An Economic Comparison in the Elderly of Adjuvanted Quadrivalent Influenza Vaccine with Recombinant Quadrivalent Influenza Vaccine in Spain
Abstract
1. Introduction
2. Materials and Methods
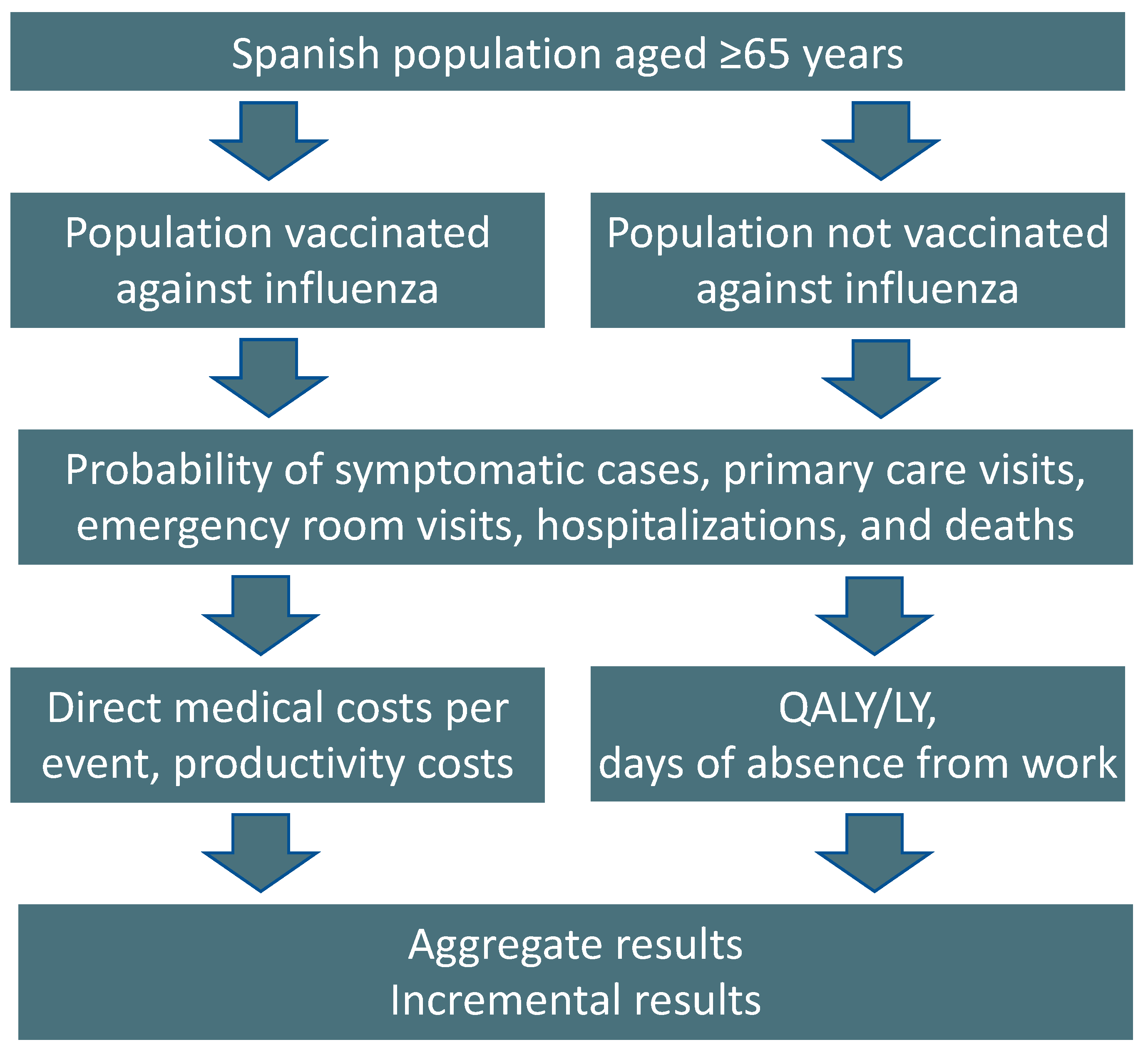
2.1. Model Structure
2.2. Model Inputs and Calculations
2.3. Vaccine Effectiveness Estimates
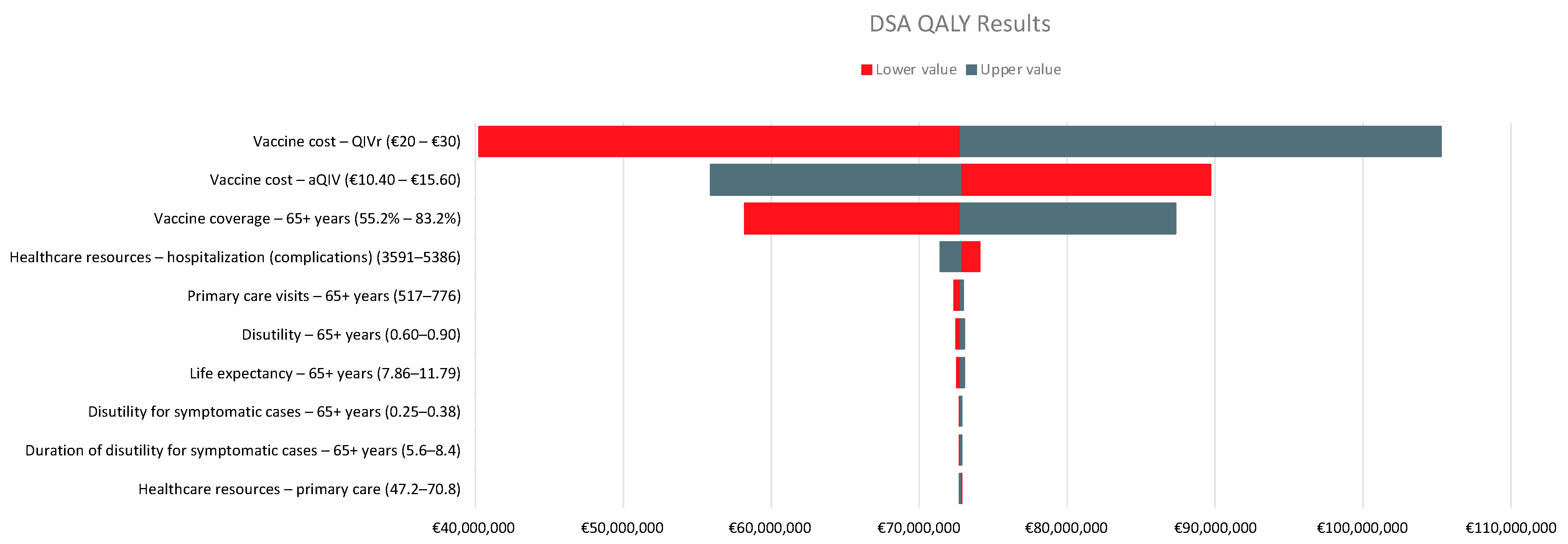
2.4. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob Health 2019, 9, 020421. [Google Scholar] [CrossRef]
- Instituto de Salud Carlos III; Sistema de Vigilancia de la Gripe en España. Impacto de la gripe en España. Temporada 2019–2020. Available online: https://vgripe.isciii.es/inicio.do (accessed on 19 September 2022).
- Oliva, J.; Delgado-Sanz, C.; Larrauri, A. Estimating the burden of seasonal influenza in Spain from surveillance of mild and severe influenza disease, 2010–2016. Influenza Other Respir. Viruses 2018, 12, 161–170. [Google Scholar] [CrossRef]
- Pérez-Rubio, A.; Platero, L.; Eiros Bouza, J.M. Seasonal influenza in Spain: Clinical and economic burden and vaccination programmes. Med. Clin. (Barc) 2019, 153, 16–27. [Google Scholar] [CrossRef]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef]
- Ministerio de Sanidad Consumo y Bienestar Social. Recomendaciones Vacunación Frente a la Gripe: Temporada 2022–2023. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/docs/Recomendaciones_vacunacion_gripe.pdf (accessed on 21 September 2022).
- European Medicines Agency. Fluad Tetra: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/fluad-tetra-epar-product-information_en.pdf (accessed on 10 August 2022).
- European Medicinces Agency. Supemtek: Quadrivalent Influenza Vaccine (Recombinant, Prepared in Cell Culture). Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/supemtek-epar-product-information_en.pdf (accessed on 21 September 2022).
- Spanish Agency of Medicines and Medical Devices. Efluelda Suspension Inyectable en Jeringa Precargada. Available online: https://cima.aemps.es/cima/publico/detalle.html?nregistro=85068 (accessed on 21 September 2022).
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Reyes, M.R.; Reynales, H.; Bermal, N.N.; Nicolay, U.; Narasimhan, V.; Forleo-Neto, E.; Arora, A.K. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014, 32, 5027–5034. [Google Scholar] [CrossRef] [PubMed]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Drago Manchón, G.; López-Belmonte, J.L.; Bricout, H.; de Courville, C. Public health benefits of switching into a recombinant quadrivalent vaccine in the Spanish Murcia and Valencia regions the recommended adult population (18+) for influenza seasonal vaccination. In Proceedings of the Eighth ESWI Influenza Conference, Virtual Conference, Salzburg, Austria, 4–7 December 2021; p. 67. [Google Scholar]
- Izurieta, H.S.; Lu, M.; Kelman, J.; Lu, Y.; Lindaas, A.; Loc, J.; Pratt, D.; Wei, Y.; Chillarige, Y.; Wernecke, M.; et al. Comparative Effectiveness of Influenza Vaccines Among US Medicare Beneficiaries Ages 65 Years and Older During the 2019–2020 Season. Clin. Infect. Dis. Off. Public Infect. Dis. Soc. Am. 2021, 73, e4251–e4259. [Google Scholar] [CrossRef]
- Jamotte, A.; Chong, C.F.; Manton, A.; Macabeo, B.; Toumi, M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health 2016, 16, 630. [Google Scholar] [CrossRef]
- Uhart, M.; Bricout, H.; Clay, E.; Largeron, N. Public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines compared to trivalent influenza vaccines in Europe. Hum. Vaccines Immunother. 2016, 12, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Meltzer, M.I.; Finelli, L.; Fiore, A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012, 30, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Chit, A.; Roiz, J.; Aballea, S. An Assessment of the Expected Cost-Effectiveness of Quadrivalent Influenza Vaccines in Ontario, Canada Using a Static Model. PLoS ONE 2015, 10, e0133606. [Google Scholar] [CrossRef] [PubMed]
- Mennini, F.S.; Bini, C.; Marcellusi, A.; Rinaldi, A.; Franco, E. Cost-effectiveness of switching from trivalent to quadrivalent inactivated influenza vaccines for the at-risk population in Italy. Hum. Vaccines Immunother. 2018, 14, 1867–1873. [Google Scholar] [CrossRef]
- Ruiz-Aragón, J.; Márquez-Peláez, S.; Gani, R.; Alvarez, P.; Guerrero-Luduena, R. Cost-Effectiveness and Burden of Disease for Adjuvanted Quadrivalent Influenza Vaccines Compared to High-Dose Quadrivalent Influenza Vaccines in Elderly Patients in Spain. Vaccines 2022, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aragón, J.; Gani, R.; Márquez, S.; Alvarez, P. Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in Spain. Hum. Vaccines Immunother. 2020, 16, 2238–2244. [Google Scholar] [CrossRef]
- López-Bastida, J.; Oliva, J.; Antoñanzas, F.; García-Altés, A.; Gisbert, R.; Mar, J.; Puig-Junoy, J. Spanish recommendations on economic evaluation of health technologies. Eur. J. Health Econ. 2010, 11, 513–520. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Portal Estadístico: Área de Inteligencia de Gestión. SIVAMIN: Cobertura de Vacunación. Available online: https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/I/sivamin/sivamin (accessed on 13 October 2022).
- Instituto Nacional de Estadistica. Tablas de Mortalidad por Año, Sexo, Edad y Funciones. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=27153 (accessed on 19 September 2022).
- Instituto Nacional de Estadistica. Población Residente por Fecha, Sexo y Edad. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=31304 (accessed on 19 September 2022).
- Ministerio de Sanidad Consumo y Bienestar Social. Acuerdo Marco para la Seleccion de Suministradores de Vacunas Frente a la Gripe Estacional (INGESA) y Ciudades de Ceuta y Melilla y Varias Comunidades Autonomas. Available online: https://contrataciondelestado.es/wps/wcm/connect/7c41cd41-00c8-4c07-be3d-272d29585268/DOC20210419131140PCAP+Gripe+2021-2025.pdf?MOD=AJPERES (accessed on 20 September 2022).
- País Vasco. Tarifas para Facturación de Servicios Sanitarios y Docentes de Osakidetza para el Año 2020. Available online: https://www.osakidetza.euskadi.eus/contenidos/informacion/osk_servic_para_empresas/es_def/adjuntos/LIBRO-DE-TARIFAS_2020_osakidetza.pdf (accessed on 19 September 2022).
- Boletin Oficial de la Region de Murcia. Comunidad Autónoma. Available online: https://www.borm.es/services/anuncio/ano/2019/numero/1263/pdf (accessed on 20 September 2022).
- Junta de Andalucía. Precios públicos de Servicios Sanitarios Prestados en el SSPA. Available online: https://www.juntadeandalucia.es/datosabiertos/portal/dataset/precios-publicos-de-servicios-sanitarios-prestados-en-el-sspa (accessed on 20 September 2022).
- Ministerio de Sanidad. Registro de Altas de los Hospitales Generales del Sistema Nacional de Salud. CMBD. Norma Estatal. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbd.htm (accessed on 19 September 2022).
- Pérez-Rubio, A.; Eiros, J.M. Economic and Health Impact of Influenza Vaccination with Adjuvant MF59 in population over 64 years in Spain. Rev. Esp. Quim. 2018, 31, 43–52. [Google Scholar]
- Instituto Nacional de Estadistica. Ocupados por Sexo y Grupo de Edad. Valores Absolutos y Porcentajes Respecto del Total de Cada Sexo. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=4076 (accessed on 19 September 2022).
- Instituto Nacional de Estadistica. Encuesta Anual de Coste Laboral (EACL). Available online: https://www.ine.es/prensa/eacl_2019.pdf (accessed on 19 September 2022).
- Instituto Nacional de Estadistica. Población por Sexo y Grupo de Edad. Valores Absolutos y Porcentajes Respecto del Total de Cada Sexo. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=4028 (accessed on 20 September 2022).
- García, A.; Ortiz de Lejarazu, R.; Reina, J.; Callejo, D.; Cuervo, J.; Morano Larragueta, R. Cost-effectiveness analysis of quadrivalent influenza vaccine in Spain. Hum. Vaccines Immunother. 2016, 12, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Dolk, C.; Eichner, M.; Welte, R.; Anastassopoulou, A.; Van Bellinghen, L.A.; Poulsen Nautrup, B.; Van Vlaenderen, I.; Schmidt-Ott, R.; Schwehm, M.; Postma, M. Cost-Utility of Quadrivalent Versus Trivalent Influenza Vaccine in Germany, Using an Individual-Based Dynamic Transmission Model. PharmacoEconomics 2016, 34, 1299–1308. [Google Scholar] [CrossRef]
- Hollmann, M.; Garin, O.; Galante, M.; Ferrer, M.; Dominguez, A.; Alonso, J. Impact of influenza on health-related quality of life among confirmed (H1N1)2009 patients. PLoS ONE 2013, 8, e60477. [Google Scholar] [CrossRef] [PubMed]
- Crépey, P.; Redondo, E.; Díez-Domingo, J.; Ortiz de Lejarazu, R.; Martinón-Torres, F.; Gil de Miguel, Á.; López-Belmonte, J.L.; Alvarez, F.P.; Bricout, H.; Solozabal, M. From trivalent to quadrivalent influenza vaccines: Public health and economic burden for different immunization strategies in Spain. PLoS ONE 2020, 15, e0233526. [Google Scholar] [CrossRef] [PubMed]
- Sacristán, J.A.; Oliva, J.; Campillo-Artero, C.; Puig-Junoy, J.; Pinto-Prades, J.L.; Dilla, T.; Rubio-Terrés, C.; Ortún, V. What is an efficient health intervention in Spain in 2020? Gac. Sanit. 2020, 34, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Torres, L.; García-Lorenzo, B.; Serrano-Aguilar, P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018, 27, 746–761. [Google Scholar] [CrossRef]
- Kirch, W. (Ed.) Human Capital Approach. In Encyclopedia of Public Health; Springer: Dordrecht, The Netherlands, 2008; pp. 697–698. [Google Scholar]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M.J. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef]
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef]
- Kohli, M.A.; Maschio, M.; Cartier, S.; Mould-Quevedo, J.; Fricke, F.-U. The Cost-Effectiveness of Vaccination of Older Adults with an MF59-Adjuvanted Quadrivalent Influenza Vaccine Compared to Other Available Quadrivalent Vaccines in Germany. Vaccines 2022, 10, 1386. [Google Scholar] [CrossRef]
- Kohli, M.A.; Maschio, M.; Mould-Quevedo, J.F.; Drummond, M.; Weinstein, M.C. The cost-effectiveness of an adjuvanted quadrivalent influenza vaccine in the United Kingdom. Hum. Vaccines Immunother. 2021, 17, 4603–4610. [Google Scholar] [CrossRef]
- Ruiz-Aragón, J.; Grande Tejada, A.M.; Márquez-Peláez, S.; García-Cenoz, M. Estimación del impacto de la vacunación antigripal con adyuvante MF59 en población mayor de 64 años para el Sistema Nacional de Salud: Efectos y costes. Vacunas 2015, 16, 6–11. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Vizzotti, C.; Uruena, A.; Giglio, N.; Magneres, C.; Richmond, H. Cost-effectiveness of introducing an MF59-adjuvanted trivalent influenza vaccine for older adults in Argentina. Vaccine 2020, 38, 3682–3689. [Google Scholar] [CrossRef]

| Event | 2017–2018 | 2018–2019 | 2019–2020 |
|---|---|---|---|
| Symptomatic cases | 22,530 | 13,697 | 10,636 |
| Primary care visits | 950 | 545 | 445 |
| ED visits | 213 | 122 | 100 |
| Hospitalizations | 668 | 489 | 324 |
| Deaths | 40 | 29 | 19 |
| Parameter | Value | Reference |
|---|---|---|
| Percentage of population aged ≥65 years who were vaccinated | 69.4% | [24] |
| Life expectancy for population aged ≥65 years | 9.8 years | [25] |
| Size of population aged ≥65 years | 9,371,743 | [26] |
| aQIV tender price | EUR 13 | [27] |
| QIVr tender price | EUR 25 | [27] |
| Primary-care physician visit costs (per visit) | EUR 59 | [28,29,30] |
| ED visit costs (per visit) | EUR 183 | [28,29,30] |
| Hospitalization costs (per event) | EUR 4467 | [30,31] |
| Co-medication costs (per influenza case) | EUR 3.21 | [32] |
| Probability of being employed (65–69 years) | 1.2% | [33] |
| Probability of being employed (≥75 years) | 0.3% | [33] |
| Productivity loss per hour | EUR 17.44 | [34] |
| Probability of requiring care at home (65–69 years) | 5.4% | [35] |
| Probability of requiring care at home (≥75 years) | 14% | [35] |
| Baseline utility for age ≥65 years | 0.65 | [36] |
| Disutility value for symptomatic patients | 0.32 | [37] |
| Disutility value for outpatient setting | 0.33 | [38] |
| Disutility value for inpatient setting | 0.6 | [38] |
| Disutility duration for symptomatic patients | 7 days | [37] |
| Disutility duration in outpatient setting | 7 days | [38] |
| Disutility duration in inpatient setting | 21 days | [38] |
| Hospital mortality rate for population aged ≥65 years | 6% | [39] |
| Discount rates for costs and outcomes * | 3% | [23] |
| Scenario | Current Tender Price | rVE | Incremental Cost | QALYs Gained | ICER |
|---|---|---|---|---|---|
| Izurieta 2021 rVE | EUR 25 | 10.70% | EUR 71,054,601.74 | 699.27 | EUR 101,612.41 |
| ICER = EUR 25,000/QALY gained | EUR 25 | 34.12% | EUR 55,747,194.30 | 2229.88 | EUR 25,000.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Aragón, J.; Márquez-Peláez, S. An Economic Comparison in the Elderly of Adjuvanted Quadrivalent Influenza Vaccine with Recombinant Quadrivalent Influenza Vaccine in Spain. Vaccines 2023, 11, 427. https://doi.org/10.3390/vaccines11020427
Ruiz-Aragón J, Márquez-Peláez S. An Economic Comparison in the Elderly of Adjuvanted Quadrivalent Influenza Vaccine with Recombinant Quadrivalent Influenza Vaccine in Spain. Vaccines. 2023; 11(2):427. https://doi.org/10.3390/vaccines11020427
Chicago/Turabian StyleRuiz-Aragón, Jesús, and Sergio Márquez-Peláez. 2023. "An Economic Comparison in the Elderly of Adjuvanted Quadrivalent Influenza Vaccine with Recombinant Quadrivalent Influenza Vaccine in Spain" Vaccines 11, no. 2: 427. https://doi.org/10.3390/vaccines11020427
APA StyleRuiz-Aragón, J., & Márquez-Peláez, S. (2023). An Economic Comparison in the Elderly of Adjuvanted Quadrivalent Influenza Vaccine with Recombinant Quadrivalent Influenza Vaccine in Spain. Vaccines, 11(2), 427. https://doi.org/10.3390/vaccines11020427






