techniques
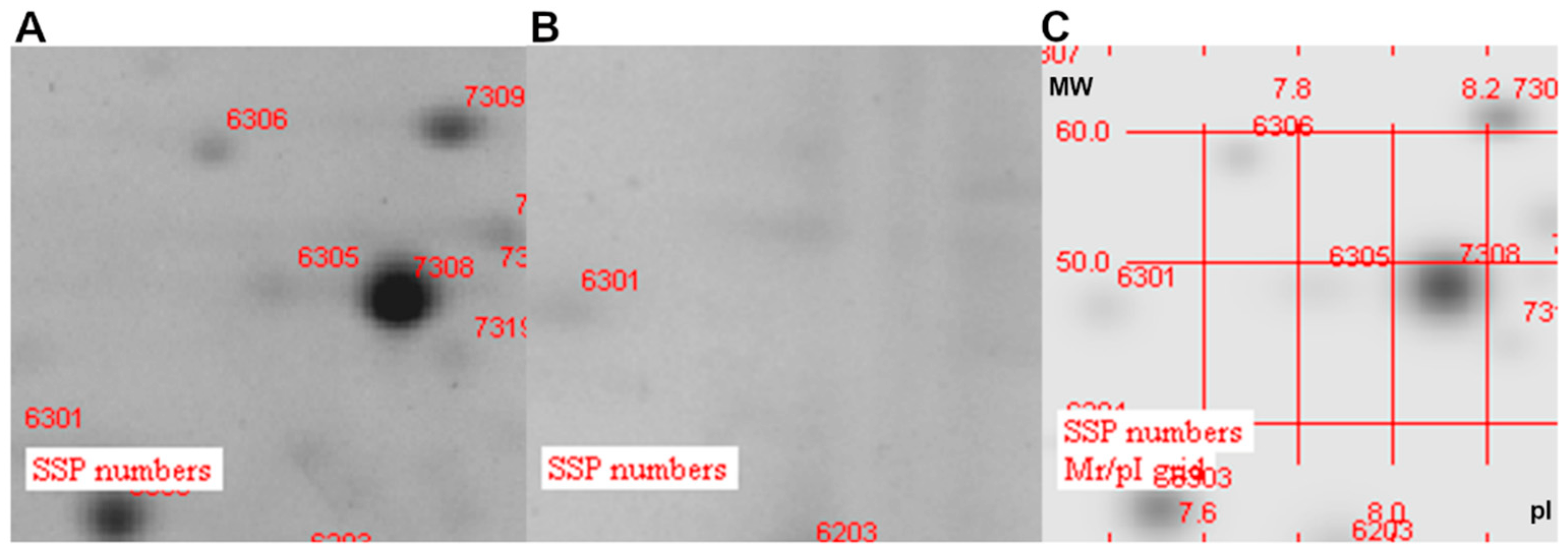
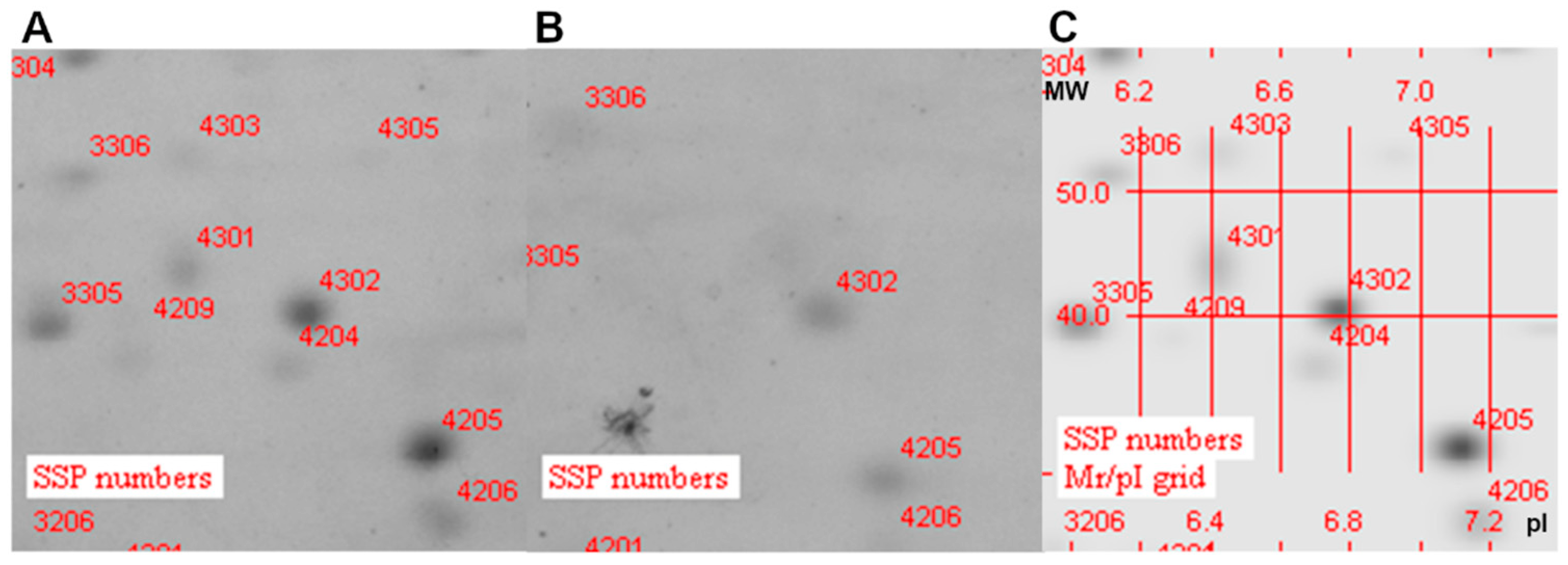
2.4. Proteomic Analysis
2.5. Antimicrobial Susceptibility Testing for Planktonic Cells
2.6. Bacterial Autoaggregation Assay
2.7. In Vitro Biofilm Formation Test in 96-Well Microtiter Plates
2.8. Biofilm Evaluation by Confocal Laser Scanning Microscopy
2.9. Swimming Motility Assay
2.10. Infection in Galleria Mellonella
2.11. Lipase Activity Assay
2.12. Rhamnolipid Analysis
2.13. Statistical Methods
3. Results and Discussion
3.1. In Silico Identification of Antigen Candidates
3.2. Construction of Deletion Mutants of the Selected Antigen Candidates
3.3. Analysis of Protein Localization
3.4. Antibiotic Susceptibility
3.5. Phenotypic Characterization of the Deleted Mutant
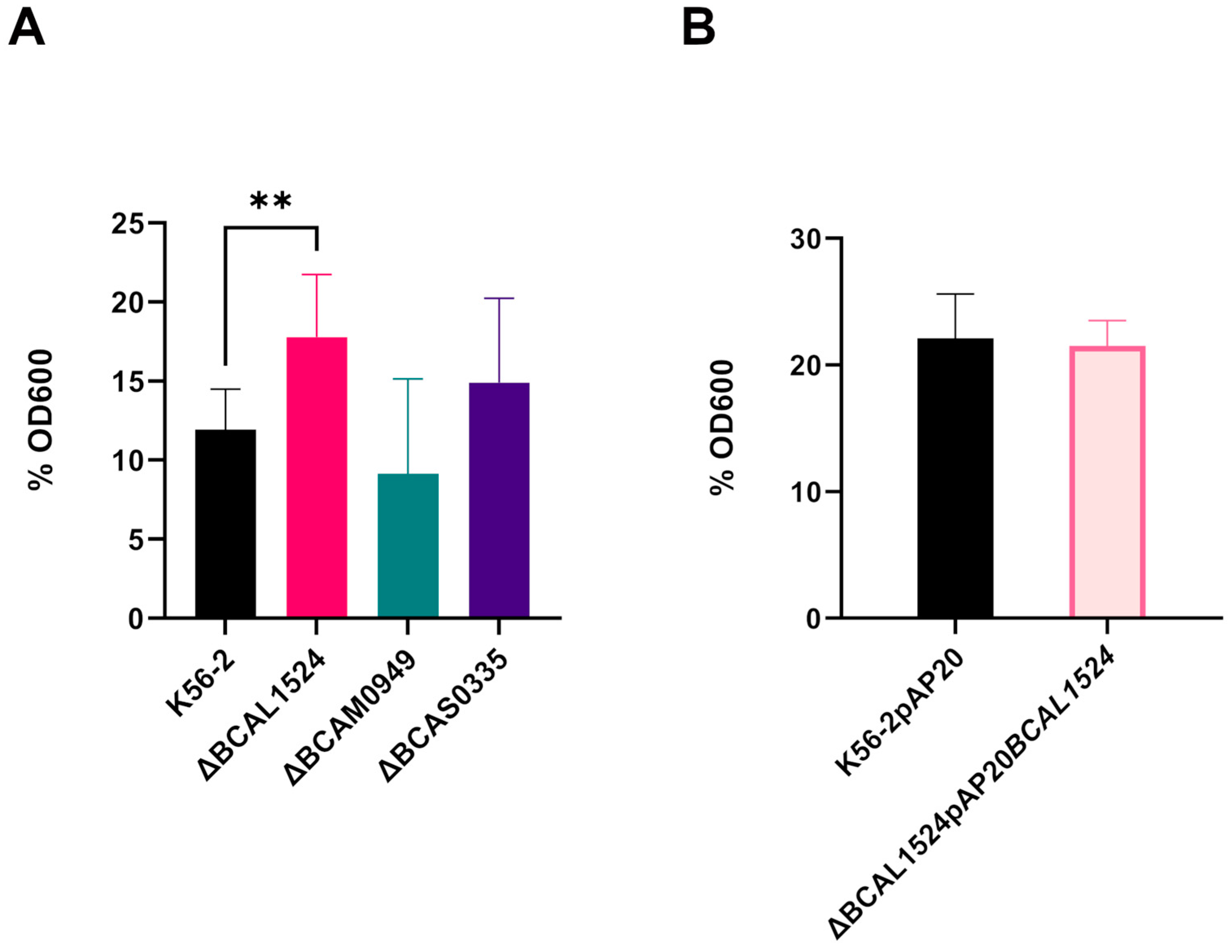
3.5.1. Biofilm Formation
3.5.2. Bacterial Autoaggregation
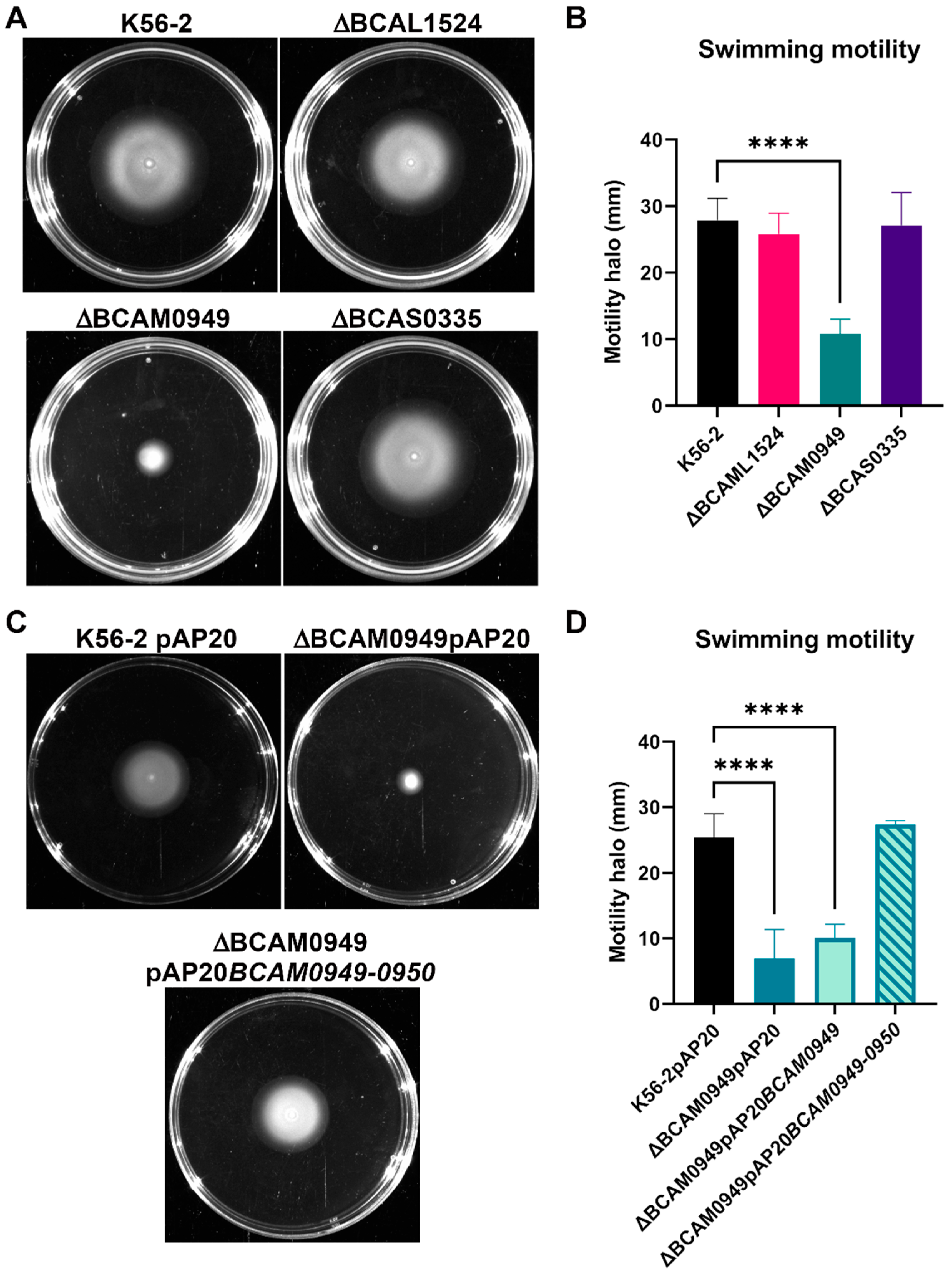
3.5.3. Swimming Motility
3.6. Infection in Galleria mellonella
3.7. Characterization of the Lipase BCAM0949
3.7.1. Lipolytic Activity
3.7.2. Rhamnolipid Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia complex bacteria: A feared contamination risk in water-based pharmaceutical products. Clin. Microbiol. Rev. 2020, 33, e00139-19. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.; Sant’Anna, F.H.; Seger, G.D.D.S.; Passaglia, L.M.P. Pangenome inventory of Burkholderia sensu lato, Burkholderia sensu stricto, and the Burkholderia cepacia complex reveals the uniqueness of Burkholderia catarinensis. Genomics 2022, 114, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Xiang, W.; Cao, K.X.; Lu, X.; Yao, S.C.; Hung, D.; Huang, R.S.; Li, L.B. Characterization of volatile organic compounds emitted from endophytic Burkholderia cenocepacia ETR-B22 by SPME-GC-MS and their inhibitory activity against various plant fungal pathogens. Molecules 2020, 25, 3765. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Patel, D.D.; Bhatt, J.; Thakor, P.; Triplett, L.R.; Thakkar, V.R. Induction of pre-chorismate, jasmonate and salicylate pathways by Burkholderia sp. RR18 in peanut seedlings. J. Appl. Microbiol. 2021, 131, 1417–1430. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Baldwin, A.; Dowson, C.G. Burkholderia cepacia complex bacteria: Opportunistic pathogens with important natural biology. J. Appl. Microbiol. 2008, 104, 1539–1551. [Google Scholar] [CrossRef]
- El Chakhtoura, N.G.; Saade, E.; Wilson, B.M.; Perez, F.; Papp-Wallace, K.M.; Bonomo, R.A. A 17-Year nationwide study of Burkholderia cepacia complex bloodstream infections among patients in the United States Veterans Health Administration. Clin. Infect. Dis. 2017, 65, 1253–1259. [Google Scholar] [CrossRef]
- Tüfekci, S.; Şafak, B.; Nalbantoğlu, B.; Samancı, N.; Kiraz, N. Burkholderia cepacia complex bacteremia outbreaks among non-cystic fibrosis patients in the pediatric unit of a university hospital. Turk. J. Pediatr. 2021, 63, 218–222. [Google Scholar] [CrossRef]
- Lubovich, S.; Zaragoza, S.; Rodríguez, V.; Buendía, J.; Camargo Vargas, B.; Alchundia Moreira, J.; Galanternik, L.; Ratto, P.; Teper, A. Risk factors associated with pulmonary exacerbations in pediatric patients with cystic fibrosis. Arch. Argent. Pediatr. 2019, 117, e466–e472. [Google Scholar]
- Somayaji, R.; Yau, Y.C.W.; Tullis, E.; LiPuma, J.J.; Ratjen, F.; Waters, V. Clinical outcomes associated with Burkholderia cepacia complex infection in patients with cystic fibrosis. Ann. Am. Thorac. Soc. 2020, 17, 1542–1548. [Google Scholar] [CrossRef]
- Akinboyo, I.C.; Sick-Samuels, A.C.; Singeltary, E.; Fackler, J.; Ascenzi, J.; Carroll, K.C.; Maldonado, Y.; Brooks, R.B.; Benowitz, I.; Wilson, L.E.; et al. Multistate outbreak of an emerging Burkholderia cepacia complex strain associated with contaminated oral liquid docusate sodium. Infect. Control Hosp. Epidemiol. 2018, 39, 237–239. [Google Scholar] [CrossRef]
- Wong, S.C.Y.; Wong, S.C.; Chen, J.H.K.; Poon, R.W.S.; Hung, D.L.L.; Chiu, K.H.Y.; So, S.Y.C.; Leung, W.S.; Chan, T.M.; Yap, D.Y.H.; et al. Polyclonal Burkholderia cepacia complex outbreak in peritoneal dialysis patients caused by contaminated aqueous chlorhexidine. Emerg. Infect. Dis. 2020, 26, 1987–1997. [Google Scholar] [CrossRef]
- Bharara, T.; Chakravarti, A.; Sharma, M.; Agarwal, P. Investigation of Burkholderia cepacia complex bacteremia outbreak in a neonatal intensive care unit: A case series. J. Med. Case Rep. 2020, 14, 76. [Google Scholar] [CrossRef]
- Lord, R.; Jones, A.M.; Horsley, A. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst. Rev. 2020, 4, CD009529. [Google Scholar] [CrossRef]
- Sousa, S.A.; Seixas, A.M.M.; Leitão, J.H. Postgenomic approaches and bioinformatics tools to advance the development of vaccines against bacteria of the Burkholderia cepacia complex. Vaccines 2018, 6, 34. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Barbieri, G.; Buroni, S.; Scarselli, M.; Pizza, M.; Rappuoli, R.; Riccardi, G. Vaccines to Overcome Antibiotic Resistance: The Challenge of Burkholderia cenocepacia. Trends Microbiol. 2020, 28, 315–326. [Google Scholar] [CrossRef]
- Pradenas, G.A.; Myers, J.N.; Torres, A.G. Characterization of the Burkholderia cenocepacia tonB mutant as a potential live attenuated vaccine. Vaccines 2017, 5, 33. [Google Scholar] [CrossRef]
- Bertot, G.M.; Restelli, M.A.; Galanternik, L.; Aranibar Urey, R.C.; Valvano, M.A.; Grinstein, S. Nasal immunization with Burkholderia multivorans outer membrane proteins and the mucosal adjuvant adamantylamide dipeptide confers efficient protection against experimental lung infections with B. multivorans and B. cenocepacia. Infect. Immun. 2007, 75, 2740–2752. [Google Scholar] [CrossRef]
- Makidon, P.E.; Knowlton, J.; Groom, J.V., 2nd; Blanco, L.P.; LiPuma, J.J.; Bielinska, A.U.; Baker, J.R., Jr. Induction of immune response to the 17 kDa OMPA Burkholderia cenocepacia polypeptide and protection against pulmonary infection in mice after nasal vaccination with an OMP nanoemulsion-based vaccine. Med. Microbiol. Immunol. 2010, 199, 81–92. [Google Scholar] [CrossRef]
- Sousa, S.A.; Morad, M.; Feliciano, J.R.; Pita, T.; Nady, S.; El-Hennamy, R.E.; Abdel-Rahman, M.; Cavaco, J.; Pereira, L.; Barreto, C.; et al. The Burkholderia cenocepacia OmpA-like protein BCAL2958: Identification, characterization, and detection of anti-BCAL2958 antibodies in serum from B. cepacia complex-infected Cystic Fibrosis patients. AMB Express 2016, 6, 41. [Google Scholar] [CrossRef]
- McClean, S.; Healy, M.E.; Collins, C.; Carberry, S.; O’Shaughnessy, L.; Dennehy, R.; Adams, Á.; Kennelly, H.; Corbett, J.M.; Carty, F.; et al. Linocin and OmpW are involved in attachment of the cystic fibrosis-associated pathogen Burkholderia cepacia complex to lung epithelial cells and protect mice against infection. Infect. Immun. 2016, 84, 1424–1437. [Google Scholar] [CrossRef]
- Sousa, S.A.; Seixas, A.M.M.; Mandal, M.; Rodríguez-Ortega, M.J.; Leitão, J.H. Characterization of the Burkholderia cenocepacia J2315 surface-exposed immunoproteome. Vaccines 2020, 8, 509. [Google Scholar] [CrossRef]
- Seixas, A.M.M.; Sousa, S.A.; Feliciano, J.R.; Gomes, S.C.; Ferreira, M.R.; Moreira, L.M.; Leitão, J.H. A polyclonal antibody raised against the Burkholderia cenocepacia OmpA-like protein BCAL2645 impairs the bacterium adhesion and invasion of human epithelial cells in vitro. Biomedicines 2021, 9, 1788. [Google Scholar] [CrossRef]
- Sousa, S.A.; Soares-Castro, P.; Seixas, A.M.M.; Feliciano, J.R.; Balugas, B.; Barreto, C.; Pereira, L.; Santos, P.M.; Leitão, J.H. New insights into the immunoproteome of B. cenocepacia J2315 using serum samples from cystic fibrosis patients. N. Biotechnol. 2020, 54, 62–70. [Google Scholar] [CrossRef]
- Dennehy, R.; Romano, M.; Ruggiero, A.; Mohamed, Y.F.; Dignam, S.L.; Mujica Troncoso, C.; Callaghan, M.; Valvano, M.A.; Berisio, R.; McClean, S. The Burkholderia cenocepacia peptidoglycan-associated lipoprotein is involved in epithelial cell attachment and elicitation of inflammation. Cell Microbiol. 2017, 19, e12691. [Google Scholar] [CrossRef]
- Pimenta, A.I.; Mil-Homens, D.; Fialho, A.M. Burkholderia cenocepacia-host cell contact controls the transcription activity of the trimeric autotransporter adhesin BCAM2418 gene. Microbiologyopen 2020, 9, e998. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Leça, M.I.; Fernandes, F.; Pinto, S.N.; Fialho, A.M. Characterization of BCAM0224, a multifunctional trimeric autotransporter from the human pathogen Burkholderia cenocepacia. J. Bacteriol. 2014, 196, 1968–1979. [Google Scholar] [CrossRef]
- Rappuoli, R. Reverse vaccinology. Curr. Opin. Microbiol. 2000, 3, 445–450. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Sette, A.; Rappuoli, R. Reverse vaccinology: Developing vaccines in the era of genomics. Immunity 2010, 33, 530–541. [Google Scholar] [CrossRef]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef]
- Tusnády, G.E.; Simon, I. The HMMTOP transmembrane topology prediction server. Bioinformatics 2001, 17, 849–850. [Google Scholar] [CrossRef]
- He, Y.; Xiang, Z.; Mobley, H.L. Vaxign: The first web-based vaccine design program for reverse vaccinology and applications for vaccine development. J. Biomed. Biotechnol. 2010, 2010, 297505. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Kirchner, S.; Fothergill, J.L.; Wright, E.A.; James, C.E.; Mowat, E.; Winstanley, C. Use of artificial sputum medium to test antibiotic efficacy against Pseudomonas aeruginosa in conditions more relevant to the cystic fibrosis lung. J. Vis. Exp. 2012, 64, e3857. [Google Scholar]
- Hamad, M.A.; Skeldon, A.M.; Valvano, M.A. Construction of aminoglycoside-sensitive Burkholderia cenocepacia strains for use in studies of intracellular bacteria with the gentamicin protection assay. Appl. Environ. Microbiol. 2010, 76, 3170–3176. [Google Scholar] [CrossRef]
- Law, R.J.; Hamlin, J.N.; Sivro, A.; McCorrister, S.J.; Cardama, G.A.; Cardona, S.T. A functional phenylacetic acid catabolic pathway is required for full pathogenicity of Burkholderia cenocepacia in the Caenorhabditis elegans host model. J. Bacteriol. 2008, 190, 7209–7218. [Google Scholar] [CrossRef] [PubMed]
- Cardona, S.T.; Valvano, M.A. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid 2005, 54, 219–228. [Google Scholar] [CrossRef]
- Biot, F.V.; Lopez, M.M.; Poyot, T.; Neulat-Ripoll, F.; Lignon, S.; Caclard, A.; Thibault, F.M.; Peinnequin, A.; Pagès, J.M.; Valade, E. Interplay between three RND efflux pumps in doxycycline-selected strains of Burkholderia thailandensis. PLoS ONE 2013, 8, e84068. [Google Scholar] [CrossRef]
- Klimentová, J.; Stulík, J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buroni, S.; Scoffone, V.C.; Fumagalli, M.; Makarov, V.; Cagnone, M.; Trespidi, G.; De Rossi, E.; Forneris, F.; Riccardi, G.; Chiarelli, L.R. Investigating the mechanism of action of diketopiperazines inhibitors of the Burkholderia cenocepacia quorum sensing synthase CepI: A site-directed mutagenesis study. Front. Pharmacol. 2018, 9, 836. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. 2-DE Gel analysis: The spot detection. Methods Mol. Biol. 2016, 1384, 155–164. [Google Scholar] [PubMed]
- EUCAST. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 509–515. [Google Scholar]
- Martin, A.; Takiff, H.; Vandamme, P.; Swings, J.; Palomino, J.C.; Portaels, F. A new rapid and simple colorimetric method to detect pyrazinamide resistance in Mycobacterium tuberculosis using nicotinamide. J. Antimicrob. Chemother. 2006, 58, 327–331. [Google Scholar] [CrossRef]
- Bhargava, S.; Johnson, B.B.; Hwang, J.; Harris, T.A.; George, A.S.; Muir, A.; Dorff, J.; Okeke, I.N. Heat-resistant agglutinin 1 is an accessory enteroaggregative Escherichia coli colonization factor. J. Bacteriol. 2009, 191, 4934–4942. [Google Scholar] [CrossRef]
- Vandecandelaere, I.; Van Acker, H.; Coenye, T. A Microplate-Based System as In Vitro Model of Biofilm Growth and Quantification. Methods Mol. Biol. 2016, 1333, 53–66. [Google Scholar]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiol. Read. 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Bernier, S.P.; Sokol, P.A. Use of suppression-subtractive hybridization to identify genes in the Burkholderia cepacia complex that are unique to Burkholderia cenocepacia. J. Bacteriol. 2005, 187, 5278–5291. [Google Scholar] [CrossRef] [PubMed]
- Seed, K.D.; Dennis, J.J. Development of Galleria mellonella as an alternative infection model for the Burkholderia cepacia complex. Infect. Immun. 2008, 76, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, W.; Otani, Y.; Hashimoto, Y.; Takagi, Y. Molecular cloning and nucleotide sequence of the lipase gene from Pseudomonas fragi. Biochem. Biophys. Res. Commun. 1986, 141, 185–190. [Google Scholar] [CrossRef]
- Rosenau, F.; Isenhardt, S.; Gdynia, A.; Tielker, D.; Schmidt, E.; Tielen, P.; Schobert, M.; Jahn, D.; Wilhelm, S.; Jaeger, K.E. Lipase LipC affects motility, biofilm formation and rhamnolipid production in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2010, 309, 25–34. [Google Scholar] [CrossRef]
- Wilhelm, S.; Gdynia, A.; Tielen, P.; Rosenau, F.; Jaeger, K.E. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J. Bacteriol. 2007, 189, 6695–6703. [Google Scholar] [CrossRef]
- Holden, M.T.; Seth-Smith, H.M.; Crossman, L.C.; Sebaihia, M.; Bentley, S.D.; Cerdeño-Tárraga, A.M.; Thomson, N.R.; Bason, N.; Quail, M.A.; Sharp, S.; et al. The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J. Bacteriol. 2009, 191, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.B.; Xiong, L.; Zhang, K.Y.; Dong, C.; Zhang, F.Z.; Woo, P.C. Identification and analysis of genomic islands in Burkholderia cenocepacia AU 1054 with emphasis on pathogenicity islands. BMC Microbiol. 2017, 17, 73. [Google Scholar] [CrossRef]
- Rosales-Reyes, R.; Aubert, D.F.; Tolman, J.S.; Amer, A.O.; Valvano, M.A. Burkholderia cenocepacia Type VI Secretion System mediates escape of Type II secreted proteins into the cytoplasm of infected macrophages. PLoS ONE 2012, 7, e41726. [Google Scholar] [CrossRef]
- Pimenta, A.I.; Kilcoyne, M.; Bernardes, N.; Mil-Homens, D.; Joshi, L.; Fialho, A.M. Burkholderia cenocepacia BCAM2418-induced antibody inhibits bacterial adhesion, confers protection to infection and enables identification of host glycans as adhesin targets. Cell Microbiol. 2021, 23, e13340. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ibrahim, M.; Qiu, H.; Kausar, S.; Ilyas, M.; Cui, Z.; Hussain, A.; Li, B.; Waheed, A.; Zhu, B.; et al. Protein profiling analyses of the outer membrane of Burkholderia cenocepacia reveal a niche-specific proteome. Microb. Ecol. 2015, 69, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Jacobsson, M.; Björck, L. Genome-based identification and analysis of collagen-related structural motifs in bacterial and viral proteins. J. Biol. Chem. 2003, 278, 32313–32316. [Google Scholar] [CrossRef]
- Pilapitiya, D.H.; Harris, P.W.R.; Hanson-Manful, P.; McGregor, R.; Kowalczyk, R.; Raynes, J.M.; Carlton, L.H.; Dobson, R.C.J.; Baker, M.G.; Brimble, M.; et al. Antibody responses to collagen peptides and streptococcal collagen-like 1 proteins in acute rheumatic fever patients. Pathog. Dis. 2021, 79, ftab033. [Google Scholar] [CrossRef] [PubMed]
- Bachert, B.A.; Choi, S.J.; Snyder, A.K.; Rio, R.V.; Durney, B.C.; Holland, L.A.; Amemiya, K.; Welkos, S.L.; Bozue, J.A.; Cote, C.K.; et al. A unique set of the Burkholderia collagen-like proteins provides insight into pathogenesis, genome evolution and niche adaptation, and infection detection. PLoS ONE 2015, 10, e0137578. [Google Scholar] [CrossRef]
- Grund, M.E.; Kramarska, E.; Choi, S.J.; McNitt, D.H.; Klimko, C.P.; Rill, N.O.; Dankmeyer, J.L.; Shoe, J.L.; Hunter, M.; Fetterer, D.P.; et al. Predictive and experimental immunogenicity of Burkholderia collagen-like protein 8-derived antigens. Vaccines 2021, 9, 1219. [Google Scholar] [CrossRef]
- Mullen, T.; Markey, K.; Murphy, P.; McClean, S.; Callaghan, M. Role of lipase in Burkholderia cepacia complex (Bcc) invasion of lung epithelial cells. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 869–877. [Google Scholar] [CrossRef]
- Straus, D.C.; Lonon, M.K.; Hutson, J.C. Inhibition of rat alveolar macrophage phagocytic function by a Pseudomonas cepacia lipase. J. Med. Microbiol. 1992, 37, 335–340. [Google Scholar] [CrossRef]
- Tielen, P.; Kuhn, H.; Rosenau, F.; Jaeger, K.E.; Flemming, H.C.; Wingender, J. Interaction between extracellular lipase LipA and the polysaccharide alginate of Pseudomonas aeruginosa. BMC Microbiol. 2013, 13, 159. [Google Scholar] [CrossRef]
- Funken, H.; Knapp, A.; Vasil, M.L.; Wilhelm, S.; Jaeger, K.E.; Rosenau, F. The lipase LipA (PA2862) but not LipC (PA4813) from Pseudomonas aeruginosa influences regulation of pyoverdine production and expression of the sigma factor PvdS. J. Bacteriol. 2011, 193, 5858–5860. [Google Scholar] [CrossRef]
- Kiessling, A.R.; Malik, A.; Goldman, A. Recent advances in the understanding of trimeric autotransporter adhesins. Med. Microbiol. Immunol. 2020, 209, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Mil-Homens, D.; Pinto, S.N.; Matos, R.G.; Arraiano, C.; Fialho, A.M. Burkholderia cenocepacia K56-2 trimeric autotransporter adhesin BcaA binds TNFR1 and contributes to induce airway inflammation. Cell Microbiol. 2017, 19, e12677. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.; Busch, M.; Reiners, J.; Hachani, E.; Baeumers, M.; Berger, J.; Schmitt, L.; Jaeger, K.E.; Kovacic, F.; Smits, S.H.J.; et al. The periplasmic chaperone Skp prevents misfolding of the secretory lipase A from Pseudomonas aeruginosa. Front. Mol. Biosci. 2022, 9, 1026724. [Google Scholar] [CrossRef] [PubMed]
- Putra, L.; Natadiputri, G.; Meryandini, A.; Suwanto, A. Isolation, cloning and co-expression of lipase and foldase genes of Burkholderia territorii GP3 from mount Papandayan soil. J. Microbiol. Biotechnol. 2019, 29, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Masignani, V.; Pizza, M.; Moxon, E.R. The Development of a Vaccine against Meningococcus B Using Reverse Vaccinology. Front. Immunol. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Delany, I.; Rappuoli, R.; Seib, K.L. Vaccines, reverse vaccinology, and bacterial pathogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a012476. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023; Online ahead of print. [Google Scholar]
- Figurski, D.H.; Helinski, D.R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 1979, 76, 1648–1652. [Google Scholar] [CrossRef] [PubMed]







| Strain | Source |
|---|---|
| B. ambifaria AMMD | cell culture |
| B. anthina AZ-4-10-S1-D7 | soil |
| B. cepacia ATCC25416 | wash glove |
| B. cenocepacia J2315 | CF sputum |
| B. cenocepacia DDS-22E-1 | aerosol sample |
| B. cenocepacia H111 | CF patient |
| B. cenocepacia HI12424 | cell culture |
| B. cenocepacia MSMB384WGS | water |
| B. cenocepacia VC12308 | CF sputum |
| B. cenocepacia YG-3 | cell culture |
| B. contaminans SK875 | pig with swine respiratory disease |
| B. diffusa RF2-nonBP9 | soil |
| B. dolosa AU0158 | CF patient |
| B. lata A05 | blood of patient with cepacia syndrome |
| B. latens AU17928 | CF Maxillary Sinus |
| B. gladioli ATCC10248 | plant |
| B. glumae 257SH-1 | rice panicles |
| B. metallica FL-6-5-30-S1-D7 | soil |
| B. multivorans ATCCBAA-247 | CF patient |
| B. pyrrocinia DSM10685 | soil |
| B. seminalis FL-5-4-10S1-D7 | soil |
| B. stabilis ATCC BAA-67 | CF sputum |
| B. stagnalis MSMB735WGS | soil |
| B. territorii RF8-non-BP5 | soil |
| B. ubonensis MSMB1471WGS | soil |
| B. vietnamiensis AU1233 | CF blood |
| Time | Voltage (V) |
|---|---|
| 1 h | 0 |
| 8 h | 30 |
| 1 h | 12 |
| 30 min | 300 |
| 3 h | Up to 3500 |
| 10 min | 500 |
| Overnight | 7950 |
| Protein Name | Functional Prediction | Protein Type | Length aa | Cellular Localization Prediction | N° of Predicted TMD | VaxiJen Score | Vaxign-ML Score |
|---|---|---|---|---|---|---|---|
| BCAL0151 | ABC-type branched-chain amino acid transport systems, periplasmic component | Extracellular ligand binding protein | 381 | unknown | Signal peptide | 0.5863 | 98.9 |
| BCAL0198 | Outer membrane protein, OmpW | Putative outer membrane protein | 278 | unknown | 8 | 0.6727 | 90.9 |
| BCAL0199 | DUF2957 domain-containing protein, putatative lipoprotein | Lipoprotein | 414 | unknown | Signal peptide | 0.6030 | 95.2 |
| BCAL0200 | DUF2957 domain-containing protein, putative lipoprotein | Lipoprotein | 477 | extracellular | ≤1 | 0.7576 | 90.9 |
| BCAL0358 | peptidase M1 family M1 metalopeptidase | Enzyme | 723 | extracellular | ≤1 | 0.4835 | 99.7 |
| BCAL1524 | Collagen-like triple helix repeat-containing protein | Lipoprotein | 558 | extracellular | ≤1 | 0.9966 | 90.9 |
| BCAL2229 | Beta-propeller fold lactonase family protein, surface antigen | Enzyme | 330 | extracellular | ≤1 | 0.3297 | 90.9 |
| BCAL2615 | Putative exported outer membrane porin protein | Putative outer membrane protein | 359 | outermembrane | 16 | 0.6883 | 91.0 |
| BCAL3279 | DUF3971 domain-containing protein, Possible exported protein | Hypothetical protein | 1400 | extracellular | 1 | 0.5901 | 90.9 |
| BCAL3353 | Putative outer membrane autotransporter | Autotransporter/adhesin protein | 1772 | outermembrane/extracellular | ≤1 | 0.8135 | 94.9 |
| BCAM0949 | LipA triacylglycerol lipase | Enzyme | 365 | extracellular | 1 | 0.4937 | 90.9 |
| BCAM1514 | Outer membrane protein | Other protein | 294 | outermembrane | ≥1 | 0.6875 | 90.9 |
| BCAM1737 | Alpha-2-macroglobulin | Other protein | 2021 | outermembrane | 1 | 0.6400 | 97.7 |
| BCAM1740 | Adhesin | Lipoprotein | 233 | extracellular | ≥1 | 0.6559 | 90.9 |
| BCAM1931 | Outer membrane porin | Putative outer membrane protein | 360 | outermembrane | 16 | 0.6868 | 92.5 |
| BCAM2311 | Outer membrane porin OmpC | Putative outer membrane protein | 380 | outermembrane | 16 | 0.5846 | 91.4 |
| BCAM2328 | Coagulation factor 5/8 type-like protein | Other protein | 470 | extracellular | 1 | 0.5850 | 95.4 |
| BCAM2418 | Cell surface protein putative haemagglutinin-related autotransporter/adhesin protein | Autotransporter/ adhesin protein | 558 | extracellular | 1 | 0.9141 | 93.5 |
| BCAM2444 | NHL-superfamily, Six-bladed beta-propeller, TolB-like | Other protein | 643 | extracellular | ≤1 | 0.5015 | 95.0 |
| BCAS0147 | YncE super family, beta-propeller fold lactonase family protein | Enzyme | 397 | outermembrane/extracellular | 1 | 0.6027 | 90.9 |
| BCAS0236 | putative haemagglutinin-related autotransporter/adhesin protein | Autotransporter/ adhesin protein | 1497 | outermembrane/extracellular | ≤1 | 0.8682 | 92.2 |
| BCAS0321 | Autotransporter | Autotransporter/ adhesin protein | 4250 | outermembrane | ≥1 | 1.115 | 90.9 |
| BCAS0335 | putative haemagglutinin-related autotransporter/adhesin protein | Autotransporter/ adhesin protein | 1198 | extracellular | ≤1 | 0.8436 | 98.9 |
| BCAS0409 | M4 family metallopeptidase | Enzyme | 566 | extracellular | ≤1 | 0.6513 | 95.0 |
| BCAS0641 | phosphatase PAP2 family protein | Enzyme | 464 | unknown | ≤1 | 0.6073 | 93.9 |
| Antibiotics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | AMK | AZT | CIP | LVX | MEM | MIN | NAL | PIP | SPX | TOB |
| K56-2 | ≥256 | ≥256 | 2 | 4 | 8 | 8 | 16 | 128 | 4 | ≥256 |
| ∆BCAL1524 | ≥256 | 256 | 2 | 4 | 8 | 4 | 8 | 128 | 4 | ≥256 |
| ∆BCAM0949 | ≥256 | 256 | 2 | 4 | 4 | 16 | 16 | 32 | 4 | ≥256 |
| ∆BCAS0335 | ≥256 | 256 | 4 | 4 | 8 | 64 | 16 | 128 | 4 | ≥256 |
| ∆BCAL1524 pSCrhaB2BCAM1524 | ≥256 | ≥256 | ≤2 | 4 | 16 | ≤2 | 16 | 64 | 4 | ≥256 |
| ∆BCAM0949 pSCrhaB2BCAM0949 | ≥256 | ≥256 | ≤2 | 4 | 4 | ≤2 | 4 | 128 | 4 | ≥256 |
| ∆BCAS0335 pSCrhaB2BCAS0335 | ≥256 | ≥256 | ≤2 | 4 | 8 | 4 | 8 | 128 | 4 | ≥256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irudal, S.; Scoffone, V.C.; Trespidi, G.; Barbieri, G.; D’Amato, M.; Viglio, S.; Pizza, M.; Scarselli, M.; Riccardi, G.; Buroni, S. Identification by Reverse Vaccinology of Three Virulence Factors in Burkholderia cenocepacia That May Represent Ideal Vaccine Antigens. Vaccines 2023, 11, 1039. https://doi.org/10.3390/vaccines11061039
Irudal S, Scoffone VC, Trespidi G, Barbieri G, D’Amato M, Viglio S, Pizza M, Scarselli M, Riccardi G, Buroni S. Identification by Reverse Vaccinology of Three Virulence Factors in Burkholderia cenocepacia That May Represent Ideal Vaccine Antigens. Vaccines. 2023; 11(6):1039. https://doi.org/10.3390/vaccines11061039
Chicago/Turabian StyleIrudal, Samuele, Viola Camilla Scoffone, Gabriele Trespidi, Giulia Barbieri, Maura D’Amato, Simona Viglio, Mariagrazia Pizza, Maria Scarselli, Giovanna Riccardi, and Silvia Buroni. 2023. "Identification by Reverse Vaccinology of Three Virulence Factors in Burkholderia cenocepacia That May Represent Ideal Vaccine Antigens" Vaccines 11, no. 6: 1039. https://doi.org/10.3390/vaccines11061039
APA StyleIrudal, S., Scoffone, V. C., Trespidi, G., Barbieri, G., D’Amato, M., Viglio, S., Pizza, M., Scarselli, M., Riccardi, G., & Buroni, S. (2023). Identification by Reverse Vaccinology of Three Virulence Factors in Burkholderia cenocepacia That May Represent Ideal Vaccine Antigens. Vaccines, 11(6), 1039. https://doi.org/10.3390/vaccines11061039








