Electrochemotherapy Plus IL-2+IL-12 Gene Electrotransfer in Spontaneous Inoperable Stage III–IV Canine Oral Malignant Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Canine Patients
2.2. Anesthetic Procedure
2.3. ECT Procedure
2.4. GET Procedure
2.5. Plasmids
2.6. Blood Sample Collection and ELISA Test
2.7. Evaluation of Treatment Outcome
2.8. Statistical Analysis
3. Results
3.1. Treatment Groups Conformation
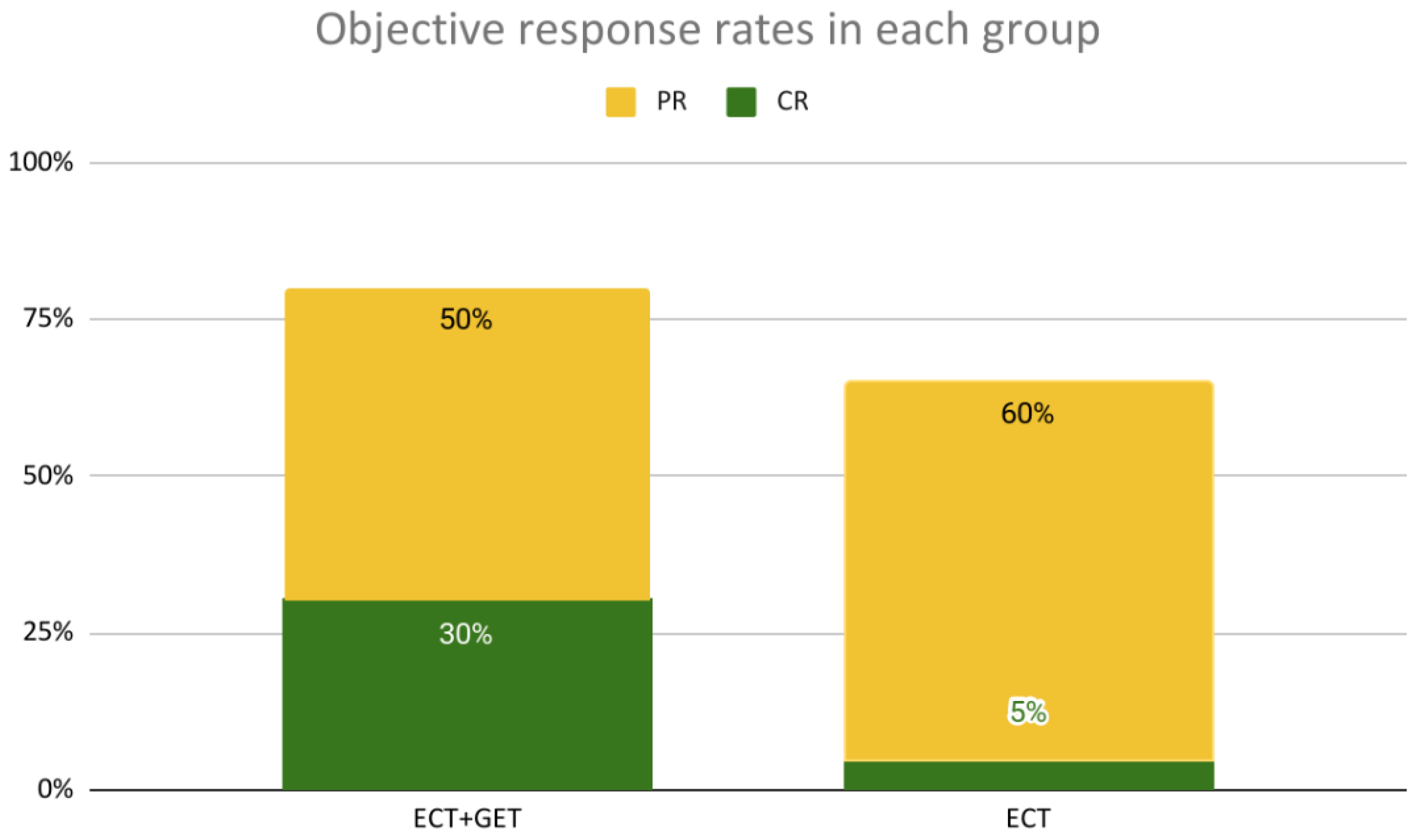
3.1.1. Local Response
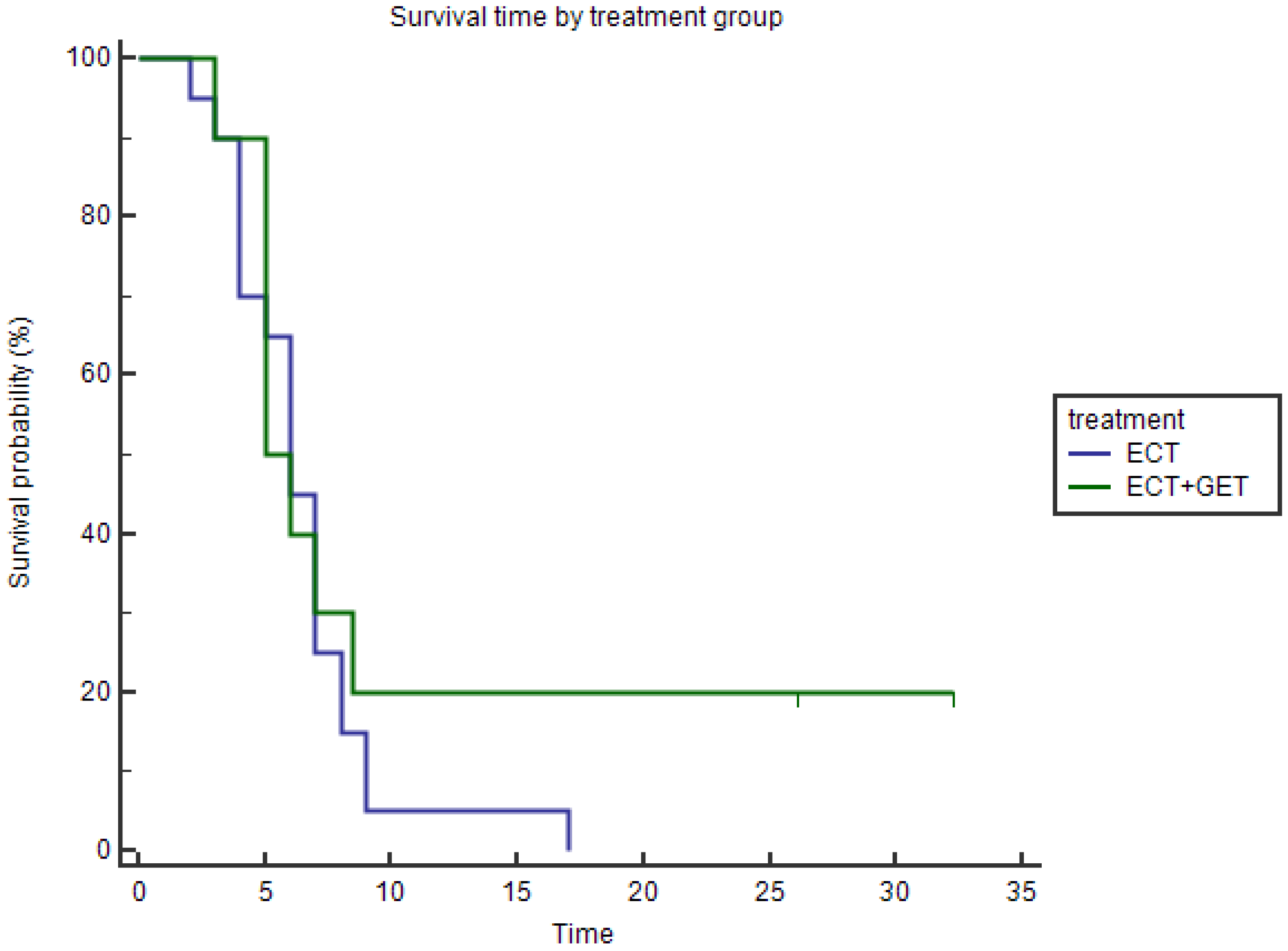
3.1.2. Overall Survival
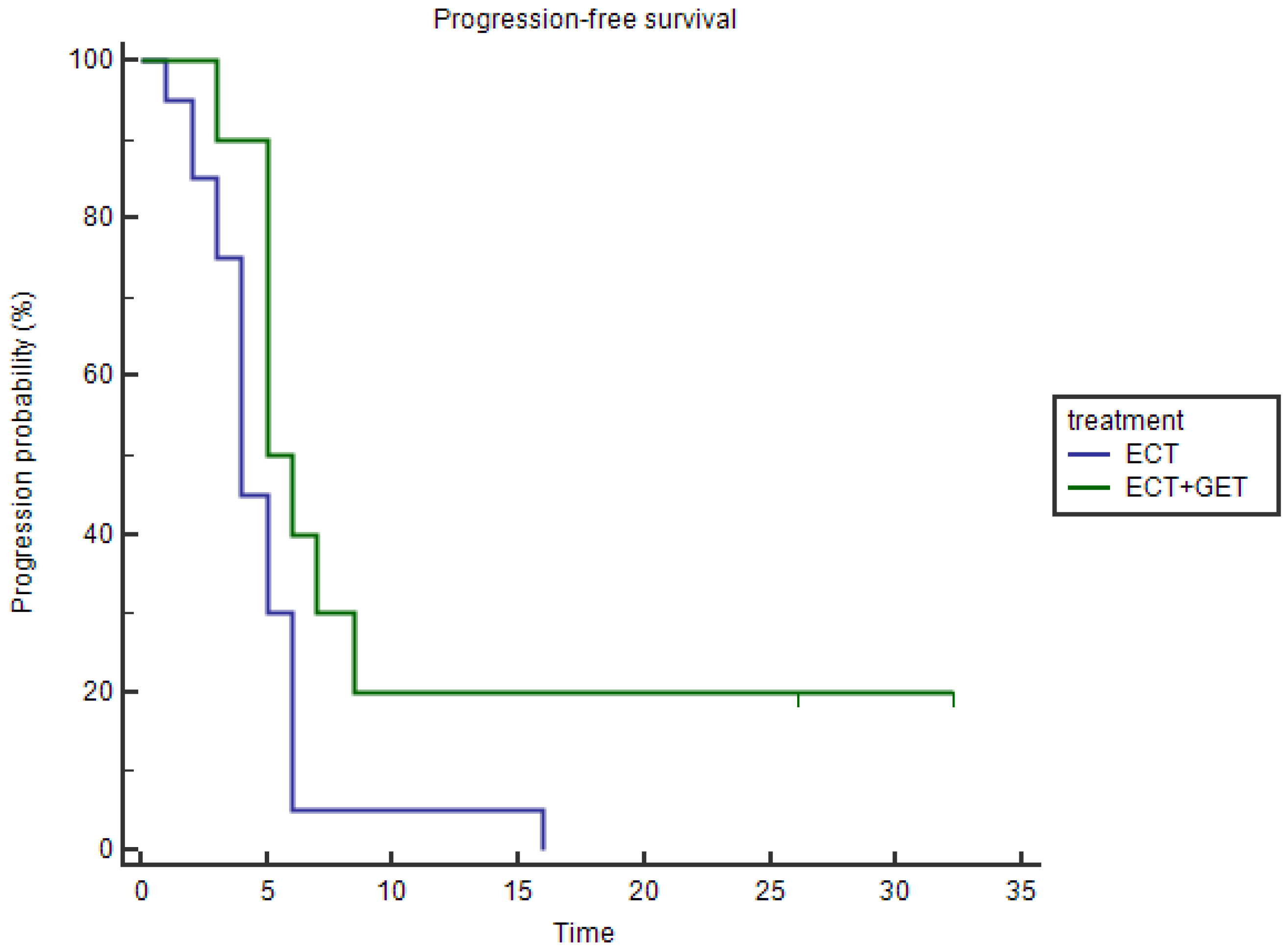
3.1.3. Progression-Free Survival
3.2. Tolerance and Side-Effects
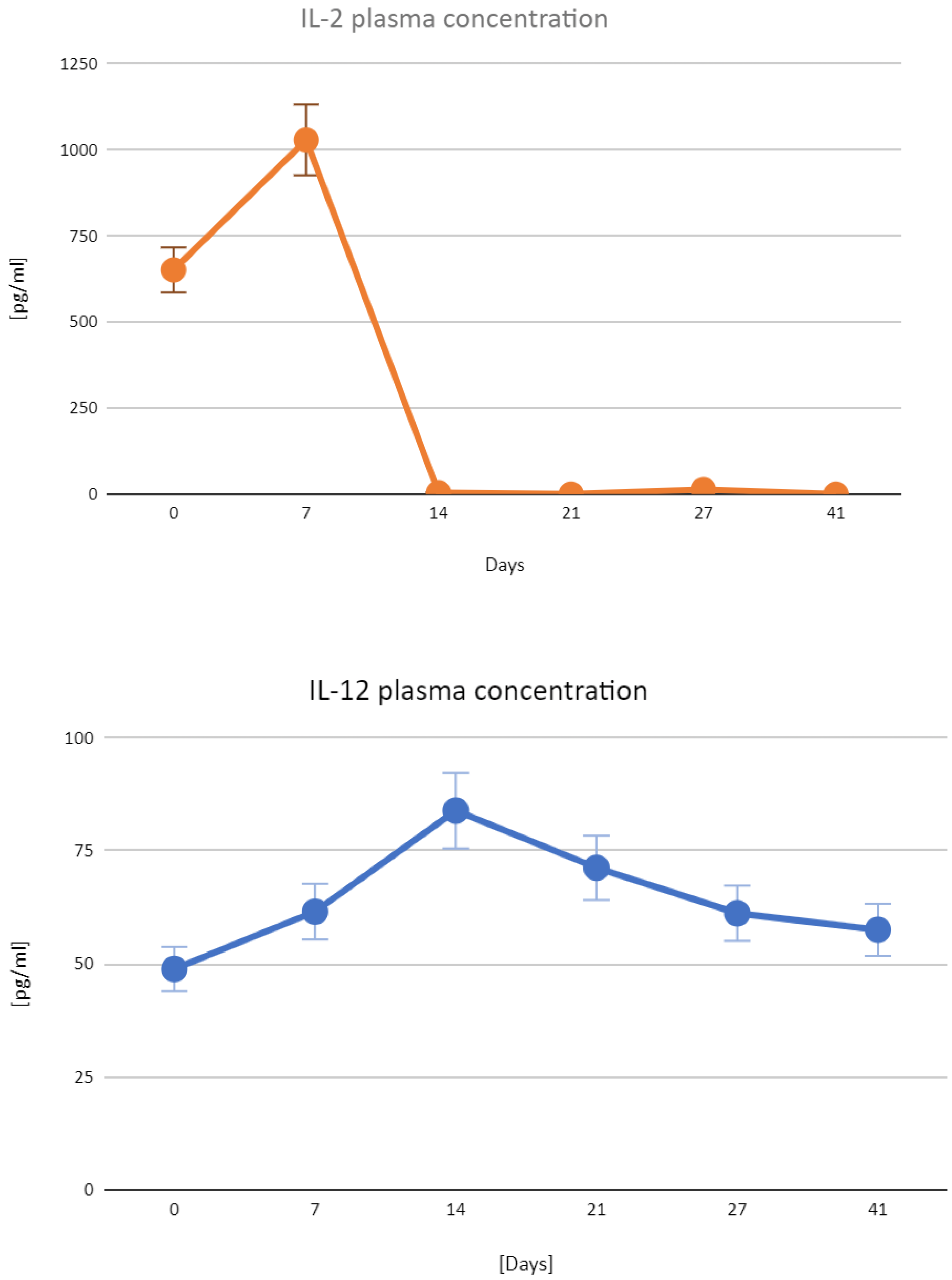
3.3. Expression of the Transfected Plasmids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLM | Bleomycin |
| GET | Gene Electrotransfer |
| ECT | Electrochemotherapy |
| OS | Overall survival |
| PFS | Progression-free survival |
| CR | Complete response |
| PR | Partial response |
| SD | Stable disease |
| PD | Progressive disease |
| OR | Objective response |
References
- Wingo, K. Histopathologic Diagnoses from Biopsies of the Oral Cavity in 403 Dogs and 73 Cats. J. Vet. Dent. 2018, 35, 7–17. [Google Scholar] [CrossRef]
- Spangler, W.L.; Kass, P.H. The Histologic and Epidemiologic Bases for Prognostic Considerations in Canine Melanocytic Neoplasia. Vet. Pathol. 2006, 43, 136–149. [Google Scholar] [CrossRef]
- Harvey, H.J.; MacEwen, E.G.; Braun, D.; Patnaik, A.K.; Withrow, S.J.; Jongeward, S. Prognostic Criteria for Dogs with Oral Melanoma. J. Am. Vet. Med. Assoc. 1981, 178, 580–582. [Google Scholar]
- Dorn, C.R.; Priester, W.A. Epidemiologic Analysis of Oral and Pharyngeal Cancer in Dogs, Cats, Horses, and Cattle. J. Am. Vet. Med. Assoc. 1976, 169, 1202–1206. [Google Scholar]
- Bergman, P.J. Canine Oral Melanoma. Clin. Tech. Small Anim. Pract. 2007, 22, 55–60. [Google Scholar] [CrossRef]
- Nishiya, A.T.; Massoco, C.O.; Felizzola, C.R.; Perlmann, E.; Batschinski, K.; Tedardi, M.V.; Garcia, J.S.; Mendonça, P.P.; Teixeira, T.F.; Zaidan Dagli, M.L. Comparative Aspects of Canine Melanoma. Vet. Sci. China 2016, 3, 7. [Google Scholar] [CrossRef]
- Pazzi, P.; Steenkamp, G.; Rixon, A.J. Treatment of Canine Oral Melanomas: A Critical Review of the Literature. Vet. Sci. China 2022, 9, 196. [Google Scholar] [CrossRef]
- Sersa, G.; Ursic, K.; Cemazar, M.; Heller, R.; Bosnjak, M.; Campana, L.G. Biological Factors of the Tumour Response to Electrochemotherapy: Review of the Evidence and a Research Roadmap. Eur. J. Surg. Oncol. 2021, 47, 1836–1846. [Google Scholar] [CrossRef]
- Ursic, K.; Kos, S.; Kamensek, U.; Cemazar, M.; Scancar, J.; Bucek, S.; Kranjc, S.; Staresinic, B.; Sersa, G. Comparable Effectiveness and Immunomodulatory Actions of Oxaliplatin and Cisplatin in Electrochemotherapy of Murine Melanoma. Bioelectrochemistry 2018, 119, 161–171. [Google Scholar] [CrossRef]
- Orlowski, S.; Belehradek, J., Jr.; Paoletti, C.; Mir, L.M. Transient Electropermeabilization of Cells in Culture. Increase of the Cytotoxicity of Anticancer Drugs. Biochem. Pharmacol. 1988, 37, 4727–4733. [Google Scholar] [CrossRef]
- Gehl, J.; Skovsgaard, T.; Mir, L.M. Enhancement of Cytotoxicity by Electropermeabilization: An Improved Method for Screening Drugs. Anticancer Drugs 1998, 9, 319–325. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard Operating Procedures of the Electrochemotherapy: Instructions for the Use of Bleomycin or Cisplatin Administered Either Systemically or Locally and Electric Pulses Delivered by the CliniporatorTM by Means of Invasive or Non-Invasive Electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated Standard Operating Procedures for Electrochemotherapy of Cutaneous Tumours and Skin Metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Tellado, M.; Mir, L.M.; Maglietti, F. Veterinary Guidelines for Electrochemotherapy of Superficial Tumors. Front. Vet. Sci. 2022, 9, 868989. [Google Scholar] [CrossRef]
- Impellizeri, J.A. Electroporation in Veterinary Oncology Practice: Electrochemotherapy and Gene Electrotransfer for Immunotherapy; Springer Nature: Berlin/Heidelberg, Germany, 2021; ISBN 9783030806682. [Google Scholar]
- Mir, L.M. Bases and Rationale of the Electrochemotherapy. Eur. J. Cancer Suppl. 2006, 4, 38–44. [Google Scholar] [CrossRef]
- Tellado, M.N.; Maglietti, F.H.; Michinski, S.D.; Marshall, G.R.; Signori, E. Electrochemotherapy in Treatment of Canine Oral Malignant Melanoma and Factors Influencing Treatment Outcome. Radiol. Oncol. 2020, 54, 68–78. [Google Scholar] [CrossRef]
- Tozon, N.; Kramaric, P.; Kos Kadunc, V.; Sersa, G.; Cemazar, M. Electrochemotherapy as a Single Treatment or Adjuvant Treatment to Surgery of Cutaneous Sarcoid Tumours in Horses: A 31-Case Retrospective Study. Vet. Rec. 2016, 179, 627. [Google Scholar] [CrossRef]
- Tellado, M.; Michinski, S.; Impellizeri, J.; Marshall, G.; Signori, E.; Maglietti, F. Electrochemotherapy Using Thin-Needle Electrode Improves Recovery in Feline Nasal Planum Squamous Cell Carcinoma—A Translational Model. Canc. Drug Resist. 2022, 5, 595–611. [Google Scholar] [CrossRef]
- Lowe, R.; Gavazza, A.; Impellizeri, J.A.; Soden, D.M.; Lubas, G. The Treatment of Canine Mast Cell Tumours with Electrochemotherapy with or without Surgical Excision. Vet. Comp. Oncol. 2017, 15, 775–784. [Google Scholar] [CrossRef]
- Cemazar, M.; Sersa, G. Recent Advances in Electrochemotherapy. Bioelectricity 2019, 1, 204–213. [Google Scholar] [CrossRef]
- Mir, L.M.; Tounekti, O.; Orlowski, S. Bleomycin: Revival of an Old Drug. Gen. Pharmacol. 1996, 27, 745–748. [Google Scholar] [CrossRef]
- Tounekti, O.; Pron, G.; Belehradek, J., Jr.; Mir, L.M. Bleomycin, an Apoptosis-Mimetic Drug That Induces Two Types of Cell Death Depending on the Number of Molecules Internalized. Cancer Res. 1993, 53, 5462–5469. [Google Scholar]
- Calvet, C.Y.; Famin, D.; André, F.M.; Mir, L.M. Electrochemotherapy with Bleomycin Induces Hallmarks of Immunogenic Cell Death in Murine Colon Cancer Cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Maglietti, F.; Tellado, M.; De Robertis, M.; Michinski, S.; Fernández, J.; Signori, E.; Marshall, G. Electroporation as the Immunotherapy Strategy for Cancer in Veterinary Medicine: State of the Art in Latin America. Vaccines 2020, 8, 537. [Google Scholar] [CrossRef]
- Mir, L.M.; Orlowski, S. Mechanisms of Electrochemotherapy. Adv. Drug Deliv. Rev. 1999, 35, 107–118. [Google Scholar] [CrossRef]
- Sersa, G.; Jarm, T.; Kotnik, T.; Coer, A.; Podkrajsek, M.; Sentjurc, M.; Miklavcic, D.; Kadivec, M.; Kranjc, S.; Secerov, A.; et al. Vascular Disrupting Action of Electroporation and Electrochemotherapy with Bleomycin in Murine Sarcoma. Br. J. Cancer 2008, 98, 388–398. [Google Scholar] [CrossRef]
- Ruzgys, P.; Navickaitė, D.; Palepšienė, R.; Uždavinytė, D.; Barauskaitė, N.; Novickij, V.; Girkontaitė, I.; Šitkauskienė, B.; Šatkauskas, S. Induction of Bystander and Abscopal Effects after Electroporation-Based Treatments. Cancers 2022, 14, 3770. [Google Scholar] [CrossRef]
- Calvet, C.Y.; Mir, L.M. The Promising Alliance of Anti-Cancer Electrochemotherapy with Immunotherapy. Cancer Metastasis Rev. 2016, 35, 165–177. [Google Scholar] [CrossRef]
- Dutcher, J.P. Current Status of Interleukin-2 Therapy for Metastatic Renal Cell Carcinoma and Metastatic Melanoma. Oncology 2002, 16, 4–10. [Google Scholar]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in Cancer Immunotherapy. Oncoimmunology 2016, 5, e1163462. [Google Scholar] [CrossRef] [PubMed]
- Quintin-Colonna, F.; Devauchelle, P.; Fradelizi, D.; Mourot, B.; Faure, T.; Kourilsky, P.; Roth, C.; Mehtali, M. Gene Therapy of Spontaneous Canine Melanoma and Feline Fibrosarcoma by Intratumoral Administration of Histoincompatible Cells Expressing Human Interleukin-2. Gene Ther. 1996, 3, 1104–1112. [Google Scholar] [PubMed]
- Boston, S.E.; Lu, X.; Culp, W.T.N.; Montinaro, V.; Romanelli, G.; Dudley, R.M.; Liptak, J.M.; Mestrinho, L.A.; Buracco, P. Efficacy of Systemic Adjuvant Therapies Administered to Dogs after Excision of Oral Malignant Melanomas: 151 Cases (2001–2012). J. Am. Vet. Med. Assoc. 2014, 245, 401–407. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Heller, L.C.; Heller, R. Electroporation Gene Therapy Preclinical and Clinical Trials for Melanoma. Curr. Gene Ther. 2010, 10, 312–317. [Google Scholar] [CrossRef]
- Komel, T.; Bosnjak, M.; Kranjc Brezar, S.; De Robertis, M.; Mastrodonato, M.; Scillitani, G.; Pesole, G.; Signori, E.; Sersa, G.; Cemazar, M. Gene Electrotransfer of IL-2 and IL-12 Plasmids Effectively Eradicated Murine B16.F10 Melanoma. Bioelectrochemistry 2021, 141, 107843. [Google Scholar] [CrossRef]
- Lee Lucas, M.; Heller, L.; Coppola, D.; Heller, R. IL-12 Plasmid Delivery by in Vivo Electroporation for the Successful Treatment of Established Subcutaneous B16.F10 Melanoma. Mol. Ther. 2002, 5, 668–675. [Google Scholar] [CrossRef]
- Lohr, F.; Lo, D.Y.; Zaharoff, D.A.; Hu, K.; Zhang, X.; Li, Y.; Zhao, Y.; Dewhirst, M.W.; Yuan, F.; Li, C.Y. Effective Tumor Therapy with Plasmid-Encoded Cytokines Combined with in Vivo Electroporation. Cancer Res. 2001, 61, 3281–3284. [Google Scholar]
- Jas, D.; Soyer, C.; De Fornel-Thibaud, P.; Oberli, F.; Vernes, D.; Guigal, P.-M.; Poulet, H.; Devauchelle, P. Adjuvant Immunotherapy of Feline Injection-Site Sarcomas with the Recombinant Canarypox Virus Expressing Feline Interleukine-2 Evaluated in a Controlled Monocentric Clinical Trial When Used in Association with Surgery and Brachytherapy. Trials Vaccinol. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Zabielska-Koczywąs, K.; Wojtalewicz, A.; Lechowski, R. Current Knowledge on Feline Injection-Site Sarcoma Treatment. Acta Vet. Scand. 2017, 59, 47. [Google Scholar] [CrossRef]
- Ottnod, J.M.; Smedley, R.C.; Walshaw, R.; Hauptman, J.G.; Kiupel, M.; Obradovich, J.E. A Retrospective Analysis of the Efficacy of Oncept Vaccine for the Adjunct Treatment of Canine Oral Malignant Melanoma. Vet. Comp. Oncol. 2013, 11, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Verganti, S.; Berlato, D.; Blackwood, L.; Amores-Fuster, I.; Polton, G.A.; Elders, R.; Doyle, R.; Taylor, A.; Murphy, S. Use of Oncept Melanoma Vaccine in 69 Canine Oral Malignant Melanomas in the UK. J. Small Anim. Pract. 2017, 58, 10–16. [Google Scholar] [CrossRef]
- Rosazza, C.; Haberl Meglic, S.; Zumbusch, A.; Rols, M.-P.; Miklavcic, D. Gene Electrotransfer: A Mechanistic Perspective. Curr. Gene Ther. 2016, 16, 98–129. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; Teissie, J.; Cemazar, M.; Signori, E.; Kamensek, U.; Marshall, G.; Miklavcic, D. Electrochemotherapy of Tumors as in Situ Vaccination Boosted by Immunogene Electrotransfer. Cancer Immunol. Immunother. 2015, 64, 1315–1327. [Google Scholar] [CrossRef]
- Daud, A.I.; DeConti, R.C.; Andrews, S.; Urbas, P.; Riker, A.I.; Sondak, V.K.; Munster, P.N.; Sullivan, D.M.; Ugen, K.E.; Messina, J.L.; et al. Phase I Trial of Interleukin-12 Plasmid Electroporation in Patients with Metastatic Melanoma. J. Clin. Oncol. 2008, 26, 5896–5903. [Google Scholar] [CrossRef]
- Cutrera, J.; King, G.; Jones, P.; Kicenuik, K.; Gumpel, E.; Xia, X.; Li, S. Safe and Effective Treatment of Spontaneous Neoplasms with Interleukin 12 Electro-Chemo-Gene Therapy. J. Cell. Mol. Med. 2015, 19, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Cutrera, J.; Torrero, M.; Shiomitsu, K.; Mauldin, N.; Li, S. Intratumoral Bleomycin and IL-12 Electrochemogenetherapy for Treating Head and Neck Tumors in Dogs. Methods Mol. Biol. 2008, 423, 319–325. [Google Scholar] [CrossRef]
- Lampreht Tratar, U.; Kos, S.; Kamensek, U.; Ota, M.; Tozon, N.; Sersa, G.; Cemazar, M. Antitumor Effect of Antibiotic Resistance Gene-Free Plasmids Encoding Interleukin-12 in Canine Melanoma Model. Cancer Gene Ther. 2018, 25, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Tozon, N.; Lampreht Tratar, U.; Znidar, K.; Sersa, G.; Teissie, J.; Cemazar, M. Operating Procedures of the Electrochemotherapy for Treatment of Tumor in Dogs and Cats. J. Vis. Exp. 2016, 116, e54760. [Google Scholar] [CrossRef]
- Cemazar, M.; Golzio, M.; Sersa, G.; Hojman, P.; Kranjc, S.; Mesojednik, S.; Rols, M.-P.; Teissie, J. Control by Pulse Parameters of DNA Electrotransfer into Solid Tumors in Mice. Gene Ther. 2009, 16, 635–644. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) Following Investigational Therapy in Dogs and Cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef] [PubMed]
- Miklavcic, D.; Pucihar, G.; Pavlovec, M.; Ribaric, S.; Mali, M.; Macek-Lebar, A.; Petkovsek, M.; Nastran, J.; Kranjc, S.; Cemazar, M.; et al. The Effect of High Frequency Electric Pulses on Muscle Contractions and Antitumor Efficiency in Vivo for a Potential Use in Clinical Electrochemotherapy. Bioelectrochemistry 2005, 65, 121–128. [Google Scholar] [CrossRef]
- Mir, L.M.; Roth, C.; Orlowski, S.; Quintin-Colonna, F.; Fradelizi, D.; Belehradek, J., Jr.; Kourilsky, P. Systemic Antitumor Effects of Electrochemotherapy Combined with Histoincompatible Cells Secreting Interleukin-2. J. Immunother. Emphas. Tumor Immunol. 1995, 17, 30–38. [Google Scholar] [CrossRef]
- Dev, S.B.; Hofmann, G.A. Electrochemotherapy—A Novel Method of Cancer Treatment. Cancer Treat. Rev. 1994, 20, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Ursic, K.; Kos, S.; Kamensek, U.; Cemazar, M.; Miceska, S.; Markelc, B.; Bucek, S.; Staresinic, B.; Kloboves Prevodnik, V.; Heller, R.; et al. Potentiation of Electrochemotherapy Effectiveness by Immunostimulation with IL-12 Gene Electrotransfer in Mice Is Dependent on Tumor Immune Status. J. Control. Release 2021, 332, 623–635. [Google Scholar] [CrossRef]
- Milevoj, N.; Tratar, U.L.; Nemec, A.; Brožič, A.; Žnidar, K.; Serša, G.; Čemažar, M.; Tozon, N. A Combination of Electrochemotherapy, Gene Electrotransfer of Plasmid Encoding Canine IL-12 and Cytoreductive Surgery in the Treatment of Canine Oral Malignant Melanoma. Res. Vet. Sci. 2019, 122, 40–49. [Google Scholar] [CrossRef]
- Daud, A.; Algazi, A.P.; Ashworth, M.T.; Fong, L.; Lewis, J.; Chan, S.E.; Buljan, M.; Molina, M.A.; Takamura, K.T.; Diep, T.T.; et al. Systemic Antitumor Effect and Clinical Response in a Phase 2 Trial of Intratumoral Electroporation of Plasmid Interleukin-12 in Patients with Advanced Melanoma. J. Clin. Oncol. 2014, 32, 9025. [Google Scholar] [CrossRef]
- Campana, L.G.; Peric, B.; Mascherini, M.; Spina, R.; Kunte, C.; Kis, E.; Rozsa, P.; Quaglino, P.; Jones, R.P.; Clover, A.J.P.; et al. Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry. Cancers 2021, 13, 4289. [Google Scholar] [CrossRef]
- Mirjačić Martinović, K.M.; Babović, N.L.; Džodić, R.R.; Jurišić, V.B.; Ninković, A.Z.; Konjević, G.M. Beneficial in-Vitro Effects of Interleukin-2, Interleukin-12, and Their Combination on Functional and Receptor Characteristics of Natural Killer Cells in Metastatic Melanoma Patients with Normal Serum Lactate Dehydrogenase Levels. Melanoma Res. 2016, 26, 551–564. [Google Scholar] [CrossRef]
- Cemazar, M.; Jarm, T.; Sersa, G. Cancer Electrogene Therapy with Interleukin-12. Curr. Gene Ther. 2010, 10, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Tevz, G.; Kranjc, S.; Cemazar, M.; Kamensek, U.; Coer, A.; Krzan, M.; Vidic, S.; Pavlin, D.; Sersa, G. Controlled Systemic Release of Interleukin-12 after Gene Electrotransfer to Muscle for Cancer Gene Therapy Alone or in Combination with Ionizing Radiation in Murine Sarcomas. J. Gene Med. 2009, 11, 1125–1137. [Google Scholar] [CrossRef]
- Tevz, G.; Pavlin, D.; Kamensek, U.; Kranjc, S.; Mesojednik, S.; Coer, A.; Sersa, G.; Cemazar, M. Gene Electrotransfer into Murine Skeletal Muscle: A Systematic Analysis of Parameters for Long-Term Gene Expression. Technol. Cancer Res. Treat. 2008, 7, 91–101. [Google Scholar] [CrossRef]
- Calvalido, J.; Wood, G.A.; Mutsaers, A.J.; Wood, D.; Sears, W.; Woods, J.P. Comparison of Serum Cytokine Levels between Dogs with Multicentric Lymphoma and Healthy Dogs. Vet. Immunol. Immunopathol. 2016, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, M.; Piccinno, M.; Roncetti, M.; Mutinati, M.; Rizzo, A.; Sciorsci, R.L. Evaluation of Serum Concentrations of Interleukin (IL)-4, IL-10, and IL-12 during Pregnancy in Bitches. Theriogenology 2013, 79, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Asano, Y.; Sajiki, Y.; Deguchi, T.; Okagawa, T.; Watari, K.; Takeuchi, H.; Takagi, S.; Hosoya, K.; et al. Exploration of Serum Biomarkers in Dogs with Malignant Melanoma Receiving Anti-PD-L1 Therapy and Potential of COX-2 Inhibition for Combination Therapy. Sci. Rep. 2022, 12, 9265. [Google Scholar] [CrossRef]
- Mir, L.M.; Roth, C.; Orlowski, S.; Belehradek, J., Jr.; Fradelizi, D.; Paoletti, C.; Kourilsky, P. Potentiation of the antitumoral effect of electrochemotherapy by immunotherapy with allogeneic cells producing interleukin 2. Comptes Rendus Acad. Sci. III 1992, 314, 539–544. [Google Scholar]
- Gothelf, A.; Eriksen, J.; Hojman, P.; Gehl, J. Duration and Level of Transgene Expression after Gene Electrotransfer to Skin in Mice. Gene Ther. 2010, 17, 839–845. [Google Scholar] [CrossRef]
- Pavlin, D.; Tozon, N.; Sersa, G.; Pogacnik, A.; Cemazar, M. Efficient Electrotransfection into Canine Muscle. Technol. Cancer Res. Treat. 2008, 7, 45–54. [Google Scholar] [CrossRef]
- Pavlin, D.; Cemazar, M.; Cör, A.; Sersa, G.; Pogacnik, A.; Tozon, N. Electrogene Therapy with Interleukin-12 in Canine Mast Cell Tumors. Radiol. Oncol. 2011, 45, 31–39. [Google Scholar] [CrossRef]
- Cemazar, M.; Sersa, G.; Pavlin, D.; Tozon, N. Intramuscular IL-12 Electrogene Therapy for Treatment of Spontaneous Canine Tumors. Targets Gene Ther. 2011, 299, 320. [Google Scholar]
- Skrombolas, D.; Frelinger, J.G. Challenges and Developing Solutions for Increasing the Benefits of IL-2 Treatment in Tumor Therapy. Expert Rev. Clin. Immunol. 2014, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B. The Two Faces of IL-2: A Key Driver of CD8+ T-Cell Exhaustion. Cell. Mol. Immunol. 2021, 18, 1641–1643. [Google Scholar] [CrossRef]
- Impellizeri, J.A. (Ed.) Electroporation in Veterinary Oncology Practice, 1st ed.; Springer Nature: Cham, Switzerland, 2021; ISBN 9783030806675. [Google Scholar]
- Impellizeri, J.; Aurisicchio, L.; Forde, P.; Soden, D.M. Electroporation in Veterinary Oncology. Vet. J. 2016, 217, 18–25. [Google Scholar] [CrossRef]
- Kishida, T.; Asada, H.; Itokawa, Y.; Yasutomi, K.; Shin-Ya, M.; Gojo, S.; Cui, F.D.; Ueda, Y.; Yamagishi, H.; Imanishi, J.; et al. Electrochemo-Gene Therapy of Cancer: Intratumoral Delivery of Interleukin-12 Gene and Bleomycin Synergistically Induced Therapeutic Immunity and Suppressed Subcutaneous and Metastatic Melanomas in Mice. Mol. Ther. 2003, 8, 738–745. [Google Scholar] [CrossRef]
- Torrero, M.N.; Henk, W.G.; Li, S. Regression of High-Grade Malignancy in Mice by Bleomycin and Interleukin-12 Electrochemogenetherapy. Clin. Cancer Res. 2006, 12, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, J.L.; Selmic, L.E.; Worley, D.R.; Ehrhart, N.P.; Withrow, S.J. Outcome Following Curative-Intent Surgery for Oral Melanoma in Dogs: 70 Cases (1998–2011). J. Am. Vet. Med. Assoc. 2014, 245, 1266–1273. [Google Scholar] [CrossRef]
- Burzykowski, T.; Buyse, M.; Piccart-Gebhart, M.J.; Sledge, G.; Carmichael, J.; Lück, H.-J.; Mackey, J.R.; Nabholtz, J.-M.; Paridaens, R.; Biganzoli, L.; et al. Evaluation of Tumor Response, Disease Control, Progression-Free Survival, and Time to Progression as Potential Surrogate End Points in Metastatic Breast Cancer. J. Clin. Oncol. 2008, 26, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.R.; Lagoni, L. End-of-Life Communication in Veterinary Medicine: Delivering Bad News and Euthanasia Decision Making. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 95–108, abstract viii–ix. [Google Scholar] [CrossRef]
- Milevoj, N.; Tozon, N.; Licen, S.; Lampreht Tratar, U.; Sersa, G.; Cemazar, M. Health-Related Quality of Life in Dogs Treated with Electrochemotherapy And/or Interleukin-12 Gene Electrotransfer. Vet. Med. Sci 2020, 6, 290–298. [Google Scholar] [CrossRef]


| Patients | ECT+GET | ECT |
|---|---|---|
| n (treatment: control) | 10 | 20 |
| Age (years) | 11 (8–13) | 11.8 (9–15) |
| Sex Ratio (F:M) | 6:4 | 11:9 |
| Body weight (kg) | 23.8 (8–45) | 23 (4–58) |
| Stage (III:IV) | 8:2 | 12:8 |
| Breeds | ||
| Mixed breed | 50% | 30% |
| Beagle | 10% | 10% |
| Labrador Retriever | 10% | 10% |
| Golden Retriever | 10% | 0% |
| Shar-pei | 10% | 0% |
| Argentinian Dogo | 10% | 0% |
| Toy poodle | 0% | 20% |
| Rottweiler | 0% | 5% |
| Cocker Spaniel | 0% | 10% |
| English Mastiff | 0% | 5% |
| Dalmatian | 0% | 5% |
| Basset Hound | 0% | 5% |
| Stage | Tumor Diameter | Lymph Node Involvement | Metastasis |
|---|---|---|---|
| I | <2 cm | No | No |
| II | 2–4 cm | No | No |
| III | > or =4 cm | No | No |
| Any | Yes | No | |
| IV | Any | Yes or No | Yes |
| Grade 1 | Grade 2 | |||
|---|---|---|---|---|
| Treatment group | ECT | ECT+GET | ECT | ECT+GET |
| Pain | 5 (25%) | 2 (20%) | 2 (10%) | 1 (10%) |
| Vomiting | 2 (10%) | 2 (20%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tellado, M.; De Robertis, M.; Montagna, D.; Giovannini, D.; Salgado, S.; Michinski, S.; Signori, E.; Maglietti, F. Electrochemotherapy Plus IL-2+IL-12 Gene Electrotransfer in Spontaneous Inoperable Stage III–IV Canine Oral Malignant Melanoma. Vaccines 2023, 11, 1033. https://doi.org/10.3390/vaccines11061033
Tellado M, De Robertis M, Montagna D, Giovannini D, Salgado S, Michinski S, Signori E, Maglietti F. Electrochemotherapy Plus IL-2+IL-12 Gene Electrotransfer in Spontaneous Inoperable Stage III–IV Canine Oral Malignant Melanoma. Vaccines. 2023; 11(6):1033. https://doi.org/10.3390/vaccines11061033
Chicago/Turabian StyleTellado, Matías, Mariangela De Robertis, Daniela Montagna, Daniela Giovannini, Sergio Salgado, Sebastián Michinski, Emanuela Signori, and Felipe Maglietti. 2023. "Electrochemotherapy Plus IL-2+IL-12 Gene Electrotransfer in Spontaneous Inoperable Stage III–IV Canine Oral Malignant Melanoma" Vaccines 11, no. 6: 1033. https://doi.org/10.3390/vaccines11061033
APA StyleTellado, M., De Robertis, M., Montagna, D., Giovannini, D., Salgado, S., Michinski, S., Signori, E., & Maglietti, F. (2023). Electrochemotherapy Plus IL-2+IL-12 Gene Electrotransfer in Spontaneous Inoperable Stage III–IV Canine Oral Malignant Melanoma. Vaccines, 11(6), 1033. https://doi.org/10.3390/vaccines11061033







