Dynamics of Anti-S IgG Antibodies Titers after the Second Dose of COVID-19 Vaccines in the Manual and Craft Worker Population of Qatar
Abstract
:1. Introduction
2. Materials and Methods
2.1. COVID-19 Vaccines
2.2. Detection of SARS-CoV-2 Antibodies
2.3. Molecular Detection of SARS-CoV-2
2.4. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
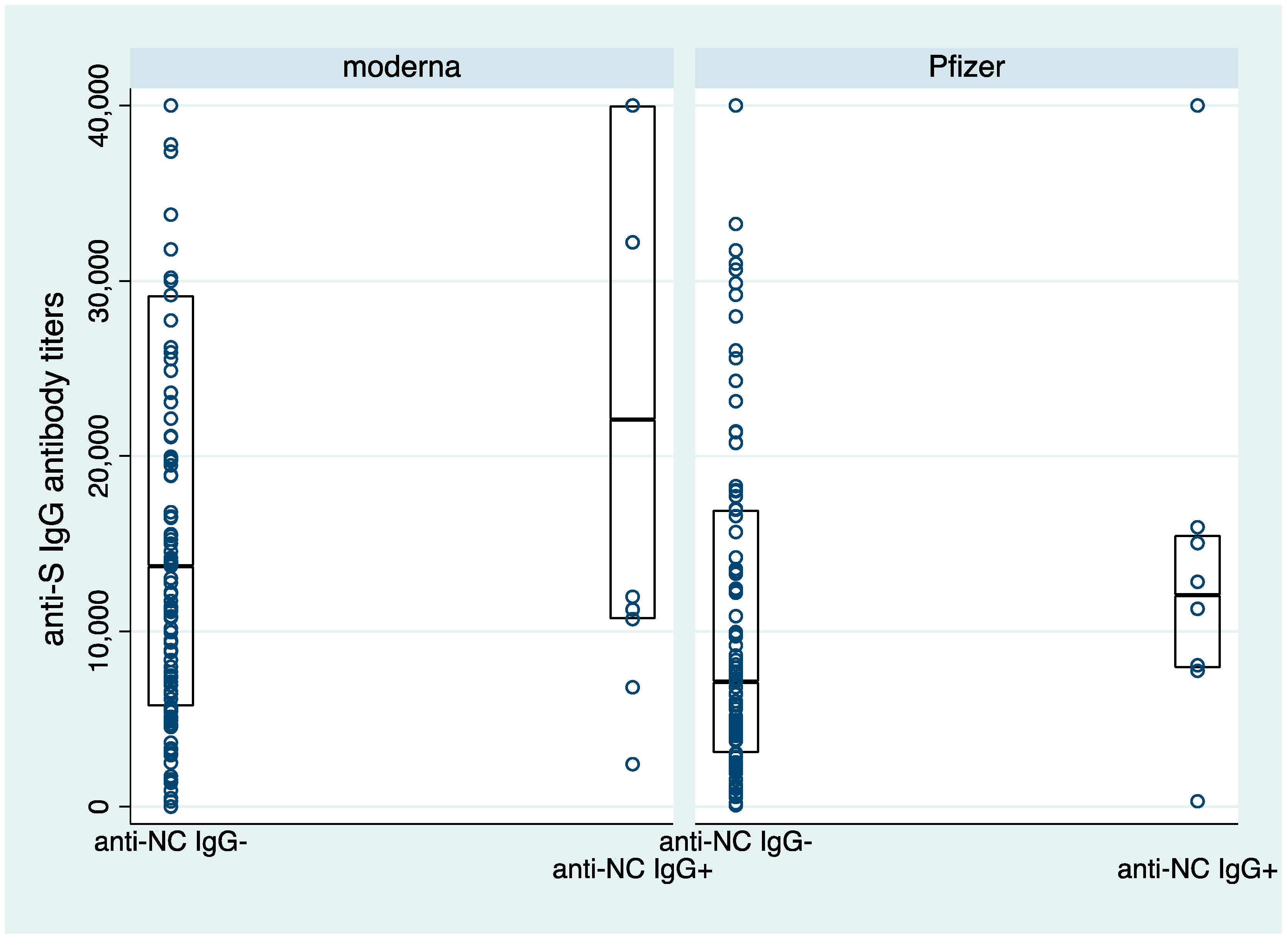
3.2. Anti-S Antibody Titers following Two Doses of COVID-19 Vaccines
3.3. Durability of Antibody Responses following Two Doses of COVID-19 Vaccines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/?gclid=EAIaIQobChMIkazL-ojJ8gIVjpGyCh1iEgKXEAAYASABEgKf9_D_BwE (accessed on 5 September 2022).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- MOPH. Ministry of Public Health Qatar 2022. Available online: https://covid19.moph.gov.qa/EN/Pages/default.aspx (accessed on 5 September 2022).
- Flacco, M.E.; Acuti Martellucci, C.; Soldato, G.; Carota, R.; Fazii, P.; Caponetti, A.; Manzoli, L. Rate of reinfections after SARS-CoV-2 primary infection in the population of an Italian province: A cohort study. J. Public Health 2021, 44, e475–e478. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Carfi, A.; Benvenuto, F.; Brandi, V.; Ciciarello, F.; Lo Monaco, M.R.; Martone, A.M.; Napolitano, C.; Pagano, F.; Paglionico, A.; et al. Predictive factors for a new positive nasopharyngeal swab among patients recovered from COVID-19. Am. J. Prev. Med. 2021, 60, 13–19. [Google Scholar] [CrossRef]
- Bansal, D.; Abdulmajeed, J.; Al-Shamali, M.H.M.A.; Albayat, S.S.A.; Himatt, S.M.; Cyprian, F.S.; Chivese, T.; Mundodan, J.M.; Khogali, H.S.; Baaboura, R.; et al. Duration of COVID-19 mRNA vaccine effectiveness against severe disease. Vaccines 2022, 10, 1036. [Google Scholar] [CrossRef]
- WHO. WHO Lists Additional COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations. WHO. 2021. Available online: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed on 1 September 2022).
- EMA. COVID-19 Vaccines: Authorised: EMA. 2021. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section (accessed on 1 September 2022).
- FDA. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine 2021. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine (accessed on 1 September 2022).
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Stellini, R.; Gianello, R.; Gomarasca, W. Durability of anti-spike antibodies after vaccination with mRNA SARS-CoV-2 vaccine is longer in subjects with previous infection: Could the booster dose be delayed? Infection 2022, 50, 1573–1577. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Laing, E.D.; Weiss, C.D.; Samuels, E.C.; Coggins, S.A.; Wang, W.; Wang, R.; Vassell, R.; Sterling, S.L.; Tso, M.S.; Conner, T.; et al. Durability of antibody response and frequency of SARS-CoV-2 infection 6 months after COVID-19 vaccination in healthcare workers. Emerg. Infect. Dis. 2022, 28, 828–832. [Google Scholar] [CrossRef]
- Sughayer, M.A.; Souan, L.; Abu Alhowr, M.M.; Al Rimawi, D.; Siag, M.; Albadr, S.; Owdeh, M.; Al Atrash, T. Comparison of the effectiveness and duration of anti-RBD SARS-CoV-2 IgG antibody response between different types of vaccines: Implications for vaccine strategies. Vaccine 2022, 40, 2841–2847. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.T.; Bryan, A.; Fink, S.L.; Goecker, E.A.; Roychoudhury, P.; Huang, M.L.; Zhu, H.; Chaudhary, A.; Madarampalli, B.; Lu, J.Y.; et al. Anti-SARS-CoV-2 antibody levels measured by the AdviseDx SARS-CoV-2 assay are concordant with previously available serologic assays but are not fully predictive of sterilizing immunity. J. Clin. Microbiol. 2021, 59, e00989-21. [Google Scholar] [CrossRef] [PubMed]
- English, E.; Cook, L.E.; Piec, I.; Dervisevic, S.; Fraser, W.D.; John, W.G. Performance of the abbott SARS-CoV-2 IgG II quantitative antibody assay including the new variants of concern, VOC 202012/V1 (United Kingdom) and VOC 202012/V2 (South Africa), and first steps towards global harmonization of COVID-19 antibody methods. J. Clin. Microbiol. 2021, 59, e00288-21. [Google Scholar] [CrossRef]
- Coyle, P.V.; Chemaitelly, H.; Ben Hadj Kacem, M.A.; Abdulla Al Molawi, N.H.; El Kahlout, R.A.; Gilliani, I.; Younes, N.; Al Anssari, G.A.; Al Kanaani, Z.; Al Khal, A.; et al. SARS-CoV-2 seroprevalence in the urban population of Qatar: An analysis of antibody testing on a sample of 112,941 individuals. iScience 2021, 24, 102646. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816. [Google Scholar] [CrossRef]
- WHO. Surface Sampling of Coronavirus Disease (COVID-19): A Practical “How To” Protocol For Health Care and Public Health Professionals; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- FDA. AdviseDx SARS-CoV-2 IgG II: FDA. 2021. Available online: https://www.fda.gov/media/146371/download (accessed on 1 September 2022).
- Goodhue Meyer, E.; Simmons, G.; Grebe, E.; Gannett, M.; Franz, S.; Darst, O.; Di Germanio, C.; Stone, M.; Contestable, P.; Prichard, A.; et al. Selecting COVID-19 convalescent plasma for neutralizing antibody potency using a high-capacity SARS-CoV-2 antibody assay. Transfusion 2021, 61, 1160–1170. [Google Scholar] [CrossRef]
- Scientific, T.F. TaqPathTM COVID-19 CE-IVD RT-PCR Kit. 2021. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0019215_TaqPathCOVID-19_CE-IVD_RT-PCR%20Kit_IFU.pdf (accessed on 1 September 2022).
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jurjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Struck, F.; Schreiner, P.; Staschik, E.; Wochinz-Richter, K.; Schulz, S.; Soutschek, E.; Motz, M.; Bauer, G. Vaccination versus infection with SARS-CoV-2: Establishment of a high avidity IgG response versus incomplete avidity maturation. J. Med. Virol. 2021, 93, 6765–6777. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Caruso, T.; Testa, D.; Tarpanelli, T.; Gentili, A.; Gioe, D.; Gioè, D.; Migliorini, P. BNT162b2 mRNA SARS-CoV-2 vaccine elicits high avidity and neutralizing antibodies in healthcare workers. Vaccines 2021, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- CDC. Science Brief: SARS-CoV-2 Infection-Induced and Vaccine-Induced Immunity 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html (accessed on 1 September 2022).

| Manufacturer | Immunoassay Name | Automated System | Detection Method/ Assay Type | Detected Antibody Targeted | SARS-CoV-2 Antigen (s) | Result Interpretation | Reference |
|---|---|---|---|---|---|---|---|
| Abbott Laboratories | Architict SARS-CoV-2 IgG II | ARCHITECT® i4000SR | CMIA * | IgG | S (S1 subunit (RBD)) * | <50.0 AU/mL Negative ≥50.0 AU/mL: Positive | [24] |
| Abbott Laboratories | Architict SARS-CoV-2 IgG | ARCHITECT® i4000SR | CMIA | IgG | N * | <1.4 S/C Negative ≥1.4 S/C: Positive | |
| Ortho Clinical Diagnostics | VITROS® Anti-SARS-CoV-2 Total Ab | VITROS® ECiQ | CLIA * | IgG, IgM, and IgA | S (S1 subunit) | <1.0 S/C: Negative ≥1.0 S/C: Positive | [25] |
| Variables | Upper Three Quartiles | Lowest Quartile | All |
|---|---|---|---|
| N | 212 | 71 | 300 |
| Age (years), median (IQR) | 37.2 (32.1, 44.7) | 35.1 (26.5, 43.1) | 36.8 (31.2, 44.7) |
| Nationality | |||
| Indian | 35.4% | 39.4% | 36.7% |
| Nepali | 37.3% | 28.2% | 33.7% |
| Others | 26.9% | 32.4% | 29.3% |
| Missing | 0.5% | 0.0% | 0.3% |
| Religion | |||
| Hindu | 62.3% | 52.1% | 59.7% |
| Muslim | 28.8% | 25.4% | 28.0% |
| Others | 7.1% | 15.5% | 9.3% |
| Missing | 1.9% | 7.0% | 3.0% |
| Occupation | |||
| Construction | 69.3% | 60.6% | 65.3% |
| Others | 30.7% | 39.4% | 34.7% |
| Vaccine group | |||
| Not mRNA | 9.0% | 28.2% | 13.0% |
| Moderna | 53.3% | 28.2% | 46.7% |
| Pfizer | 37.7% | 40.8% | 39.7% |
| None | 0.0% | 2.8% | 0.7% |
| Interval between two doses (days), median (IQR) | 28.0 (22.0, 28.0) | 22.0 (21.0, 28.0) | 28.0 (21.0, 28.0) |
| Duration between sample collection and last vaccine dose (days), median (IQR) | 125.0 (71.5, 158.0) | 146.0 (89.0, 191.0) | 129.0 (72.0, 172.0) |
| Anti-S IgG antibody titre (AU/mL), median (IQR) | 13,477.0 (7328.5, 26,117.2) | 1551.7 (835.6, 2822.7) | 8927.7 (3766.4, 19,964.2) |
| Vaccine group | |||
| Not mRNA | 5693.5 (4798.9, 15,020.5) | 2131 (1053.8, 2948.4) | 3759.7 (2059.7–5693.5) |
| Moderna | 15,545.8 (9501.7, 37,778) | 1644.6 (1145.1, 3124.85) | 13,720.9 (6426.5, 30,185.6) |
| Pfizer | 11,079.45 (6637.15, 20,757.45) | 1549.5 (692.9, 2360.7) | 7570.9 (3757.9, 16,577.4) |
| None | NA | 632.6 (184.3, 1080.9) | 632.6 (184.3, 1080.9) |
| Anti-NC IgG antibody status * | |||
| Absent | 90.6% | 87.3% | 88.3% |
| Present | 9.4% | 12.7% | 9.7% |
| Test not done (Sample Rejected) | - | - | 2.0% |
| Anti-S Total (IgG, IgM and IgA) antibody status ** | |||
| Reactive | 100% | 98.6% | 99.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, D.; Atia, H.; Al Badr, M.; Nour, M.; Abdulmajeed, J.; Hasan, A.; Al-Hajri, N.; Ahmed, L.; Ibrahim, R.; Zamel, R.; et al. Dynamics of Anti-S IgG Antibodies Titers after the Second Dose of COVID-19 Vaccines in the Manual and Craft Worker Population of Qatar. Vaccines 2023, 11, 496. https://doi.org/10.3390/vaccines11030496
Bansal D, Atia H, Al Badr M, Nour M, Abdulmajeed J, Hasan A, Al-Hajri N, Ahmed L, Ibrahim R, Zamel R, et al. Dynamics of Anti-S IgG Antibodies Titers after the Second Dose of COVID-19 Vaccines in the Manual and Craft Worker Population of Qatar. Vaccines. 2023; 11(3):496. https://doi.org/10.3390/vaccines11030496
Chicago/Turabian StyleBansal, Devendra, Hassan Atia, Mashael Al Badr, Mohamed Nour, Jazeel Abdulmajeed, Amal Hasan, Noora Al-Hajri, Lina Ahmed, Rumissa Ibrahim, Reham Zamel, and et al. 2023. "Dynamics of Anti-S IgG Antibodies Titers after the Second Dose of COVID-19 Vaccines in the Manual and Craft Worker Population of Qatar" Vaccines 11, no. 3: 496. https://doi.org/10.3390/vaccines11030496
APA StyleBansal, D., Atia, H., Al Badr, M., Nour, M., Abdulmajeed, J., Hasan, A., Al-Hajri, N., Ahmed, L., Ibrahim, R., Zamel, R., Mohamed, A., Pattalaparambil, H., Daraan, F., Chaudhry, A., Oraby, S., El-Saleh, S., El-Shafie, S. S., Al-Farsi, A. F., Paul, J., ... Farooqui, H. H. (2023). Dynamics of Anti-S IgG Antibodies Titers after the Second Dose of COVID-19 Vaccines in the Manual and Craft Worker Population of Qatar. Vaccines, 11(3), 496. https://doi.org/10.3390/vaccines11030496










