Retrospective Analysis of Vaccinated and Unvaccinated COVID-19 Patients Treated with Monoclonal Antibodies (mAb) and Their Emergent Needs (RAVEN)
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Cohort
2.2. Vaccination Status
2.3. Outcomes
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Cohorts Description
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
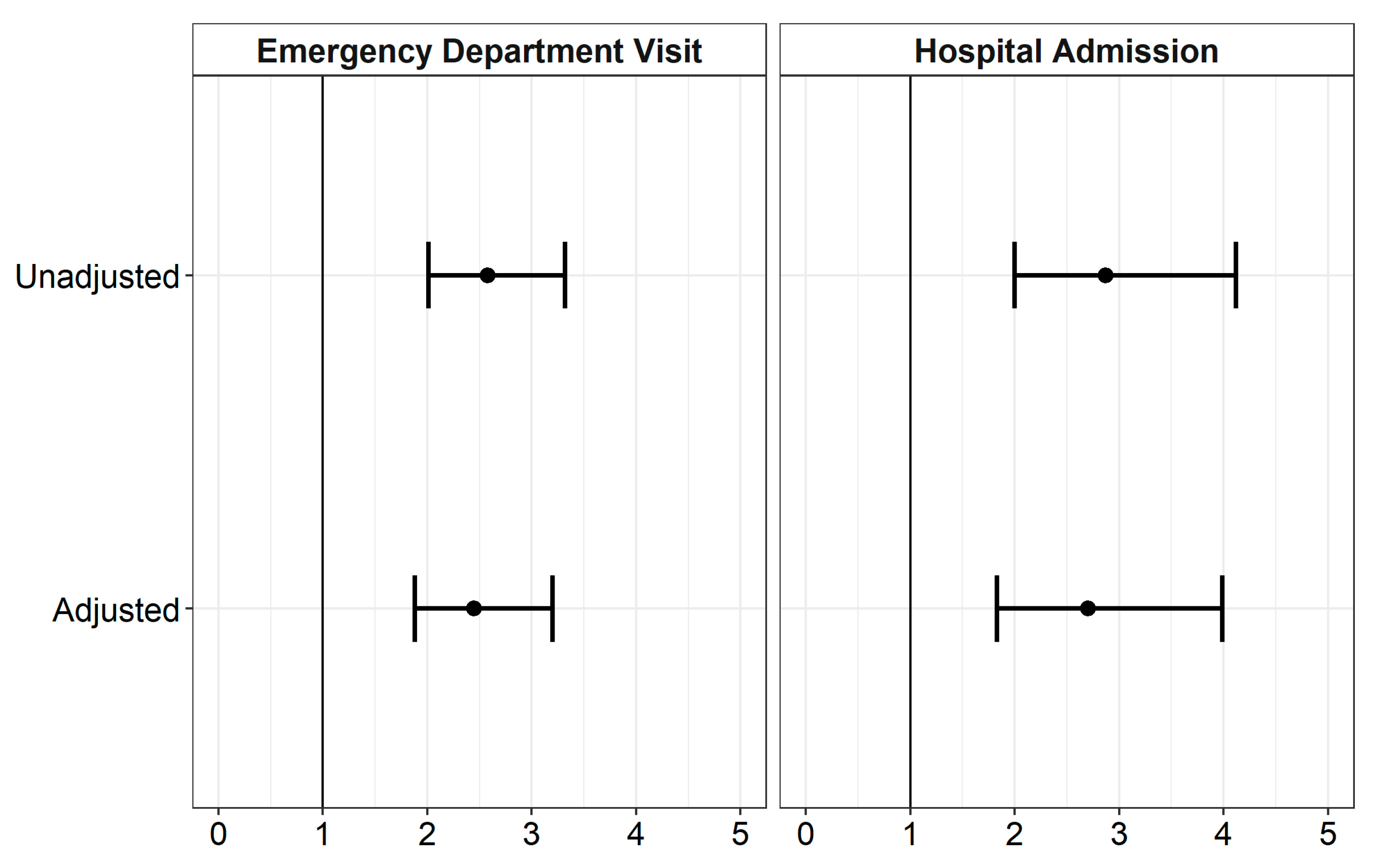
| Outcome | Unadjusted (95% CI) | Adjusted (95% CI) |
|---|---|---|
| Emergency Department Visit | 2.58 (2.01, 3.32) | 2.45 (1.88, 3.20) |
| Hospital Admissions | 2.87 (2.00, 4.12) | 2.70 (1.83, 3.99) |
| CI: Confidence Interval | ||
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Determination that a Public Health Emergency Exists (hhs.gov). Available online: https://aspr.hhs.gov/legal/PHE/Pages/2019-nCoV.aspx (accessed on 14 December 2022).
- U.S. Department of Health and Human Services. Notice of Emergency Use Authorization Declaration. (fda.gov). Emergency Use Authorization|FDA. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization (accessed on 14 December 2022).
- U.S. Food and Drug administration. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment (fda.gov). Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment (accessed on 16 March 2023).
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19.(fda.gov). Available online: www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 (accessed on 16 March 2023).
- U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers. Emergency Use Authorization (EUA) for Casirivmab and Imdevimab. Regeneron EUA HCP Fact Sheet 01242022 (fda.gov). Available online: https://www.fda.gov/media/145611/download (accessed on 14 December 2022).
- U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers. Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. Available online: https://www.fda.gov/media/145802/download (accessed on 14 December 2022).
- U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers. Emergency Use Authorization (EUA) of Sotrovimab. Available online: https://www.fda.gov/media/149534/download (accessed on 14 December 2022).
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Xiao, J.; Hooper, A.T.; Hamilton, J.D.; Musser, B.J.; et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N. Engl. J. Med. 2021, 385, e81. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Bierle, D.M.; Ganesh, R.; Razonable, R.R. Breakthrough COVID-19 and casirivimab-imdevimab treatment during a SARS-CoV-2 B1. 617.2 (Delta) surge. J. Clin. Virol. 2021, 145, 105026. [Google Scholar] [CrossRef]
- Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (accessed on 14 December 2022).
- Food and Drug Administration. Moderna COVID-19 Vaccine Emergency Use Authorization; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/Moderna-covid-19-vaccine (accessed on 14 December 2022).
- Food and Drug Administration. Janssen COVID-19 Vaccine Emergency Use Authorization; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2021. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/Janssen-covid-19-vaccine (accessed on 14 December 2022).
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Patel, M.M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; Gaglani, M.; McNeal, T.; et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin. Infect. Dis. 2022, 74, 1515–1524. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Naioti, E.A.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March–July 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1156. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Tsutsui, T.; Kakizaki, Y.; Miyashita, Y.; Iwase, F.; Maejima, M.; Sugiura, H.; Mochizuki, H.; Omata, M. Active immunization by COVID-19 mRNA vaccine results in rapid antibody response and virus reduction in breakthrough infection by Delta (B. 1.617. 2). Res. Sq. 2021. [Google Scholar] [CrossRef]
- Bierle, D.M.; Ganesh, R.; Tulledge-Scheitel, S.; Hanson, S.N.; Arndt, L.L.; Wilker, C.G.; Razonable, R.R. Monoclonal antibody treatment of breakthrough COVID-19 in fully vaccinated individuals with high-risk comorbidities. J. Infect. Dis. 2022, 225, 598–602. [Google Scholar] [CrossRef]
- Zitek, T.; Jodoin, K.; Kheradia, T.; Napolillo, R.; Dalley, M.T.; Quenzer, F.; Farcy, D.A. Vaccinated patients have reduced rates of hospitalization after receiving casirivimab and imdevimab for COVID-19. Am. J. Emerg. Med. 2022, 56, 370. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cowman, K.; Chang, M.; Bao, H.; Golia, A.; Mcsweeney, T.; Bard, L.; Simpson, R.; Andrews, E.; Pirofski, L.-A.; et al. Assessment of unvaccinated and vaccinated patients with coronavirus disease 2019 (COVID-19) treated with monoclonal antibodies during the delta wave (July 1–August 20, 2021): A retrospective observational monocentric study. BMC Infect. Dis. 2022, 22, 645. [Google Scholar] [CrossRef]
- Keshishian, E.; Kuge, E.; Memmott, J.; Hasenbalg, P.; Belford, N.; Matlock, A.; Schritter, S.; Agbayani, G.; Dietrich, T.; Santarelli, A.; et al. Casirivimab/imdevimab treatment for outpatient COVID-19 during a SARS-CoV-2 B1.617.2 (Delta) surge at a community hospital. J. Osteopath. Med. 2022, 122, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef]
- Westreich, D.; Greenland, S. The Table 2 fallacy: Presenting and interpreting confounder and modifier coefficients. Am. J. Epidemiol. 2013, 177, 292–298. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Srinivasan, V.; Weinstein, S.E.; Bhimani, A.; Clemons, N.C.; Dinolfo, M.; Shin, C.S.; Grier, J.; Lopez, A.; Braggs, J.; Boucher, J.; et al. On Variants and Vaccines: The Effectiveness of COVID-19 Monoclonal Antibody Therapy during Two Distinct Periods in the Pandemic. PLoS ONE 2022, 17, e0278394. [Google Scholar] [CrossRef]
- Douin, D.J.; Wogu, A.F.; Beaty, L.E.; Carlson, N.E.; Bennett, T.D.; Aggarwal, N.R.; Mayer, D.A.; Ong, T.C.; Russell, S.; Steele, J.; et al. Association between Treatment Failure and Hospitalization after Receipt of Neutralizing Monoclonal Antibody Treatment for COVID-19 Outpatients. BMC Infect. Dis. 2022, 22, 818. [Google Scholar] [CrossRef]
- Population and Demographics|Greater Grand Rapids. The Right Place. Available online: www.rightplace.org/why-greater-grand-rapids/talent/demographics (accessed on 25 February 2023).
- Anderson-Carpenter, K.D.; Neal, Z.P. Racial disparities in COVID-19 impacts in Michigan, USA. J. Racial Ethn. Health Disparities 2022, 9, 156–164. [Google Scholar] [CrossRef]
- Raifman, M.A.; Raifman, J.R. Disparities in the population at risk of severe illness from COVID-19 by race/ethnicity and income. Am. J. Prev. Med. 2020, 59, 137–139. [Google Scholar] [CrossRef]
- Hooper, M.W.; Nápoles, A.M.; Pérez-Stable, E.J. COVID-19 and racial/ethnic disparities. JAMA 2020, 323, 2466–2467. [Google Scholar] [CrossRef] [PubMed]
- Selden, T.M.; Berdahl, T.A. COVID-19 And Racial/Ethnic Disparities in Health Risk, Employment, and Household Composition: Study examines potential explanations for racial-ethnic disparities in COVID-19 hospitalizations and mortality. Health Aff. 2020, 39, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Elze, M.C.; Gregson, J.; Baber, U.; Williamson, E.; Sartori, S.; Mehran, R.; Nichols, M.; Stone, G.W.; Pocock, S.J. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J. Am. Coll. Cardiol. 2017, 69, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Boston, R.; Farrar, J.T.; Strom, B.L. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am. J. Epidemiol. 2003, 158, 280–287. [Google Scholar] [CrossRef] [PubMed]

| Total n = 3898 | Unvaccinated n = 2009 | Vaccinated n = 1889 | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 1692 (43.1) | 862 (42.9) | 830 (43.9) |
| Female | 2206 (56.4) | 1147 (57.0) | 1059 (56.1) |
| Age, years ^ | 55.1 ± 16.4 | 52.4 ± 16.4 | 58.0 ± 18.8 |
| Race, n (%) | |||
| Non-Hispanic Caucasian | 3525 (90.4) | 1783 (88.7) | 1742 (92.2) |
| Black or African American | 111 (2.9) | 72 (3.6) | 39 (2.1) |
| Hispanic | 99 (2.5) | 62 (3.1) | 37 (2.0) |
| Other | 74 (1.9) | 40 (2) | 34 (1.8) |
| Unknown/Missing | 89 (2.3) | 52 (2.6) | 37 (2.0) |
| Insurance, n (%) | |||
| Commercial | 2209 (56.7) | 1147 (57.1) | 1062 (56.2) |
| Medicaid | 301 (7.7) | 223 (11.1) | 78 (4.1) |
| Medicare | 1115 (28.6) | 459 (22.8) | 656 (34.7) |
| Self-pay/Other/Unknown | 273 (7.0) | 180 (9.0) | 93 (4.9) |
| Infusion Location, n (%) | |||
| Infusion Clinic | 2876 (73.4) | 1264 (62.9) | 1612 (85.3) |
| Emergency Department/Observation | 741 (19.0) | 590 (29.4) | 151 (8.0) |
| Mobile Unit/In-home | 281 (7.2) | 155 (7.7) | 126 (6.7) |
| Infusion Prior to First Symptoms *, n (%) | |||
| ≤7 Days | 3074 (78.9) | 1540 (76.7) | 1534 (81.2) |
| >7 Days | 824 (21.1) | 469 (23.3) | 355 (18.8) |
| Unvaccinated n = 2009 | Vaccinated n = 1889 | |
|---|---|---|
| Elevated BMI * (>35 kg/m2) | 1441 (71.7) | 1446 (76.5) |
| Hypertension | 527 (26.2) | 775 (41.0) |
| Smoker | 547 (27.2) | 550 (29.1) |
| Lung Disease | 336 (16.7) | 402 (21.3 |
| Cardiovascular Disease | 243 (12.1) | 380 (20.1) |
| Diabetes | 222 (11.1) | 324 (17.2) |
| Cancer | 118 (5.9) | 200 (13.8) |
| Immunosuppression | 114 (7.2) | 147 (7.8) |
| Chronic Kidney Disease | 70 (3.5) | 119 (6.3) |
| Neurological Condition | 67 (3.3) | 79 (4.2) |
| Pregnancy | 33 (1.6) | 13 (0.7) |
| Transplant | 9 (0.4) | 27 (1.4) |
| Other | 34 (1.7) | 45 (2.4) |
| Number of Comorbidities per Patient ^ | ||
| One | 940 (46.8) | 560 (29.6) |
| Two | 569 (28.3) | 566 (30.0) |
| Three | 298 (14.8) | 404 (21.4) |
| Four or more | 202 (10.1) | 359 (19.0) |
| Total n = 3898 | Unvaccinated n = 2009 | Vaccinated n = 1889 | p-Value | |
|---|---|---|---|---|
| Hospital Admissions | 154 (4.0) | 116 (5.8) | 38 (2.0) | <0.0001 |
| Emergency Department Visit | 296 (7.6) | 217 (10.8) | 79 (4.2) | <0.0001 |
| Progression to Severe Disease | 25 (0.6) | 19 (0.9) | 6 (0.3) | 0.016 |
| ICU Admission | 18 (0.46) | 16 (0.8) | 2 (0.1) | 0.015 |
| 30-Day Mortality | 12 (0.3) | 8 (0.4) | 4 (0.2) | 0.2543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeunovic, G.; Polega, J.; Toor, S.; Andersen, N.J. Retrospective Analysis of Vaccinated and Unvaccinated COVID-19 Patients Treated with Monoclonal Antibodies (mAb) and Their Emergent Needs (RAVEN). Vaccines 2023, 11, 688. https://doi.org/10.3390/vaccines11030688
Simeunovic G, Polega J, Toor S, Andersen NJ. Retrospective Analysis of Vaccinated and Unvaccinated COVID-19 Patients Treated with Monoclonal Antibodies (mAb) and Their Emergent Needs (RAVEN). Vaccines. 2023; 11(3):688. https://doi.org/10.3390/vaccines11030688
Chicago/Turabian StyleSimeunovic, Gordana, James Polega, Subhan Toor, and Nicholas J. Andersen. 2023. "Retrospective Analysis of Vaccinated and Unvaccinated COVID-19 Patients Treated with Monoclonal Antibodies (mAb) and Their Emergent Needs (RAVEN)" Vaccines 11, no. 3: 688. https://doi.org/10.3390/vaccines11030688
APA StyleSimeunovic, G., Polega, J., Toor, S., & Andersen, N. J. (2023). Retrospective Analysis of Vaccinated and Unvaccinated COVID-19 Patients Treated with Monoclonal Antibodies (mAb) and Their Emergent Needs (RAVEN). Vaccines, 11(3), 688. https://doi.org/10.3390/vaccines11030688







