The First Assessments of Pediatric HBV Immunization Coverage in Mauritania and Persistence of Antibody Titers Post Infant Immunizations
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of Vaccine Coverage
2.2. Study Population
2.3. Sampling
2.4. Hepatitis B Virus Serology
2.5. Data Analysis
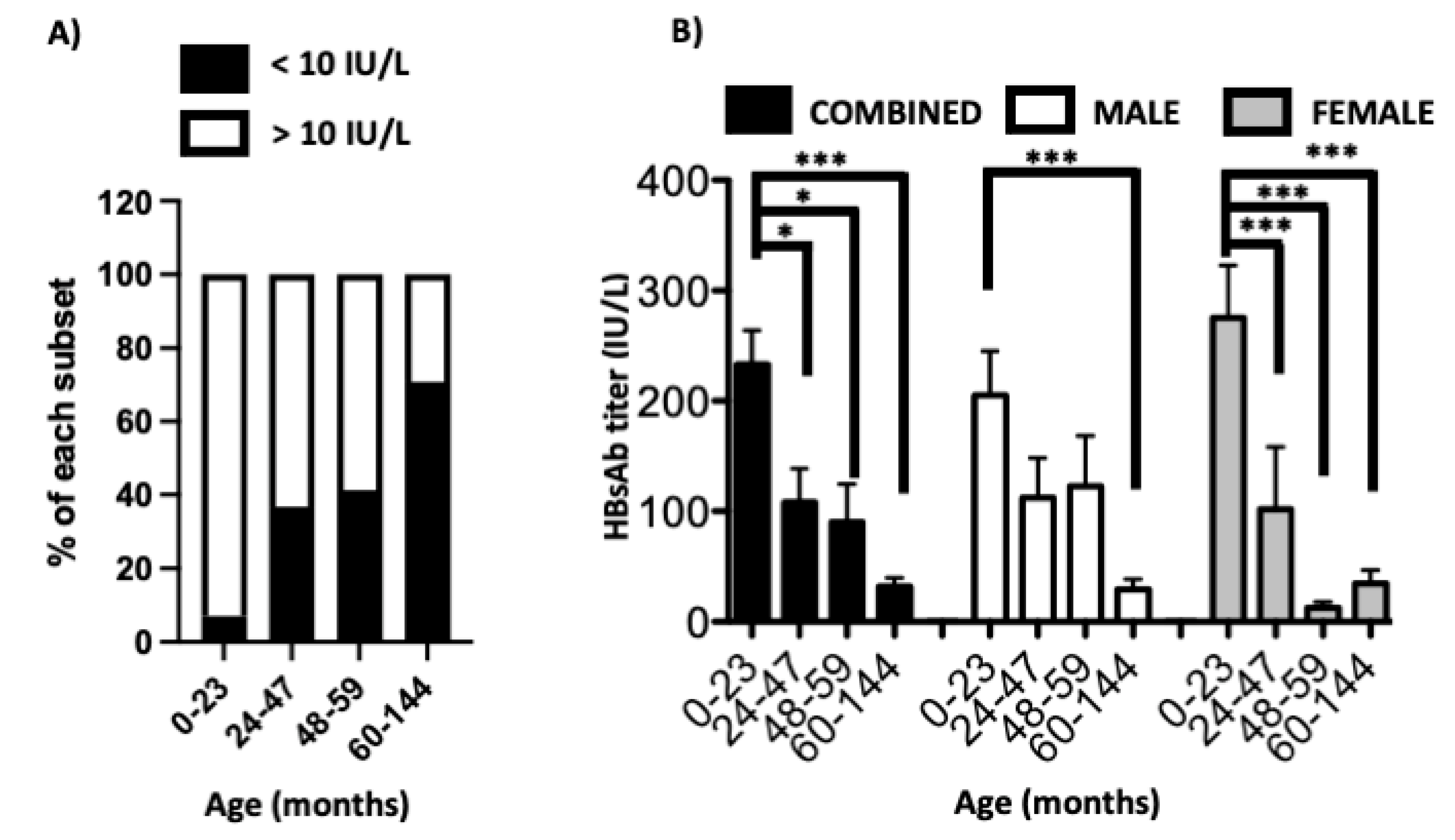
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nshimirimana, D.; Mihigo, R.; Clements, C.J. Routine immunization services in Africa: Back to basics. J. Vaccines Immun. 2013, 1, 6–12. [Google Scholar]
- Maman, K.; Zöllner, Y.; Greco, D.; Duru, G.; Sendyona, S.; Remy, V. The value of childhood combination vaccines: From beliefs to evidence. Hum. Vaccines Immunother. 2015, 11, 2132–2141. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S.; Buchanan, R.M.; Babiuk, L.A.; Gerdts, V. Maternal immunity provides protection against pertussis in newborn piglets. Infect. Immun. 2006, 74, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection; WHO: Geneva, Switzerland, 2015; pp. 1–166. [Google Scholar]
- Indolfi, G.; Easterbrook, P.; Dusheiko, G.; Siberry, G.; Chang, M.-H.; Thorne, C.; Bulterys, M.; Chan, P.-L.; El-Sayed, M.H.; Giaquinto, C.; et al. Hepatitis B Virus Infection in Children and Adolescents. Lancet Gastroenterol. Hepatol. 2019, 4, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Armstrong, G.L.; Hu, D.; Wasley, A.; Painter, J.A. The Increasing Burden of Imported Chronic Hepatitis B—United States, 1974–2008. PLoS ONE 2011, 6, e27717. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for the Western Pacific. Expert Consultation on Triple Elimination of Mother-to-Child Transmission of HIV, Hepatitis B and Syphilis in the Western Pacific, Manila, Philippines, 20–21 February 2017; Meeting Report; WHO Regional Office for the Western Pacific: Manila, India, 2017; Available online: http://iris.wpro.who.int/handle/10665.1/1359415 (accessed on 26 May 2022).
- World Health Organization. Hepatitis B vaccines: WHO position paper, July 2017—Recommendations. Vaccine 2019, 37, 223–225. [Google Scholar] [CrossRef]
- Dziuban, E.; Marton, S.; Hughey, A.; Mbingo, T.L.; Draper, H.; Schutze, G. Seroprevalence of hepatitis B in a cohort of HIV-infected children and adults in Swaziland. Int. J. STD AIDS 2013, 24, 561–565. [Google Scholar] [CrossRef]
- Mazahi, M.M.E.; Maksoud, H.M.A.; Hady, M.A.; Ali, M.A.; Nawawy, A.N.E.; Ahmad, S.M.A. Long Term Immunity to Hepatitis B Vaccine Among a Sample of Secondary School Students in Damietta. J. Pharmacol. Toxicol. 2015, 11, 27–32. [Google Scholar] [CrossRef]
- Razavi-Shearer, D.; Gamkrelidze, I.; Nguyen, M.H.; Chen, D.-S.; Van Damme, P.; Abbas, Z.; Abdulla, M.; Abou Rached, A.; Adda, D.; Aho, I.; et al. Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, E.H.; Park, S.E.; Kim, Y.J.; Jo, D.S.; Kim, Y.K.; Eun, B.W.; Lee, J.; Lee, S.Y.; Lee, H.; et al. Recommended immunization schedule for children and adolescents: Immunization Guideline (8th edition) released by the Korean Pediatric Society in 2015. Korean J. Pediatr. 2016, 59, 461–465. [Google Scholar] [CrossRef]
- Thio, C.L.; Guo, N.; Xie, C.; Nelson, K.E.; Ehrhardt, S. Global elimination of mother-to-child transmission of hepatitis B: Revisiting the current strategy. Lancet Infect. Dis. 2015, 15, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.P.; Zeng, Y.L.; Zhou, M.; Chen, L.L.; Hu, R.; Wang, L.; Tang, H. Factors Associated with Mother-to-child Transmission of Hepatitis B Virus Despite Immunoprophylaxis. Intern. Med. 2015, 54, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Pulcini, C.; Massin, S.; Launay, O.; Verger, P. Factors associated with vaccination for hepatitis B, pertussis, seasonal and pandemic influenza among French general practitioners: A 2010 survey. Vaccine 2013, 31, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Zingone, F.; Morisco, F.; Zanetti, A.; Romanò, L.; Portella, G.; Capone, P.; Andreozzi, R.T.; Ciacci, C. Long-term antibody persistence and immune memory to hepatitis B virus in adult celiac patients vaccinated as adolescents. Vaccine 2011, 29, 1005–1008. [Google Scholar] [CrossRef]
- Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy. Available online: https://www.who.int/publications-detail-redirect/978-92-4-000270-8 (accessed on 23 October 2022).
- World Health Organization. GHO | By Category | Hepatitis B (HepB3)—Immunization Coverage Estimates by Country. Available online: https://apps.who.int/gho/data/view.main.80300 (accessed on 15 May 2022).
- Mahmud, S.; Gulshan, J.; Tasneem, F.; Ahmed, S.S. Seroconversion of Hepatitis B Vaccine in Young Bangladeshi Children: A Tertiary Centre Experience. World J. Vaccines 2021, 11, 7–18. [Google Scholar] [CrossRef]
- Perveen, I.; Raza, M.A.; Zaidi, S.S.Z.; Ahmed, S. Hepatitis B Seroconversion after Vaccination in Infants in Rural and Urban Areas of Rawalpindi, Pakistan. J. Adv. Med. Med. Res. 2015, 5, 1557–1561. [Google Scholar] [CrossRef]
- Available online: https://pdf.usaid.gov/pdf_docs/PA00Z537.pdf (accessed on 5 June 2022).
- Rui, W.Z.; Lo, B.B.; N’Diaye, M. Hepatitis B virus infection in the school milieu of Kiffa and Selibaby, Mauritania. Bull. Soc. Pathol. Exot. 1998, 91, 247–248. [Google Scholar]
- Lô, G.; Sow-Sall, A.; Diop-Ndiaye, H.; Babacar, N.; Diouf, N.N.; Daffé, S.M.; Ndao, B.; Thiam, M.; Mbow, M.; Lemoine, M.; et al. Hepatitis B virus (HBV) infection amongst children in Senegal: Current prevalence and seroprotection level. Pan. Afr. Med. J. 2019, 32, 140. Available online: http://www.panafrican-med-journal.com/content/article/32/140/full/ (accessed on 5 June 2022). [CrossRef]
- Chen, D.S. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J. Hepatol. 2009, 50, 805–816. [Google Scholar] [CrossRef]
- Utsumi, T.; Yano, Y.; Lusida, M.I.; Amin, M.; Hotta, H.; Hayashi, Y. Serologic and Molecular Characteristics of Hepatitis B Virus among School Children in East Java, Indonesia. Am. J. Trop. Med. Hyg. 2010, 83, 189–193. [Google Scholar] [CrossRef]
- Périères, L.; Protopopescu, C.; Lo, G.; Marcellin, F.; Ba, E.; Coste, M.; Touré Kane, C.; Diallo, A.; Sokhna, C.; Boyer, S. Sibling status, home birth, tattoos and stitches are risk factors for chronic hepatitis B virus infection in Senegalese children: A cross-sectional survey. J. Viral. Hepat. 2021, 28, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Seyed Alinaghi, S.; Karimi, A.; Mojdeganlou, H.; Alilou, S.; Mirghaderi, S.P.; Noori, T.; Shamsabadi, A.; Dadras, O.; Vahedi, F.; Mohammadi, P. Impact of COVID-19 pandemic on routine vaccination coverage of children and adolescents: A systematic review. Health Sci. Rep. 2022, 5, e00516. [Google Scholar] [PubMed]
- Mahmood, S.; Shah, K.U.; Khan, T.M. Immune Persistence After Infant Hepatitis-B Vaccination: A Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12550. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Alam, A.N.; Siddiqua, M.; Siddika, A.; Nessa, A. Immune response among the children to hepatitis B vaccination: A community-based study in Bangladesh. Bangladesh Med. Res. Counc. Bull. 2018, 44, 103–108. [Google Scholar] [CrossRef]
- Freitas da Motta, M.S.; Mussi-Pinhata, M.M.; Jorge, S.M.; Yoshida, C.F.T.; Sandoval de Souza, C.B. Immunogenicity of Hepatitis B vaccine in preterm and full term infants vaccinated within the first week of life. Vaccine 2002, 20, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Perera, J.; Perera, B.; Gamage, S. Seroconversion after hepatitis B vaccination in healthy young adults, and the effect of a booster dose. Ceylon Med. J. 2014, 47, 6. [Google Scholar] [CrossRef]
- Dassah, S.; Sakyi, S.A.; Frempong, M.T.; Luuse, A.T.; Ephraim, R.K.; Anto, E.O.; Oduro, A. Seroconversion of hepatitis B vaccine in young children in the Kassena Nankana district of Ghana: A cross-sectional study. PLoS ONE 2015, 10, e0145209. [Google Scholar] [CrossRef]
- Sanou, A.M.; Ilboudo, A.K.; Meda, C.Z.; Togozia, A.; Coulibaly, A.; Cisse, A.; Sagna, T.; Kania, D.; Tarnagda, Z. Hepatitis B vaccination in Burkina Faso: Prevalence of HBsAg carriage and immune response in children in the western region. J. Infect. Dev. Ctries. 2018, 12, 1002–1008. [Google Scholar] [CrossRef]
- Lee, K.H.; Shim, K.S.; Lim, I.S.; Chae, S.A.; Yun, S.W.; Lee, N.M.; Choi, Y.B.; Yi, D.Y. Changes in hepatitis B virus antibody titers over time among children: A single center study from 2012 to 2015 in an urban of South Korea. BMC Pediatr. 2017, 17, 164. [Google Scholar] [CrossRef]
- Alssamei, F.A.A.; Al-Sonboli, N.A.; Alkumaim, F.A.; Alsayaad, N.S.; Al-Ahdal, M.S.; Higazi, T.B.; Elagib, A.A. Assessment of Immunization to Hepatitis B Vaccine among Children under Five Years in Rural Areas of Taiz, Yemen. Hepat. Res. Treat. 2017, 2017, 2131627. [Google Scholar] [CrossRef]
- Yue, X.; Ge, C.; Zhuge, S.; He, H.; Yang, H.; Xu, H.; Huang, A.; Zhao, Y. Changes and analysis of anti-HBs titres after primary immunization in 1- to 16-year-old Chinese children: A hospital-based study. J. Viral Hepat. 2018, 25, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Qawasmi, M.; Samuh, M.; Glebe, D.; Gerlich, W.H.; Azzeh, M. Age-dependent decrease of anti-HBs titers and effect of booster doses using 2 different vaccines in Palestinian children vaccinated in early childhood. Hum. Vaccines Immunother. 2015, 11, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Sami, S.; Salama, I.; Abdel-Latif, G.; Etreby, L.; Metwally, A.; Abd, N. Hepatitis B Seroprotection and the Response to a Challenging Dose among Vaccinated Children in Red Sea Governorate. Open Access Maced. J. Med. Sci. 2016, 4, 219–225. [Google Scholar]
- Leuridan, E.; van Damme, P. Hepatitis B and the Need for a Booster Dose. Clin. Infect. Dis. 2011, 53, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.G.; Bruden, D.; Hurlburt, D.; Zanis, C.; Thompson, G.; Rea, L.; Toomey, M.; Townshend-Bulson, L.; Rudolph, K.; Bulkow, L.; et al. Antibody levels and protection after hepatitis B vaccine: Results of a 30-year follow-up study and response to a booster dose. J. Infect. Dis. 2016, 214, 16–22. [Google Scholar] [CrossRef]
- Qu, C.; Chen, T.; Fan, C.; Zhan, Q.; Wang, Y.; Lu, J.; Lu, L.-L.; Ni, Z.; Huang, F.; Yao, H.; et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: A cluster randomized controlled trial. PLoS Med. 2014, 11, e1001774. [Google Scholar] [CrossRef]
- Lin, A.W.C.; Wong, K.H. Long-term protection of neonatal hepatitis B vaccination in a 30-year cohort in Hong Kong. J. Hepatol. 2013, 59, 1363–1364. [Google Scholar] [CrossRef]
- Wijaya, R.S.; Read, S.A.; Truong, N.R.; Han, S.; Chen, D.; Shahidipour, H.; Fewings, N.L.; Schibeci, S.; Azardaryany, M.K.; Parnell, G.P.; et al. HBV vaccination and HBV infection induces HBV-specific natural killer cell memory. Gut 2021, 70, 369–375. [Google Scholar] [CrossRef]
- Lee, G.-H.; Lim, S.-G. CpG-Adjuvanted Hepatitis B Vaccine (HEPLISAV-B®) Update. Expert Rev. Vaccines 2021, 20, 487–495. [Google Scholar] [CrossRef]
- Querec, T.; Bennouna, S.; Alkan, S.; Laouar, Y.; Gorden, K.; Flavell, R.; Akira, S.; Ahmed, R.; Pulendran, B. Yellow Fever Vaccine YF-17D Activates Multiple Dendritic Cell Subsets via TLR2, 7, 8, and 9 to Stimulate Polyvalent Immunity. J. Exp. Med. 2006, 203, 413–424. [Google Scholar] [CrossRef]
- Jamshidi, E.; Asgary, A.; Shafiekhani, P.; Khajeamiri, Y.; Mohamed, K.; Esmaily, H.; Jamal Rahi, S.; Mansouri, N. Longevity of Immunity Following COVID-19 Vaccination: A Comprehensive Review of the Currently Approved Vaccines. Hum. Vaccines Immunother. 2022, 18, 2037384. [Google Scholar] [CrossRef] [PubMed]
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|
| Nouakchott North | ||||||
| Total population | 399,368 | 413,194 | 427,531 | 442,363 | 457,735 | 473,654 |
| Estimated number of children < 1 year | 14,377 | 14,875 | 15,391 | 15,925 | 16,478 | 17,052 |
| Number of fully vaccinated children | 10,064 | 11,751 | 12,621 | 11,785 | 13,018 | 11,595 |
| Estimated proportion of fully immunized children | 70% | 79% | 82% | 74% | 79% | 68% |
| Nouakchott West | ||||||
| Total population | 180,482 | 186,730 | 193,209 | 199,912 | 206,859 | 214,053 |
| Estimated number of children < 1 year | 6497 | 6722 | 6956 | 7197 | 7447 | 7706 |
| Number of fully vaccinated children | 6722 | 8806 | 8347 | 8708 | 8341 | 7629 |
| Estimated proportion of fully immunized children | 103% | 131% | 120% | 121% | 112% | 99% |
| Nouakchott South | ||||||
| Total population | 463,327 | 479,367 | 496,000 | 513,208 | 531,042 | 549,510 |
| Estimated number of children < 1 year | 16,680 | 17,257 | 17,856 | 18,475 | 19,118 | 19,782 |
| Number of fully vaccinated children | 11,342 | 11,735 | 12,677.755 | 12,748 | 13,573 | 12,265 |
| Estimated proportion of fully immunized children | 68% | 68% | 71% | 69% | 71% | 62% |
| Mauritania | ||||||
| Total population | 3,720,125 | 3,805,659 | 3,893,775 | 3,984,233 | 4,077,347 | 4,173,077 |
| Estimated number of children < 1 year | 132,719 | 135,769 | 138,912 | 142,137 | 145,458 | 148,872 |
| Number of fully vaccinated children | 96,885 | 116,762 | 123,631 | 120,817 | 129,457 | 120,586 |
| Estimated proportion of fully immunized children | 73% | 86% | 89% | 85% | 89% | 81% |
| Age Group [Months] | N, Frequency [%] | Sex [n, % of Female] |
|---|---|---|
| 0–23 | 42 [22.7] | 17 [40.5] |
| 24–47 | 30 [16.2] | 11 [36.7] |
| 48–59 | 17 [9.2] | 5 [29.4] |
| 60–144 | 96 [51.9] | 50 [52.1] |
| Total | 185 [100] | 83 [44.9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hachimi, H.; El Alem, M.M.M.; Haimoudane, E.; Yebouk, C.; Pedersen, J.; Fall-Malick, F.-Z.; Khiddi, F.; Abdawe, M.; Sadegh, S.A.; Fausther-Bovendo, H.; et al. The First Assessments of Pediatric HBV Immunization Coverage in Mauritania and Persistence of Antibody Titers Post Infant Immunizations. Vaccines 2023, 11, 588. https://doi.org/10.3390/vaccines11030588
El Hachimi H, El Alem MMM, Haimoudane E, Yebouk C, Pedersen J, Fall-Malick F-Z, Khiddi F, Abdawe M, Sadegh SA, Fausther-Bovendo H, et al. The First Assessments of Pediatric HBV Immunization Coverage in Mauritania and Persistence of Antibody Titers Post Infant Immunizations. Vaccines. 2023; 11(3):588. https://doi.org/10.3390/vaccines11030588
Chicago/Turabian StyleEl Hachimi, Hala, Mohamed Mahmoud Mohamed El Alem, Esma Haimoudane, Cheikh Yebouk, Jannie Pedersen, F-Zahra Fall-Malick, Fatimetou Khiddi, Mohamed Abdawe, Sidi Ahmed Sadegh, Hugues Fausther-Bovendo, and et al. 2023. "The First Assessments of Pediatric HBV Immunization Coverage in Mauritania and Persistence of Antibody Titers Post Infant Immunizations" Vaccines 11, no. 3: 588. https://doi.org/10.3390/vaccines11030588
APA StyleEl Hachimi, H., El Alem, M. M. M., Haimoudane, E., Yebouk, C., Pedersen, J., Fall-Malick, F.-Z., Khiddi, F., Abdawe, M., Sadegh, S. A., Fausther-Bovendo, H., & Mohamed Abdellahi, M. V. (2023). The First Assessments of Pediatric HBV Immunization Coverage in Mauritania and Persistence of Antibody Titers Post Infant Immunizations. Vaccines, 11(3), 588. https://doi.org/10.3390/vaccines11030588








