Encephalitis following COVID-19 Vaccination: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Database Search
2.2. Screening and Inclusion Criteria
2.3. Statistical Analysis
2.4. Quality Assessment
3. Results
3.1. Patient Characteristics
3.2. Clinical Presentation
3.3. Investigations and Diagnostic Results
3.4. Treatment Plan and Its Outcomes
3.5. Quality Assessment
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granerod, J.; Ambrose, H.E.; Davies, N.W.S.; Clewley, J.P.; Walsh, A.L.; Morgan, D.; Cunningham, R.; Zuckerman, M.; Mutton, K.J.; Solomon, T.; et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect. Dis. 2010, 10, 835–844. [Google Scholar] [CrossRef]
- Ellul, M.; Solomon, T. Acute encephalitis—Diagnosis and management. Clin. Med. 2018, 18, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?adgroupsurvey={adgroupsurvey}&gclid=Cj0KCQiAiJSeBhCCARIsAHnAzT88OV4oFdsSZEUH8dm2dTrqizrL4qFUaSGociASz3fTLjYEhyp3vw0aAmayEALw_wcB (accessed on 12 November 2022).
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Abufares, H.I.; Oyoun Alsoud, L.; Alqudah, M.A.Y.; Shara, M.; Soares, N.C.; Alzoubi, K.H.; El-Huneidi, W.; Bustanji, Y.; Soliman, S.S.M.; Semreen, M.H.; et al. COVID-19 Vaccines, Effectiveness, and Immune Responses. Int. J. Mol. Sci. 2022, 23, 15415. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021, 36, 427–439. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2022, 43, 3–40. [Google Scholar] [CrossRef]
- Meppiel, E.; Peiffer-Smadja, N.; Maury, A.; Bekri, I.; Delorme, C.; Desestret, V.; Gorza, L.; Hautecloque-Raysz, G.; Landre, S.; Lannuzel, A.; et al. Neurologic manifestations associated with COVID-19: A multicentre registry. Clin. Microbiol. Infect. 2021, 27, 458–466. [Google Scholar] [CrossRef]
- Vences, M.A.; Canales, D.; Albujar, M.F.; Barja, E.; Araujo-Chumacero, M.M.; Cardenas, E.; Alvarez, A.; Urrunaga-Pastor, D. Post-Vaccinal Encephalitis with Early Relapse after BNT162b2 (COMIRNATY) COVID-19 Vaccine: A Case Report. Vaccines 2022, 10, 1065. [Google Scholar] [CrossRef]
- Gao, J.-J.; Tseng, H.-P.; Lin, C.-L.; Hsu, R.-F.; Lee, M.-H.; Liu, C.-H. Acute encephalitis after COVID-19 vaccination: A case report and literature review. Hum. Vaccines Immunother. 2022, 18, 2082206. [Google Scholar] [CrossRef] [PubMed]
- Shyu, S.; Fan, H.-T.; Shang, S.-T.; Chan, J.-S.; Chiang, W.-F.; Chiu, C.-C.; Chen, M.-H.; Shyu, H.-Y.; Hsiao, P.-J. Clinical Manifestation, Management, and Outcomes in Patients with COVID-19 Vaccine-Induced Acute En-cephalitis: Two Case Reports and a Literature Review. Vaccines 2022, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, Y.; Gadoth, A.; Abu-Salameh, I.; Horev, A.; Novoa, R.; Ifergane, G. Case Report: Anti-LGI1 Encephalitis Following COVID-19 Vaccination. Front. Immunol. 2021, 12, 813487. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Purkayastha, B.; Alam, M.M.J.; Chakraborty, S.R.; Roy, S.; Ahmed, N. COVID-19 mRNA vaccine-associated encephalopathy, myocarditis, and thrombocytopenia with ex-cellent response to methylprednisolone: A case report. J. Neuroimmunol. 2022, 368, 577883. [Google Scholar] [CrossRef]
- Nagaratnam, S.A.; Ferdi, A.C.; Leaney, J.; Lee, R.L.K.; Hwang, Y.T.; Heard, R. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol. 2022, 22, 54. [Google Scholar] [CrossRef]
- Rinaldi, V.; Bellucci, G.; Romano, A.; Bozzao, A.; Salvetti, M. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Mult. Scler. J. 2022, 28, 1151–1154. [Google Scholar] [CrossRef]
- Permezel, F.; Borojevic, B.; Lau, S.; de Boer, H.H. Acute disseminated encephalomyelitis (ADEM) following recent Ox-ford/AstraZeneca COVID-19 vaccination. Forensic Sci. Med. Pathol. 2022, 18, 74–79. [Google Scholar] [CrossRef]
- Al-Quliti, K.; Qureshi, A.; Quadri, M.; Abdulhameed, B.; Alanazi, A.; Alhujeily, R. Acute Demyelinating Encephalomyelitis Post-COVID-19 Vaccination: A Case Report and Literature Review. Diseases 2022, 10, 13. [Google Scholar] [CrossRef]
- Lazaro, L.G.; Cossio, J.E.P.; Luis, M.B.; Tamagnini, F.; Mejia, D.A.P.; Solarz, H.; Liguori, N.A.F.; Alonso, R.N. Acute disseminated encephalomyelitis following vaccination against SARS-CoV-2: A case report. Brain Behav. Immun. Health 2022, 20, 100439. [Google Scholar] [CrossRef]
- Yazdanpanah, F.; Iranpour, P.; Haseli, S.; Poursadeghfard, M.; Yarmahmoodi, F. Acute disseminated encephalomyelitis (ADEM) after SARS-CoV-2 vaccination: A case report. Radiol. Case Rep. 2022, 17, 1789–1793. [Google Scholar] [CrossRef]
- Cao, L.; Ren, L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. Acta Neurol. Belg. 2022, 122, 793–795. [Google Scholar] [CrossRef]
- Dutta, S.; Kaur, R.; Charan, J.; Bhardwaj, P.; Ambwani, S.R.; Babu, S.; Goyal, J.P.; Haque, M. Analysis of Neurological Adverse Events Reported in VigiBase From COVID-19 Vaccines. Cureus 2022, 14, e21376. [Google Scholar] [CrossRef] [PubMed]
- Zuhorn, F.; Graf, T.; Klingebiel, R.; Schäbitz, W.; Rogalewski, A. Postvaccinal Encephalitis after ChAdOx1 nCov-19. Ann. Neurol. 2021, 90, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-T.; Lin, Y.-Y.; Chiang, W.-F.; Lin, C.-Y.; Chen, M.-H.; Wu, K.-A.; Chan, J.-S.; Kao, Y.-H.; Shyu, H.-Y.; Hsiao, P.-J. COVID-19 vaccine-induced encephalitis and status epilepticus. Qjm Int. J. Med. 2022, 115, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Al-Mashdali, A.F.; Ata, Y.M.; Sadik, N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: A case report. Ann. Med. Surg. 2021, 69, 102803. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Rabin, K.; Ratzan, S.C.; Leigh, J.P.; Hu, J.; El-Mohandes, A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 2022, 13, 3801. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Venkatesan, A.; Tunkel, A.R.; Bloch, K.C.; Lauring, A.S.; Sejvar, J.; Bitnun, A.; Stahl, J.-P.; Mailles, A.; Drebot, M.; Rupprecht, C.E.; et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: Consensus statement of the international encephalitis consortium. Clin. Infect. Dis. 2013, 57, 1114–1128. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Ho, T.-C.; Chang, C.-C.; Shen, D.H.-Y.; Chan, H.-P.; Chuang, K.-P.; Tyan, Y.-C.; Yang, M.-H. A Rare Adverse Effect of the COVID-19 Vaccine on Autoimmune Encephalitis. Vaccines 2022, 10, 1114. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reac-togenicity. Npj Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.; Shrestha, A.K.; Colantonio, M.A.; Liberio, R.N.; Sriwastava, S. Acute transverse myelitis following SARS-CoV-2 vaccination: A case report and review of literature. J. Neurol. 2022, 269, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Maramattom, B.V.; Lotlikar, R.S.; Sukumaran, S. Central nervous system adverse events after ChAdOx1 vaccination. Neurol. Sci. 2022, 43, 3503–3507. [Google Scholar] [CrossRef]
- Liotta, E.M.; Batra, A.; Clark, J.R.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Koralnik, I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in COVID-19 patients. Ann. Clin. Transl. Neurol. 2020, 7, 2221–2230. [Google Scholar] [CrossRef]

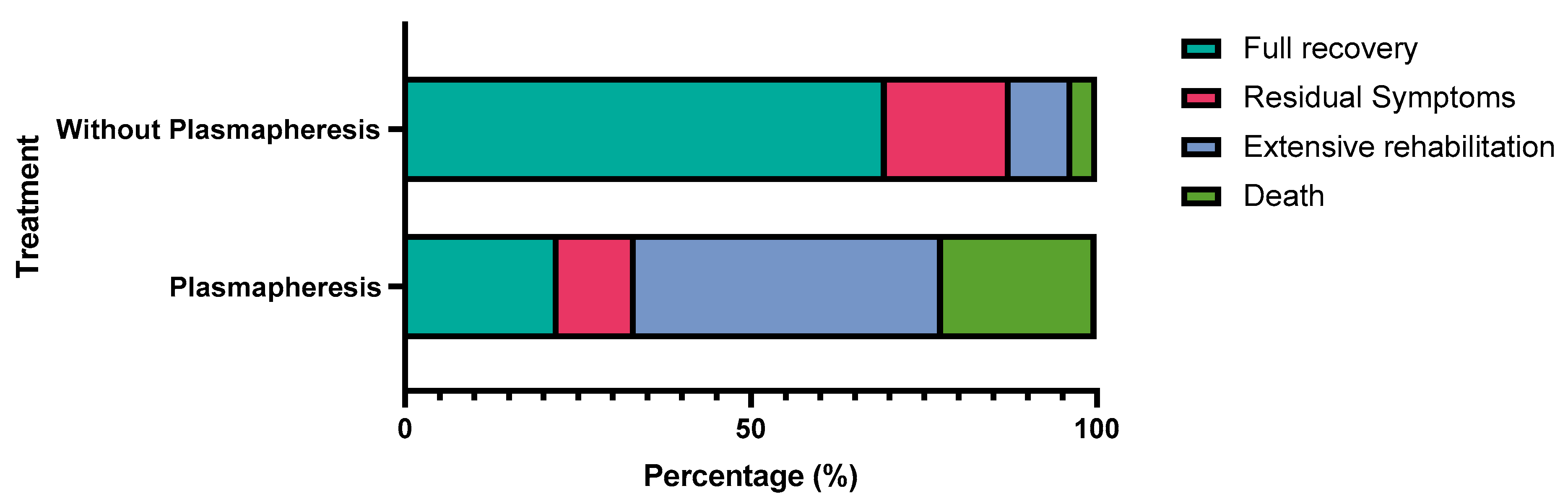
| No. | Author | Vaccine | Age/Sex | Type of Encephalitis | Onset Time (Days) | Dose | Clinical Features | Comorbidities | Other Complaints | Neuroimaging and CSF Analysis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ahmed et al. | Pfizer BioNTech | 61/F | ADEM | 7 | 1 | Progressive generalized weakness and difficulty with communication | Hypertension | - | MRI: nonspecific acute versus subacute leukoencephalopathy involving the brainstem and deep white matter | Methylprednisolone, IVIG | Full recovery |
| 2 | Ahmed et al. | Pfizer BioNTech | 62/F | Meningoencephalitis | 5 | 2 | Headache, fever, and rigor for 4 days, inability to stand up and walk, did not obey commands | Ceftriaxone allergy | - | CSF: high protein, pleocytosis | Acyclovir | Full recovery |
| 3 | Ahn et al. | AstraZeneca | 53/M | AE | 7 | 2 | Gait disturbance, dysarthria, cognitive decline, Hoffman sign, and ankle clonus | - | - | MRI: Increased FLAIR signal intensity in the bilateral hippocampus, Multiple enhanced lesions of the spinal cord (C6, T1 level) CSF: pleocytosis, elevated protein, oligoclonal band type 2 (+) | Acyclovir, IVIG, and rituximab | Favorable |
| 4 | Albsheer et al. | Moderna | 35/F | LE | 2 | 2 | Seizures | - | Anisocoria | CSF: pleocytosis CT: temporal lobe hypodensities ANA (+) | Steroids, IVIG, and rituximab | Responded well with no additional neurological sequelae |
| 5 | Aljamea et al. | Pfizer BioNTech | 38/M | Bickerstaff Brainstem Encephalitis | 10 | 1 | Generalized fatigue, weakness, slowing of movement, hypophonia | - | Aspiration pneumonia, constipation | CSF: elevated proteins and albumin, GD1a (+), MRI: T2 hyperintensity within the distal spinal cord/conus medullaris | IVIG, plasmapheresis, and corticosteroids | Management and rehabilitation in a long-term care facility |
| 6 | Aljamea et al. | Pfizer BioNTech | 54/M | Bickerstaff Brainstem Encephalitis | 14 | 2 | Dysphagia, altered mental status, progressive weakness, limb ataxia, disturbed conscious level | DM, hypertension | Aspiration pneumonia, ophthalmoplegia | CSF: pleocytosis, oligoclonal bands (+), GQ1b and GM1 antibodies (+) | IVIG, plasmapheresis, Rituximab | Extensive rehabilitation in a long-term care facility |
| 7 | Al-Quliti et al. | AstraZeneca | 56/F | ADEM | 10 | Generalized weakness, lower extremity myalgia, difficulty in the articulation of speech, dysmetria | - | Anorexia | MRI: T2 and FLAIR showed hyperintensities in the subcortical and deep white matter involving basal ganglia CSF: protein and glucose elevated | Hypertonic saline, methylprednisolone | Neck stiffness and bilateral-adduction-gaze deficit were resolved, as well as minimal improvement in her lower limbs’ weakness was observed, able to mobilize freely without assistance | |
| 8 | Ancau et al. | AstraZeneca | 61/M | AHEM | 4 | 1 | Fever, headache, apathy, generalized seizure, unconsciousness, bedridden, foaming around the mouth | Hypothyroidism, polymyalgia rheumatica | CT: diffuse hypodense areas in the right subcortical, frontotemporal, and right thalamic region. MRI: bilateral confluent cortical and subcortical FLAIR hyperintense lesions with hemorrhagic involvement of the basal ganglia CSF: moderate disturbance of the BBB | Methylprednisolone, plasmapheresis | Vegetative state | |
| 9 | Ancau et al. | AstraZeneca | 25/F | AHEM | 2 | 1 | Cephalgia, fatigue, lack of sensation in legs, paraplegic syndrome, absent tendon reflexes, detrusor areflexia, difficulty urinating, mild weakness, ascending numbness in legs | - | Thoracic back pain | MRI: longitudinal edema along the thoracic spinal cord with contrast enhancement, focal central hemorrhage, bi-hemispheric white matter lesions with focal contrast enhancement CSF: pleocytosis, increased albumin, intrathecal IgM synthesis | Methylprednisolone, Plasmapheresis | Persistent paraplegia |
| 10 | Ancau et al. | AstraZeneca | 55/F | AHEM | 9 | 1 | Meningism, spastic tetraparesis, coma | - | Nausea, dizziness | MRI: multiple FLAIR hyperintense hemorrhagic lesions in the right temporal and parietal lobes, bilaterally in fronto-temporal distribution and in the right occipital lobe and left fronto-basal region CSF: pleocytosis, intrathecal IgM, IgG and IgA, trans-tenetorial herniation, and hydrocephalus occlusion | Right-sided decompressive hemicraniectomy, Methylprednisolone | Death |
| 11 | Asaduzzaman et al. | Pfizer BioNTech | 15/F | Autoimmune encephalitis | 1 | 2 | Fever, agitation, altered consciousness, convulsions | - | Diarrhea, dehydration, palpitation, myocarditis | CSF: pleocytosis, raised protein level, and normal glucose level MRI: no abnormality | Acyclovir, Ceftriaxone, methylprednisolone | Full recovery after 4 weeks |
| 12 | Asioli et al. | AstraZeneca | 73/F | Anti-LGI1 encephalitis | 14 | 1 | FBDS, behavioral disturbances | - | - | CSF: Anti-LGI1 (+), EEG: Bilateral fronto-temporal sharp waves; electrographic temporal seizures, MRI: Bilateral mesial temporal lobe T2-weighted hyper-intensity with swelling in the left hippocampus | Methylprednisolone, Valproate | Seizure-free, normal mental status |
| 13 | Asioli et al. | Pfizer BioNTech | 66/M | Anti-LGI1 encephalitis | 6 | 2 | Cognitive impairment, behavioral disturbances | Hypertension | - | CSF: Anti-LGI1 (+), high protein, RBCs (+), pleocytosis MRI: Bilateral mesial temporal lobe T2-weighted hyper-intensity with swelling and contrast enhancement in the right amygdala and hippocampus, EEG: Right fronto-temporal sharp waves; electrographic temporal seizures | Methylprednisolone, Levetiracetam | Normal mental status in 7 months |
| 14 | Asioli et al. | Pfizer BioNTech | 66/M | Anti-LGI1 encephalitis | 9 | 2 | FBDS, focal seizures, behavioral disturbances | Polyallergy | Hypersomnia | CSF: Anti-LGI1 (+), EEG: Bilateral fronto-temporal epileptiform discharges | Methylprednisolone, Lacosamide | Seizure-free, normal mental status in 3 months |
| 15 | Asioli et al. | Moderna | 18/F | Anti-LGI1 encephalitis | 23 | 3 | Focal seizures, short-term memory impairment | - | - | MRI: Right fronto-temporal sharp waves, CSF: Anti-LGI1 (+) | Methylprednisolone, Lacosamide | Seizure-free, normal mental status in 3 months |
| 16 | Autjimanon et al. | Pfizer BioNTech | 14/F | Acute encephalopathy | 9 | 1 | Fever, headaches, drowsiness, tonic-clonic focal and generalized seizures, status epilepticus | - | - | ANA (+) | Anti-convulsants, immunosuppressants | Discharged home but had residual memory problems |
| 17 | Ballout et al. | Pfizer BioNTech | 27/M | Autoimmune Encephalitis | 6 | 1 | Fatigue, confusion and anxiety, headache, agitation, dysfluent speech with paraphasic errors, and difficulty with writing | - | - | CSF: Pleocytosis with lymphocytic predominance MRI: no abnormalities EEG: mild generalized slowing without epileptiform abnormalities | Methylprednisolone | Full recovery after 1 month |
| 18 | Ballout et al. | Moderna | 81/M | ADEM | 13 | 1 | Change in mental status with severe encephalopathy, fever, absent pupillary response to light, absent right corneal reflex, diffuse hypertonicity, extensor plantar responses bilaterally | - | Fatigue, myalgia | CSF: pleocytosis, elevated protein, elevated myelin basic protein (MBP) MRI: diffusion restriction in the right dorsal medulla with corresponding T2 FlAIR hyperintensity, faint T2 hyperintensities in the left pons, midbrain, and thalamus, and minimal T2 sulcal hyperintensity without contrast enhancement | Methylprednisolone, IVIG therapy, plasmapheresis | Death |
| 19 | Bastide et al. | AstraZeneca | 49/F | ADEM | 2 | 1 | Fever, flu-like symptoms, Lhermitte’s phenomenon, sensory ataxia, Romberg sign, impaired tandem walking, paraparesis, pallesthesia, hypoesthesia, and Sphincter dysfunction | - | - | Brain MRI: large ill-defined T2 FLAIR hyperintensities of periventricular and deep white matter with smaller lesions infratentorially, spared cortex, deep gray matter and subcortical U fibers; SSEPs: abnormal conduction above the sensory decussation in the lower brainstem CSF: mild pleocytosis | Methylprednisolone, rituximab | Improved and MRI showed stability or regression of most lesions |
| 20 | Cao et al. | Sinopharm and Sputnik V | 24/F | ADEM | 14 | 1 | Reduced memory, headache, fever, spasticity, weakness in extremities | - | Loss of appetite | CSF: pleocytosis, oligoclonal band (+) MRI: abnormal signals in the B/L temporal cortex, lesions EEG: epileptiform waves | Immunoglobulin, diazepam, levetiracetam | Full recovery after 1 month |
| 21 | Escola et al. | AstraZeneca | 43/F | Encephalomyelitis | 9 | 1 | Headache, meningism, and fever, sensorimotor tetraparesis, subacute sensorimotor paraparesis, urinary retention, hyperreflexia | Migraine | - | MRI: T2 hyperintense lesions involving frontal cortex, periventricular space, pulvinar thalamic nuclei, brain stem, and cerebellar peduncles CSF: extensive predominant granulocytic pleocytosis, elevated lactate and protein | Methylprednisolone, ceftriaxone, ampicillin, plasma exchange, meropenem, tocilizumab | Patient developed a stuporous to a comatose state and was discharged to a rehabilitation center; after 3 months, she improved with a light cerebellar syndrome (Rt hand intention tremor) |
| 22 | Etemadifar et al. | Sinopharm and Sputnik V | 50/F | Anti-NMDAR encephalitis | 20 | 2 | Behavioral disturbances, muscle pain, limb weakness, ataxia, dizziness, weakness, agitation, (+) Babinski sign | Rituximab-treated MS | Vomiting | MRI: plaques in the periventricular, juxtacortical, and cortical area | Methylprednisolone | Full recovery |
| 23 | Fan et al. | Moderna | 22/M | AE | 6 | 2 | Fever, blurry vision, consciousness disturbance, status epilepticus, slurred speech, memory loss | - | Sinus tachycardia | CSF: no pleocytosis, elevated protein, SARS-CoV-2 spike S1 RBD IgG; EEG: continuous diffuse slowing in theta and delta ranges; CT: mild hypoperfusion in right temporal region | Levetiracetam, acyclovir, valproate sodium, and methylprednisolone | Full recovery |
| 24 | Fernandes et al. | Pfizer BioNTech | 16/M | Anti-GAD encephalitis | 7 | 1 | Generalized tonic clonic seizures | DM | - | EEG: bitemporal focal slowing with admixed sharp waves CSF: pleocytosis, protein elevation | Dexamethasone | Improved with minimal right focal slowing |
| 25 | Flannery et al. | Pfizer BioNTech | 20–30/F | Anti-NMDAR encephalitis | 7 | 1 | Anxiety, decreased mental acuity, insomnia, COVID-19 hypochondria, motor dysfunction, aphasia, accusatory auditory hallucinations, spontaneous defecation, psychosis, catatonia, grand mal seizure, lethargy | Irritable bowels and kidney disease | Tachycardia, hypertension | CSF: mild lymphocyte pleocytosis, anti-NMDA titer 1:20 | Olanzapine, haloperidol, lithium, Risperidone, IVIG, methylprednisolone, metoprolol, and rituximab | Improved with minor neurological deficits after 45 days |
| 26 | Gao et al. | Moderna | 82/F | AE | 5 | 1 | Fever, headache, behavioral changes | DM, hypertension | - | EEG: slow waves in the right frontoparietal regions MRI: signal change in the right middle and posterior temporal lobe CSF: elevated protein | Pulse steroid | Full recovery |
| 27 | Garibashvili et al. | AstraZeneca | 71/M | Anti-LGI1 encephalitis | 28 | 1 | Faciobrachial dystonic seizures, mild pallhypaethesia | Heart disease, hypertension and hyperlipidemia | Cardiac pauses | LG1 antibodies: marked increase in serum, normal in CSF | Prednisolone | Full recovery |
| 28 | Gogu et al. | Johnson & Johnson | 45/M | AHEM | 30 | 1 | Right hemiparesis, mixed aphasia, severe headache, agitation, depressed level of consciousness, coma | SARS-CoV-2 (+), DM, Tolosa–Hunt Syndrome | Incomplete left ophthalmoplegia | MRI: multiple ischemic strokes, meningitis, infectious vasculitis, and hemorrhagic encephalitis with extension of the lesion to left fronto-parieto-temporal lobes with hypersignal aspects on the T2, Flair, and DWI images | Methylprednisolone | Death |
| 29 | Grossi et al. | Pfizer BioNTech | /M | AE | 17 | 1 | Fever, agitation, confusion, headache, and Tonic-Clonic seizures | CLL, herpetic trigeminal rash | Vomiting | CSF: pleocytosis, high albumin, oligoclonal bands (+) | Dexamethasone Acyclovir, Ceftriaxone, Vancomycin, and Levetiracetam | Free from focal neurological impairment after 4 months |
| 30 | Huang et al. | AstraZeneca | 38/F | Autoimmune Encephalitis | 14 | 1 | Acute-onset amnesia, fever, and general malaise for 2 days, incoherent speech, difficulty typing using communication software, tonic–clonic seizure | - | - | MRI: a subacute infarction at the right internal capsule and irregular vascular contour, which indicated a vasculopathy, such as vasculitis CSF: inflammation without pleocytosis EEG: diffuse background slowing with sharp transients at the right temporal region | Levetiracetam + steroid pulse therapy | Full recovery without neurological deficit or sequelae |
| 31 | Jarius et al. | Pfizer BioNTech | 67/M | MOG encephalomyelitis | 10 | Color desaturation in the left eye associated with left-sided temporal headache and pain upon eye movement | Arterial hypertension and benign prostate hyperplasia | Optic neuritis | MRI showed swelling and contrast enhancement of the anterior part of the left optic nerves. Visual evoked potentials (VEP) demonstrated prolonged absolute and relative P100 latency and marked amplitude reduction in the left eye (by 40 ms and 73%, respectively, compared with the right eye) | Methylprednisolone | Favorable | |
| 32 | Kania et al. | Moderna | 19/F | ADEM | 14 | 1 | Headache, fever, urinary retention | - | Nausea, vomiting, back and neck pain | MRI: multiple hyperintense lesions in T2 weighted and FLAIR images located in both brain hemispheres, pons, the medulla oblongata, and cerebellum CSF: pleocytosis, elevated protein and RBC | Methylprednisolone | Full recovery |
| 33 | Kobayashi et al. | Pfizer BioNTech | 46/F | Brainstem encehalitis | 5 | 2 | Diplopia | Vasculitis | - | MRI: lesion on the dorsal pons across the midline and no gadolinium enhancement | Methyprednisolone | Full recovery |
| 34 | Kwon et al. | AstraZeneca | 57/F | Autoimmune encephalitis | 5 | 1 | Headache, fever, generalized convulsive seizure, cognitive decline including attention and memory deficits along with gradually worsening dysphasia | Hypertension | Myalgia | MRI: restricted diffusion through the left insular and mesial temporal cortices, contrast enhancement CSF: pleocytosis, elevated protein, oligoclonal band (+) EEG: intermittent generalized delta activity | Methylprednisolone, Immunoglobulin, Rituximab | The patient’s language function slowly improved substantially following rituximab therapy, but the memory dysfunction hardly improved. Encephalomalacia change was observed in the left temporal lobe |
| 35 | Lazaro et al. | Sinopharm and Sputnik V | 26/F | ADEM | 28 | 1 | Disorientation, inappropriate behavior, headache, gait imbalance declined memory, hypoprosexia, anosognosia, incoherent speech, visuospatial failures, right upper limb weakness, gait ataxia | - | - | CSF: normal, OCB (+) MRI: nodular hyperintense lesions on T2/FLAIR without restricted diffusion | Methylprednisolone | Full recovery and clear MRI after 3 months |
| 36 | Li et al. | AstraZeneca | 55/M | AE | 7 | 1 | Fever, progressive weakness, consciousness disturbance | Hypertension, hyperlipidemia, and sleep apnea under medication | - | CSF: pleocytosis, elevated protein, ANA (+); high D-dimer | Dexamethasone and subcutaneous fondaparinux | Normal, 4 months later he received Moderna vaccine with no sequelae |
| 37 | Maramattom et al. | AstraZeneca | 64/M | LE | 10 | 1 | Headache, altered sensorium, fever | Glomerulonephritis, Subsegmental pulmonary embolism | - | CT chest; normal MRI brain: hyperintensities in bilateral medial temporal lobe and head and proximal body of hippocampus (L > R) CSF: pleocytosis | Methylprednisolone, plasma exchange, and rituximab | Improved |
| 38 | Maramattom et al. | AstraZeneca | 46/M | ADEM | 5 | 1 | Fever, urinary complaints, progressive paraparesis | A brisk jaw jerk, spastic quadriparesis paresis, loss of posterior column sensations till the T6 level | - | MRI spine: longitudinally extensive transverse myelitis MRI brain: T2, FLAIR hyperintensities in bilateral middle cerebellar peduncle (left > right), pontine tegmentum, right paramedian medulla, and left thalamocapsular region CSF: encephalitis panel: negative | methylprednisolone, plasma exchange | Improved |
| 39 | Maramattom et al. | AstraZeneca | 64/M | ADEM | 20 | 2 | Progressive paresthesia of legs, followed by UL, spastic paraparesis | - | - | MRI brain and spine: bilateral corticospinal tract hyperintensities Dorsal cord hyperintensity at D8–9 CT: normal | methylprednisolone and Rituximab | Improved |
| 40 | Maramattom et al. | AstraZeneca | 42/F | LE | 5 | 1 | Persistent daily headache | - | Papilledema | CSF: opening pressure 32 cm H O2 CSF parameters normal serum and CSF autoimmune MRI: initial MRI: leptomeningeal and sulcal enhancement; 25 days later: large right temporal irregular enhancing lesion with significant perilesional edema | Decompression of lesion Excisional biopsy, prednisolone | Good recovery with some symptoms |
| 41 | Monte et al. | Pfizer BioNTech | 15/F | Bickerstaff brainstem encephalitis | 3 | 2 | Diplopia, dysarthria, consciousness disturbance, fever, asthenia, limb paresthesia, cranial nerve paresis, and gait unsteadiness | Previous recent mycoplasma pneumoniae infection | Cough, vomiting, ophthalmoplegia, abnormal blink reflex | Both normal | Methylprednisolone, immunoglobulins | After 2 weeks, he was able to walk without assistance and the neurological examination was normal. Six weeks after the disease onset, the blink reflex was normal |
| 42 | Moslemi et al. | AstraZeneca | 27/M | HSE | 8 | 1 | Agitation, headache delirium, and disorientation to time, location, and people, slowed psychomotor activity, and loss of alertness | - | Vomiting | CT scans: normal findings with no evidence of involvement MRI: all normal | Acyclovir | Improved |
| 43 | Nagaratnam et al. | AstraZeneca | 36/F | ADEM | 14 | 1 | Headache, fatigue, photophobia, bilateral visual disablement, subjective color desaturation, aching eye movements | - | Photophobia, blurred vision in the right eye | MRI: T1/FLAIR hyperintense lesions involving the subcortical white matter, posterior limb of bilateral internal capsules, pons and left middle cerebellar peduncle, multiple internal punctuate foci of gadolinium contrast enhancement | Methylprednisolone | Improvement in response bilaterally with responses being detectable on the left eye |
| 44 | Naz et al. | Sinopharm and Sputnik V | 33/F | Anti-NMDAR encephalitis | 4 | 2 | Constitutional symptoms, memory disturbance, confusion, fish mouthing movement and seizures | - | - | CSF: pleocytosis, oligoclonal band (+), CT and MRI (−), blood NMDA test (−) | Steroids, IVIG, T-PLEX, and Rituximab | Full recovery |
| 45 | Permezel et al. | AstraZeneca | 63/M | ADEM | 12 | 1 | Vertigo, fatigue, disorientation, declining cognition, impaired attention, poor responsiveness | DM, ischemic heart disease and atrial fibrillation | Abdominal pain, fatigue, ketoacidosis, and silent myocardial infarction | MRI: bilateral foci (>20) of high T2 and FLAIR signal in the white matter | Corticosteroids, plasmapheresis | Death |
| 46 | Rastogi et al. | Moderna | 59/F | Rhombencephalitis | 12 | 2 | Binocular diplopia, paresthesia, hand numbness, decreased sensation, cerebellar dysfunction | Fibromyalgia, migraines, and carpel tunnel syndrome | Dizziness, lethargy | CSF: elevated protein, elevated glucose, lymphocytic pleocytosis; MRI: multiple focal poorly defined regions of contrast enhancement in the cerebral cortex, deep grey matter, brainstem, and cerebellum | Expectant, without empiric corticosteroids or antimicrobials | Ongoing gradual improvement |
| 47 | Rinaldi et al. | AstraZeneca | 45/M | ADEM | 12 | 1 | Numbness of limbs, trunk and legs, slurred speech, difficulty swallowing, clumsy right-hand movements, dysarthria, dysphagia, urge incontinence | - | Reduced visual acuity | MRI: large, poorly marginated T2-weighted hyperintensities in the pons, right cerebellar peduncle, right thalamus, and multiple spinal cord segments. All lesions, except the thalamic one and a single dorsal spinal area, showed blurred gadolinium enhancement on T1-weighted images CSF: pleocytosis | Methylprednisolone | Complete clinical recovery and no relapses, almost entire resolution of the brainstem and spinal cord lesions at the dorsal/conus medullaris level, and further shrinkage of cervical areas |
| 48 | Saad et al. | Pfizer BioNTech | 69/F | Acute encephalopathy | 5 | 1 | Coma, seizure, status epilepticus | - | - | CSF: high protein, MRI: pyriform-pattern diffusion restriction in the right hemisphere and left frontoparietal region | Methylprednisolone, antibiotics, and antivirals | Discharged in a deeply comatose status on day 30 of hospital admission |
| 49 | Sawczyńska et al. | Unknown | 77/F | AE | 14 | 1 | Fever, attention and cognition disturbances, confusion, hyperactivity, delusions, chorea, orofacial dyskinesia, psychomotor slowing, seizures, hemiparesis, loss of consciousness | COVID-19 infection, hypertension, DM, hypothyroidis, urinary incontinence, and multiple malignancies in remission, slight cognition disturbances | Atrial fibrillation, pneumonia, sepsis, pulmonary embolism, urinary tract infection, reactive arthritis | MRI: features of cerebral small vessel disease, diffuse white matter hyperintensities, cortical and subcortical atrophy EEG: FIRDA pattern | Methylprednisolone, diazepam, remdesivir, IVIG, and antiepileptic | Hospitalization for non-neurological complications |
| 50 | Senda et al. | Pfizer BioNTech | 72/F | Acute Meningoencephalitis | 3 | 1 | Depressed level of consciousness, headache | Rheumatoid vasculitis, DM, and hyperlipidemia | General fatigue | CSF: a cell count of four cells/mm3 (all mononuclear leukocytes), an increased protein level, IgG index was elevated (1.13) MRI: hyperintensities in white matter of the bilateral frontotemporal areas on DWI, more on the right side FLAIR images: diffuse cerebral cortex swelling in bilateral frontotemporal areas, also stronger on the right side | Intravenous steroid pulse and gammaglobulin therapies | Improved |
| 51 | Shinet al. | AstraZeneca | 35/F | Autoimmune encephalitis | 5 | 1 | Dysarthria, abnormal movements, anxiety, fever, rigidity, dystonia, catatonia, motor aphasia, jaw-opening dystonia, hypophonia, drooling, reduced voluntary movements | - | Sinus tachycardia | MRI: swelling of the hippocampus, encephalomalacia in frontoparietal lobes EEG: diffuse beta wave activity, intermittent generalized delta activity | Methylprednisolone, immunoglobulins, rituximab | After 1 week, her catatonia, rigidity, and drooling had improved, and she could walk for a short distance without assistance; however, she still had significant rigidity |
| 52 | Shyu et al. | Moderna | 58/F | AE | 7 | 1 | Fever, cognitive deficits, left deviation of the head and eyeballs, and mild weakness of the right UL | - | - | CSF: pleocytosis, elevated protein, CSF/serum albumin ratio of 19.7 | Dexamethasone | Regained normal cognitive function and was discharged in 13 days |
| 53 | Shyu et al. | Moderna | 21/M | AE | 7 | 1 | Coma, status epilepticus | - | - | CSF: elevated protein and microalbumin EEG: continuous diffuse slowing in the theta and delta ranges, indicating moderate diffuse cerebral dysfunction SPECT: hypoperfusion in the right temporal region | Methylprednisolone | Healthy and seizure free after 3 months |
| 54 | Sluyts et al. | Moderna | 48/3 | AE | 6 | booster dose | Agitation, physical aggression, mutism, left arm: paretic and atactic, confusion | - | Bradyphrenia | CSF: pleocytosis, elevated protein MRI: small left internal capsule developmental venous anomaly | Ceftriaxone, amoxicilline, and acyclovir | Full recovery after 3 days from steroids admission |
| 55 | Takata et al. | AstraZeneca | 22/F | Autoimmune encephalitis | Few | 2 | Headache, fatigue, confusion, agitation, hallucinations, fever, disorientation | - | - | CSF: opening pressure of 30 cm H2O, pleocytosis, IgG oligoclonal bands (+ve) | Ceftriaxone, acyclovir, lorazepam, and olanzepine | She remains on low-dose olanzapine and is functionally well with independent activities of daily living, but her family reports that she has not recovered back to her pre-morbid state |
| 56 | Torrealb a-Acosta G et al. | Moderna | 77/M | Meningoencephalitis | 2 | 1 | Dizziness, fever, rashes, headache, double vision, confusion | Coronary artery disease, hyperlipidemia, and hypothyroidism | Edematous erythematous papules and plaques with overlying pustules on the trunk and abdomen | CSF: pleocytosis, increased protein vEEG: generalized slow theta range with state changes and reactivity | Methylprednisolone following prednisone | Full recovery |
| 57 | Vences et al. | Pfizer BioNTech | 72/M | AE | 1 | 1, relapse in 4 2 | Malaise, headache, fever, confusion, aggressiveness, and gait alterations | - | - | CSF: elevated protein MRI: circumscribed encephalitis at the anterior frontal and bilateral temporal lobes | Methylprednisolone | Favorable |
| 58 | Vogrig et al. | Pfizer BioNTech | 56/F | ADEM | 14 | 1 | Malaise, chills, unsteadiness of gait | - | - | MRI: hyperintensities on FLAIR sequences involving the left cerebellar peduncle, with moderate mass effect on the fourth ventricle | Prednisone | Full recovery |
| 59 | Walter et al. | Pfizer BioNTech | 30/M | RE | 21 | 2 | Malaise, headache, taste disorder, facial paralysis (left side), gait disturbance by ataxia, hypoglossal nerve paralysis | - | - | MRI: weak FLAIR hyperintensity of the brainstem, mesencephalon and cerebellar around the fourth ventricle without contrast enhancement CSF: pleocytosis | Methylprednisolone | Full recovery within a few weeks |
| 60 | Werner et al. | Pfizer BioNTech | 35/F | Autoimmune encephalitis | 2 | 2 | Fever, headache, visual impairment, behavioral changes, recurrent focal to bilateral tonic-clonic seizures, reduced level of consciousness, and choreatic movements | - | Skin rash | Cerebral magnetic resonance imaging: swelling in the (para-) hippocampal region predominantly on the left hemisphere and bilateral subcortical subinsular FLAIR hyperintensities. Cerebrospinal fluid analysis: a lymphocytic pleocytosis of 7 cells/μL and normal protein and immunoglobulin parameters | Levetiracetam, lacosamide, methylprednisolone, and plasma exchange | Partial recovery |
| 61 | Yazdanpanah et al. | Sinopharm and Sputnik V | 37/M | ADEM | Few | 1 | Intermittent myalgia, drooling, progressive weakness of 4 limbs, bilateral f, dysphagia | - | Nausea, vomiting | Brain MRI: Hyperintense foci within the left cerebral peduncle, left corticospinal tract, right and left sides of pons and medulla Spine MRI: unremarkable Magnetic resonance spectroscopy (MRS): confirmed the demyelination process by the presence of Myoinositol and Choline peaks | Heparin, Pantoprazole, Clindamycin, Paracetamol, and Methylprednisolone | Full recovery |
| 62 | Zlotnik et al. | Pfizer BioNTech | 48/M | Anti-LGI1 encephalitis | 18 | 2 | Fatigue, memory deficit, anterograde amnesia | - | - | MRI: hyperintense signal on both medial temporal lobes | Methylprednisolone | Recovered, but still faces some executive skills difficulties |
| 63 | Zuhorn et al. | AstraZeneca | 21/F | Autoimmune encephalitis | 5 | 1 | Headache, concentration difficulties, fever, malaise, epileptic seizure | - | - | CSF: pleocytosis EEG: diffuse slow theta rhythm | Dexamethasone | Normal state of the parenchyma without sequelae |
| 64 | Zuhorn et al. | AstraZeneca | 63/F | Autoimmune encephalitis | 6 | - | Gait deterioration, twitching, opsoclonus-myoclonus syndrome | ‘ | Oral anticoagulation, vigilance disorder | EEG: diffuse slow theta rhythm CSF: pleocytosis | Methylprednisolone | Normal state of the parenchyma |
| 65 | Zuhorn et al. | AstraZeneca | 63/M | Autoimmune encephalitis | 8 | - | Fever, aphasia | - | - | CSF: pleocytosis | - | Further improvement could be observed, no evidence of structural lesions |
| Variable | Descriptive Statistics | |
|---|---|---|
| Sex (%) | Male | 36 (55.4%) |
| Female | 28 (43.1%) | |
| Transgender male | 1 (1.5%) | |
| Age (mean ± SD) | 46.82 ± 19.25 | |
| Period after vaccination in days (mean ± SD) | 9.97 ± 7.16 | |
| Vaccine Subtypes (%) | AstraZeneca | 25 (38.5%) |
| Pfizer BioNTech | 22 (33.8%) | |
| Moderna | 11 (16.9%) | |
| Sinopharm and Sputnik | 5 (7.7%) | |
| Johnson & Johnson | 1 (1.5%) | |
| Unknown | 1 (1.5%) | |
| Vaccine dose (%) | 1st | 41 (66.1%) |
| 2nd | 18 (29%) | |
| 3rd | 1 (1.6%) | |
| 4th | 2 (3.2%) | |
| Encephalitis Subtypes (%) | Acute encephalitis | 11 (16.9%) |
| ADEM | 14 (21.5%) | |
| AHEM | 4 (6.2%) | |
| Other | 36 (55.4%) | |
| Headache (%) | 20 (30.8 %) | |
| Fever (%) | 23 (35.4 %) | |
| Seizure (%) | 15 (23.1 %) | |
| Abnormal movement (%) | 24 (36.9 %) | |
| CSF findings (%) | ||
| Pleocytosis | 32 (49.2 %) | |
| High protein | 7 (10.8 %) | |
| Antibodies positive | 6 (9.2 %) | |
| MRI findings (%) | ||
| Abnormal | 40 (61.5 %) | |
| Treatment (%) | Steroids | 56 (86.2 %) |
| Immunoglobulins | 15 (23.1 %) | |
| Plasmapheresis | 9 (13.8 %) | |
| Antiviral | 10 (15.4 %) | |
| Immunosuppressive drug | 53 (81.5 %) | |
| Treatment outcome (%) | Full recovery | 41 (63.1 %) |
| Residual Symptoms | 11 (16.9 %) | |
| Extensive rehabilitation | 9 (13.8 %) | |
| Death | 4 (6.2 %) | |
| Death-associated comorbidities | Hemicraniectomy, Tolosa Hunt Syndrome, Diabetes type 2, ischemic heart disease, atrial fibrillation |
| Outcome | ||||||
|---|---|---|---|---|---|---|
| Vaccine | Full Recovery | Residual Symptoms | Extensive Rehabilitation | Death | Total | p-Value |
| AstraZeneca | 13 | 4 | 6 | 2 | 25 | 0.124 * |
| 52.0% | 16.0% | 24.0% | 8.0% | 100.0% | ||
| Pfizer BioNTech | 14 | 5 | 3 | 0 | 22 | |
| 63.6% | 22.7% | 13.6% | 0.0% | 100.0% | ||
| Moderna | 9 | 1 | 0 | 1 | 11 | |
| 81.8% | 9.1% | 0.0% | 9.1% | 100.0% | ||
| Sinopharm and Sputnik V | 5 | 0 | 0 | 0 | 5 | |
| 100.0% | 0.0% | 0.0% | 0.0% | 100.0% | ||
| Johnson & Johnson | 0 | 0 | 0 | 1 | 1 | |
| 0.0% | 0.0% | 0.0% | 100.0% | 100.0% | ||
| Unknown mRNA vaccine | 0 | 1 | 0 | 0 | 1 | |
| 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | ||
| Outcome | ||||||
|---|---|---|---|---|---|---|
| Encephalitis Subtype | Full Recovery | Residual Symptoms | Extensive Rehabilitation | Death | Total | p-Value |
| Acute encephalitis | 10 | 1 | 0 | 0 | 11 | <0.001 * |
| 90.9% | 9.1% | 0.0% | 0.0% | 100.0% | ||
| ADEM | 10 | 2 | 1 | 2 | 15 | |
| 66.7% | 13.3% | 6.7% | 13.3% | 100.0% | ||
| AHEM | 0 | 0 | 2 | 2 | 4 | |
| 0.0% | 0.0% | 50.0% | 50.0% | 100.0% | ||
| Other | 21 | 8 | 6 | 0 | 35 | |
| 60.0% | 22.9% | 17.1% | 0.0% | 100.0% | ||
| Outcome | ||||||
|---|---|---|---|---|---|---|
| Plasmapheresis | Full Recovery | Residual Symptoms | Extensive Rehabilitation | Death | Total | p-Value |
| Plasmapheresis | 2 | 1 | 4 | 2 | 9 | 0.002 * |
| 22.2% | 11.1% | 44.4% | 22.2% | 100.0% | ||
| Without Plasmapheresis | 39 | 10 | 5 | 2 | 56 | |
| 69.6% | 17.9% | 8.9% | 3.6% | 100.0% | ||
| Author’s Name | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Total | % | ROB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Ahmed et al. | No | No | Yes | Yes | Yes | Yes | No | Yes | 5 | 63% | Moderate risk |
| Ahn et al. | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 6 | 75% | Low risk |
| Albsheer et al. | No | Yes | Yes | Yes | No | No | No | Yes | 4 | 50% | Moderate risk |
| Aljamea et al. | UC | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Al-Quliti et.al | No | Yes | Yes | Yes | Yes | No | No | Yes | 5 | 63% | Moderate risk |
| Ancau et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Asaduzzaman et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Autjimanon et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Bastide et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Cao et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Ebadi et al. | No | Yes | Yes | No | No | No | No | Yes | 3 | 38% | High risk |
| Escolà et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Etemadifaret et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Fan et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Fernandes et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Flannery et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Gao et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Garibashvili et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Gogu et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Grossi et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Huang et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Jarius et al. | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 | 88% | Low risk |
| K. Kania et al. | No | Yes | Yes | Yes | No | Yes | UC | Yes | 5 | 63% | Moderate risk |
| Kobayashi et al. | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 | 88% | Low risk |
| Kobayashi et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Kwon et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Lagioia et al. | No | Yes | No | No | No | No | No | No | 1 | 13% | High risk |
| Lazaro et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Li et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Mörz et al. | No | Yes | Yes | No | No | No | No | Yes | 3 | 38% | High risk |
| Moslemi et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Nagaratnam et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Naz et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Permezel et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Rastogi et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Rinaldi et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Saad et al. | No | No | Yes | No | Yes | Yes | Yes | Yes | 5 | 63% | Moderate risk |
| Sawczyńska et al. | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 6 | 75% | Low risk |
| Senda et al. | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 8 | 100% | Low risk |
| Shin HR et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Shyu et al. | No | No | Yes | Yes | Yes | Yes | No | Yes | 5 | 63% | Moderate risk |
| Sluyts et al. | No | Yes | Yes | Yes | No | No | No | Yes | 4 | 50% | Moderate risk |
| Takata et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Torrealba-Acosta et al. | No | Yes | Yes | Yes | No | Yes | UC | Yes | 5 | 63% | Moderate risk |
| Vences et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Vogrig et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Walter et al. | No | No | Yes | Yes | Yes | Yes | NA | Yes | 5 | 63% | Moderate risk |
| Werner et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Yazdanpanah et al. | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 6 | 75% | Low risk |
| Zlotnik et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Zuhorn et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Author’s Name | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Total | % | ROB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asioli et al. | Yes | Yes | Yes | Yes | Yes | No | UC | Yes | Yes | NA | 7 | 70% | Moderate risk |
| Ballout et al. | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | NA | 6 | 60% | Moderate risk |
| Maramattom et al. | Yes | UC | Yes | No | UC | UC | No | Yes | Yes | Yes | 5 | 50% | Moderate risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhady, M.; Husain, M.A.; Hawas, Y.; Elazb, M.A.; Mansour, L.S.; Mohamed, M.; Abdelwahab, M.M.; Aljabali, A.; Negida, A. Encephalitis following COVID-19 Vaccination: A Systematic Review. Vaccines 2023, 11, 576. https://doi.org/10.3390/vaccines11030576
Abdelhady M, Husain MA, Hawas Y, Elazb MA, Mansour LS, Mohamed M, Abdelwahab MM, Aljabali A, Negida A. Encephalitis following COVID-19 Vaccination: A Systematic Review. Vaccines. 2023; 11(3):576. https://doi.org/10.3390/vaccines11030576
Chicago/Turabian StyleAbdelhady, Mariam, Muhammad Ashraf Husain, Yousef Hawas, Mahmoud Abdelsalam Elazb, Lena Said Mansour, Mohamed Mohamed, Maya Magdy Abdelwahab, Ahmed Aljabali, and Ahmed Negida. 2023. "Encephalitis following COVID-19 Vaccination: A Systematic Review" Vaccines 11, no. 3: 576. https://doi.org/10.3390/vaccines11030576
APA StyleAbdelhady, M., Husain, M. A., Hawas, Y., Elazb, M. A., Mansour, L. S., Mohamed, M., Abdelwahab, M. M., Aljabali, A., & Negida, A. (2023). Encephalitis following COVID-19 Vaccination: A Systematic Review. Vaccines, 11(3), 576. https://doi.org/10.3390/vaccines11030576







