Serological Protection Rates against TBEV Infection in Blood Donors from a Highly Endemic Region in Southern Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Samples
2.2. Active Vaccination Status
2.3. Serological Methods
2.4. Data Analysis
3. Results
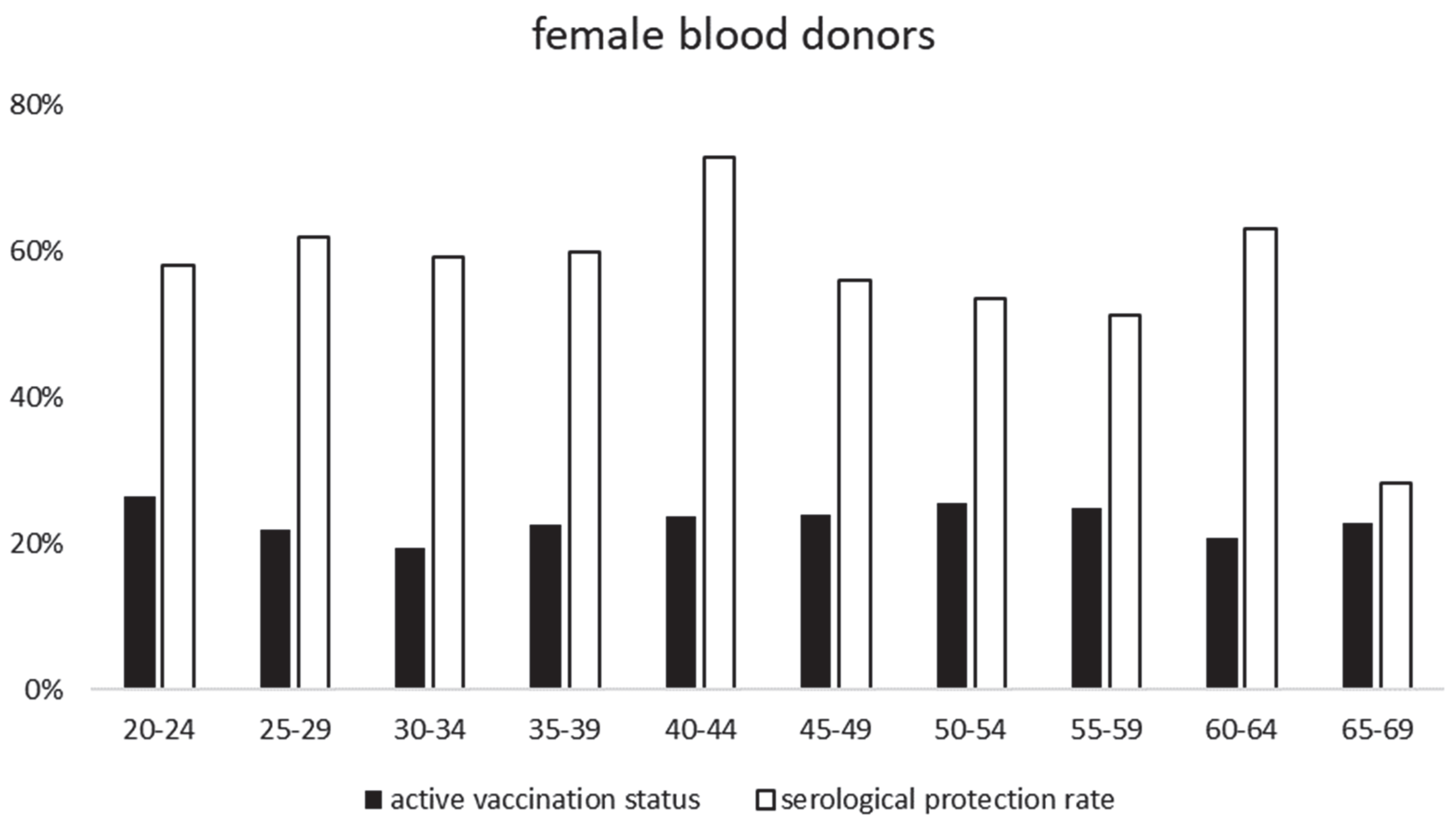
- TBEV-IgG protection rates
- Optical density (OD) values, mSNA titers and age
- Optical density (OD) values and age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogovic, P.; Strle, F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015, 3, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Ruzek, D.; Avsic Zupanc, T.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res. 2019, 164, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Ecker, M.; Allison, S.L.; Meixner, T.; Heinz, F.X. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol 1999, 80 Pt 1, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, I.V.; Verkhozina, M.M.; Demina, T.V.; Dzhioev, Y.P.; Tkachev, S.E.; Karan, L.S.; Doroshchenko, E.K.; Lisak, O.V.; Suntsova, O.V.; Paramonov, A.I.; et al. Genetic and Biological Properties of Original TBEV Strains Group Circulating in Eastern Siberia. Encephalitis 2013, 283, 95–112. [Google Scholar]
- Dai, X.; Shang, G.; Lu, S.; Yang, J.; Xu, J. A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China. Emerg. Microbes Infect 2018, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, S.O.; Oehme, R.; Buckenmaier, T.; Beer, M.; Jeffery-Smith, A.; Spannenkrebs, M.; Haag-Milz, S.; Wagner-Wiening, C.; Schlegel, C.; Fritz, J.; et al. A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in Germany, May 2016. Eurosurveillance 2018, 23, 17–00336. [Google Scholar] [CrossRef] [PubMed]
- Borde, J.P.; Zajkowska, J. Chapter 5: TBE in adults. In Tick-Borne Encephalitis—The Book; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2021. [Google Scholar] [CrossRef]
- Mickiene, A.; Laiskonis, A.; Gunther, G.; Vene, S.; Lundkvist, A.; Lindquist, L. Tickborne encephalitis in an area of high endemicity in lithuania: Disease severity and long-term prognosis. Clin. Infect Dis. 2002, 35, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Euringer, K.; Girl, P.; Kaier, K.; Peilstöcker, J.; Schmidt, M.; Müller-Steinhardt, M.; Rauscher, B.; Bressau, E.; Kern, W.V.; Dobler, G.; et al. Tick-borne encephalitis virus IgG antibody surveillance: Vaccination- and infection-induced seroprevalence rates, south-western Germany. Eurosurveillance 2023, in press. [Google Scholar]
- Zent, O.; Jilg, W.; Plentz, A.; Schwarz, T.F.; Fruhwein, N.; Kuhr, H.B.; Banzhoff, A. Kinetics of the immune response after primary and booster immunization against tick-borne encephalitis (TBE) in adults using the rapid immunization schedule. Vaccine 2003, 21, 4655–4660. [Google Scholar] [CrossRef]
- Harabacz, I.; Bock, H.; Jungst, C.; Klockmann, U.; Praus, M.; Weber, R. A randomized phase II study of a new tick-borne encephalitis vaccine using three different doses and two immunization regimens. Vaccine 1992, 10, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Erber, W.; Schmitt, H.J. Self-reported tick-borne encephalitis (TBE) vaccination coverage in Europe: Results from a cross-sectional study. Ticks Tick Borne Dis. 2018, 9, 768–777. [Google Scholar] [CrossRef]
- Girl, P.; Bestehorn-Willmann, M.; Zange, S.; Borde, J.P.; Dobler, G.; Buttlar, H.V. Tick-borne encephalitis virus (TBEV): Non-structural protein (NS1) IgG ELISA differentiating infection vs. vaccination antibody responses. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, B.; Ronnberg, B.; Vene, S.; Lundkvist, A. Antibody responses to tick-borne encephalitis virus non-structural protein 1 and whole virus antigen-a new tool in the assessment of suspected vaccine failure patients. Infect Ecol. Epidemiol. 2019, 9, 1696132. [Google Scholar] [CrossRef] [PubMed]
- Nygren, T. pers. Communication, Robert Koch Institute (RKI), Berlin, Germany.
- Coroian, M.; Mihalca, A.D.; Dobler, G.; Euringer, K.; Girl, P.; Borsan, S.D.; Kalmar, Z.; Tincuta Briciu, V.; Flonta, M.; Topan, A.; et al. Seroprevalence Rates against West Nile, Usutu, and Tick-Borne Encephalitis Viruses in Blood-Donors from North-Western Romania. Int. J. Environ. Res. Public Health 2022, 19, 8182. [Google Scholar] [CrossRef] [PubMed]
- Kollaritsch, H.; Dobler, G.; Schmidt, A.J.; Krech, T.; Steffen, R. Tick-Borne Encephalitis (TBE)—Fundamentals. Ther. Umsch. 2022, 79, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C. Vaccination against TBE in Austria: The success story continues. Int. J. Med. Microbiol. 2002, 291 (Suppl. 33), 56–57. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C. TBE vaccination and the Austrian experience. Vaccine 2003, 21 (Suppl. 1), S50–S55. [Google Scholar] [CrossRef] [PubMed]
- Schosser, R.; Reichert, A.; Mansmann, U.; Unger, B.; Heininger, U.; Kaiser, R. Irregular tick-borne encephalitis vaccination schedules: The effect of a single catch-up vaccination with FSME-IMMUN. A prospective non-interventional study. Vaccine 2014, 32, 2375–2381. [Google Scholar] [CrossRef] [PubMed]


| Age-Group (Years) | TBEV-IgG Positive | TBEV-IgG Negative | TBEV-IgG Borderline | TBEV-IgG Positive or Borderline and NS1 Negative or Borderline and: | ||
|---|---|---|---|---|---|---|
| SNA Titer > 20 (Vaccinees) | SNA Titer ≤ 10 | No IgG-Antibodies or NS1 pos. (no mSNA Performed) | ||||
| 18 | 70% (16/23) | 30% (7/23) | 0% (0/23) | 65% (15/23) | 0% (0/23) | 35% (7/23) |
| 19–24 | 64% (180/281) | 33% (94/281) | 2% (7/281) | 58% (162/281) | 0% (1/281) | 42% (118/281) |
| 25–29 | 63% (167/266) | 34% (91/266) | 3% (8/266) | 57% (152/266) | 1% (2/266) | 42% (112/266) |
| 30–34 | 58% (110/191) | 39% (74/191) | 4% (7/191) | 55% (106/191) | 1% (1/191) | 44% (84/191) |
| 35–39 | 60% (64/106) | 35% (37/106) | 5% (5/106) | 58% (62/106) | 0% (0/106) | 41% (44/106) |
| 40–44 | 63% (97/154) | 29% (45/154) | 8% (12/154) | 60% (92/154) | 5% (8/154) | 35% (54/154) |
| 45–49 | 58% (94/162) | 35% (56/162) | 7% (12/162) | 58% (94/162) | 2% (4/162) | 40% (64/162) |
| 50–54 | 56% (173/311) | 40% (123/311) | 5% (15/311) | 51% (160/311) | 3% (9/311) | 55% (142/311) |
| 55–59 | 48% (148/308) | 44% (136/308) | 8% (24/308) | 50% (154/308) | 2% (7/308) | 48% (147/308) |
| 60–64 | 56% (122/217) | 39% (85/217) | 5% (10/217) | 57% (123/217) | 1% (3/217) | 42% (91/217) |
| >65 | 43% (86/201) | 51% (103/201) | 6% (12/201) | 43% (87/201) | 1% (2/201) | 56% (112/201) |
| Total | 57% 1257/2220 | 38% 851/2220 | 5% 112/2220 | 55% 1207/2220 | 2% 37/2220 | 43% 975/2220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobler, G.; Euringer, K.; Kaier, K.; Borde, J.P. Serological Protection Rates against TBEV Infection in Blood Donors from a Highly Endemic Region in Southern Germany. Vaccines 2023, 11, 522. https://doi.org/10.3390/vaccines11030522
Dobler G, Euringer K, Kaier K, Borde JP. Serological Protection Rates against TBEV Infection in Blood Donors from a Highly Endemic Region in Southern Germany. Vaccines. 2023; 11(3):522. https://doi.org/10.3390/vaccines11030522
Chicago/Turabian StyleDobler, Gerhard, Kathrin Euringer, Klaus Kaier, and Johannes P. Borde. 2023. "Serological Protection Rates against TBEV Infection in Blood Donors from a Highly Endemic Region in Southern Germany" Vaccines 11, no. 3: 522. https://doi.org/10.3390/vaccines11030522
APA StyleDobler, G., Euringer, K., Kaier, K., & Borde, J. P. (2023). Serological Protection Rates against TBEV Infection in Blood Donors from a Highly Endemic Region in Southern Germany. Vaccines, 11(3), 522. https://doi.org/10.3390/vaccines11030522







