The Dynamic Linkage between Provirus Integration Sites and the Host Functional Genome Property Alongside HIV-1 Infections Associated with Antiretroviral Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Procession of Public Datasets
2.2. Data and Bioinformatic Analyses
2.2.1. MsigDb Over-Representation Analysis

2.2.2. KEGG Pathway Over-Representation Analysis
2.2.3. Determination of Provirus-Targeted Genes Interacting with HIV-1
2.2.4. Statistics
3. Results
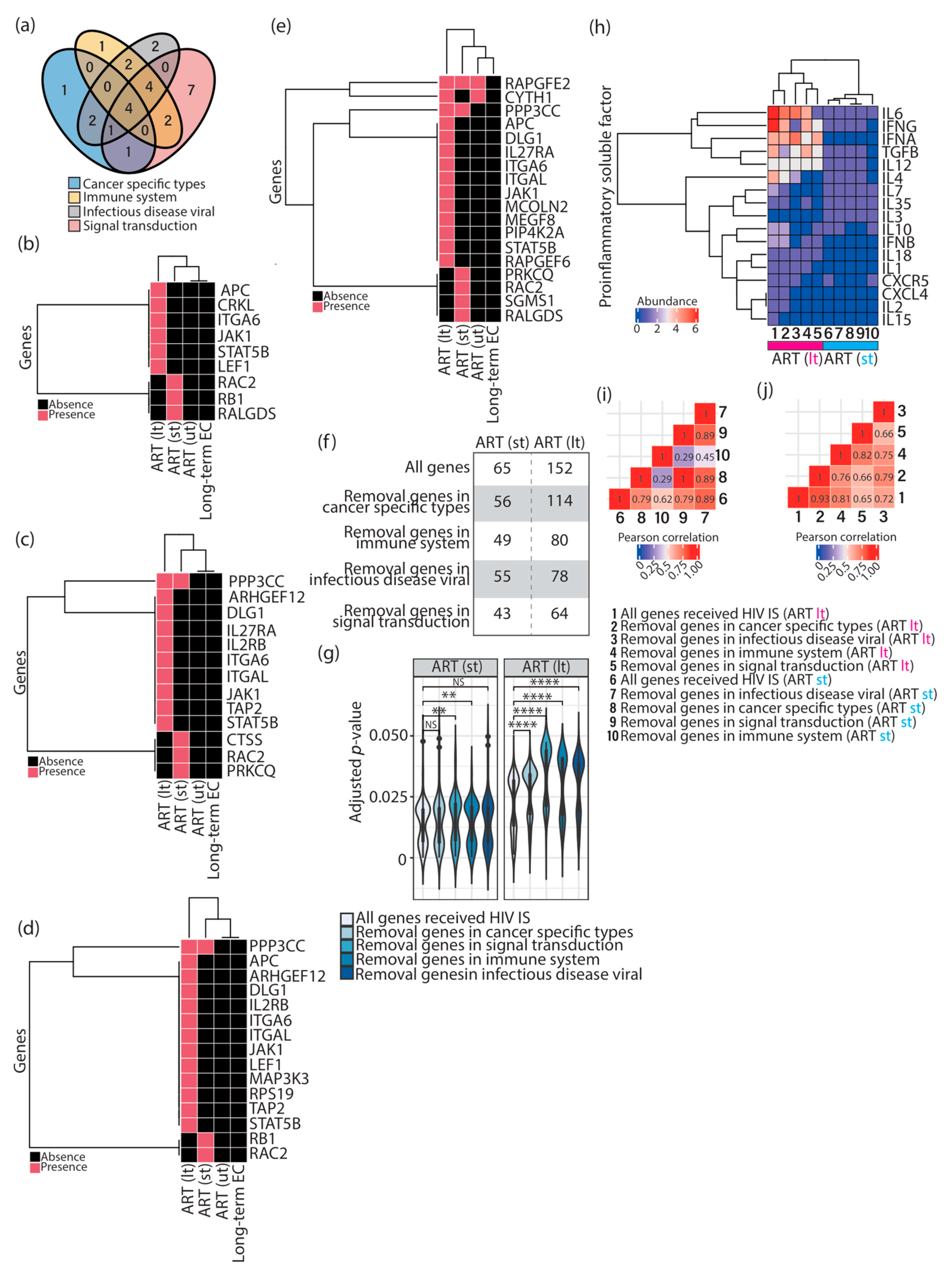
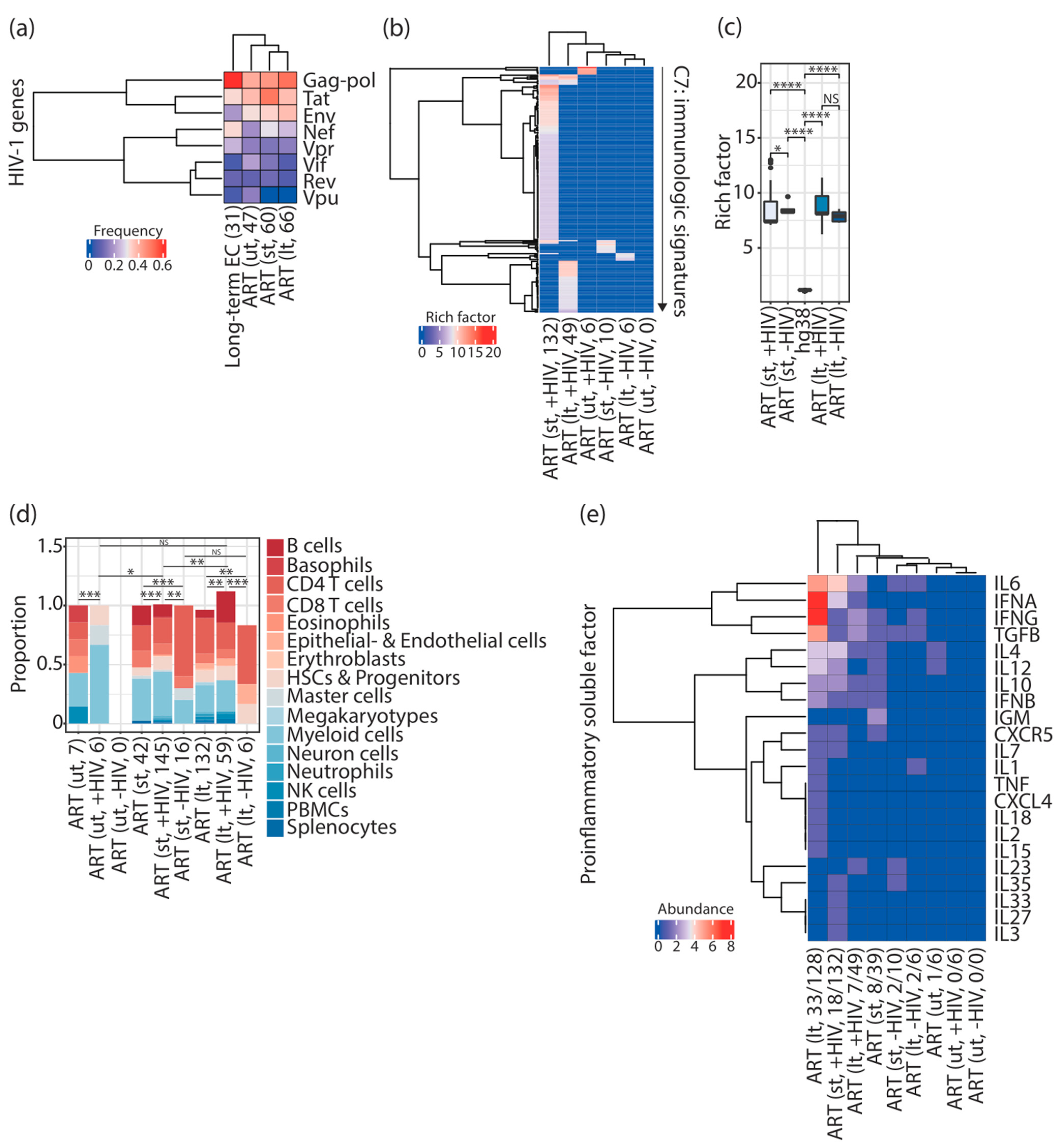
3.1. Different Immunologic Signatures Were Enriched Alongside HIV-1 Infections Associated with ART
3.2. Different Combinations of Immune Cell Signatures Were Observed Alongside HIV-1 Infections Associated with ART
3.3. Different Combinations of Proinflammatory Soluble Factor Signatures Were Observed Alongside HIV-1 Infections Associated with ART
3.4. The Majority of Enriched Immunologic Signatures Resulted from Gene Sets That Interact with HIV-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikeda, T.; Shibata, J.; Yoshimura, K.; Koito, A.; Matsushita, S. Recurrent HIV-1 Integration at theBACH2Locus in Resting CD4 T Cell Populations during Effective Highly Active Antiretroviral Therapy. J. Infect. Dis. 2007, 195, 716–725. [Google Scholar] [CrossRef]
- Maldarelli, F.; Wu, X.; Su, L.; Simonetti, F.R.; Shao, W.; Hill, S.; Spindler, J.; Ferris, A.L.; Mellors, J.W.; Kearney, M.F.; et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014, 345, 179–183. [Google Scholar] [CrossRef]
- Wagner, T.A.; McLaughlin, S.; Garg, K.; Cheung, C.Y.K.; Larsen, B.B.; Styrchak, S.; Huang, H.C.; Edlefsen, P.T.; Mullins, J.I.; Frenkel, L.M. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014, 345, 570–573. [Google Scholar] [CrossRef]
- Zhyvoloup, A.; Melamed, A.; Anderson, I.; Planas, D.; Lee, C.-H.; Kriston-Vizi, J.; Ketteler, R.; Merritt, A.; Routy, J.-P.; Ancuta, P.; et al. Digoxin reveals a functional connection between HIV-1 integration preference and T-cell activation. PLoS Pathog. 2017, 13, e1006460. [Google Scholar] [CrossRef]
- Einkauf, K.B.; Osborn, M.R.; Gao, C.; Sun, W.; Sun, X.; Lian, X.; Parsons, E.M.; Gladkov, G.T.; Seiger, K.W.; Blackmer, J.E.; et al. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell 2022, 185, 266–282.e15. [Google Scholar] [CrossRef]
- Jiang, C.; Lian, X.; Gao, C.; Sun, X.; Einkauf, K.B.; Chevalier, J.M.; Chen, S.M.Y.; Hua, S.; Rhee, B.; Chang, K.; et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 2020, 585, 261–267. [Google Scholar] [CrossRef]
- Pastor-Satorras, R.; Vázquez, A.; Vespignani, A. Dynamical and correlation properties of the internet. Phys. Rev. Lett. 2001, 87, 258701. [Google Scholar] [CrossRef]
- Girvan, M.; Newman, M.E.J. Community structure in social and biological networks. Proc. Natl. Acad. Sci. USA 2002, 99, 7821–7826. [Google Scholar] [CrossRef]
- Krause, J.; Croft, D.P.; James, R. Social network theory in the behavioural sciences: Potential applications. Behav. Ecol. Sociobiol. 2007, 62, 15–27. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Ferreira, A.-M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Boyle, E.I.; Weng, S.; Gollub, J.; Jin, H.; Botstein, D.; Cherry, J.M.; Sherlock, G. GO::TermFinder--open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 2004, 20, 3710–3715. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdottir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Ptak, R.G.; Fu, W.; Sanders-Beer, B.E.; Dickerson, J.E.; Pinney, J.W.; Robertson, D.L.; Rozanov, M.N.; Katz, K.S.; Maglott, D.R.; Pruitt, K.D.; et al. Short Communication: Cataloguing the HIV Type 1 Human Protein Interaction Network. AIDS Res. Hum. Retrovir. 2008, 24, 1497–1502. [Google Scholar] [CrossRef]
- Fu, W.; Sanders-Beer, B.E.; Katz, K.S.; Maglott, D.R.; Pruitt, K.D.; Ptak, R.G. Human immunodeficiency virus type 1, human protein interaction database at NCBI. Nucleic Acids Res. 2009, 37, D417–D422. [Google Scholar] [CrossRef]
- Pinney, J.W.; Dickerson, J.E.; Fu, W.; Sanders-Beer, B.E.; Ptak, R.G.; Robertson, D.L. HIV-host interactions: A map of viral perturbation of the host system. AIDS 2009, 23, 549–554. [Google Scholar] [CrossRef]
- Clerici, M.; Shearer, G.M. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol. Today 1993, 14, 107–111. [Google Scholar] [CrossRef]
- Hiener, B.; Horsburgh, B.A.; Eden, J.-S.; Barton, K.; Schlub, T.E.; Lee, E.; Stockenstrom, S.; Odevall, L.; Milush, J.M.; Liegler, T.; et al. Identification of Genetically Intact HIV-1 Proviruses in Specific CD4 T Cells from Effectively Treated Participants. Cell Rep. 2017, 21, 813–822. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.S.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef]
- Sun, W.; Gao, C.; Hartana, C.A.; Osborn, M.R.; Einkauf, K.B.; Lian, X.; Bone, B.; Bonheur, N.; Chun, T.-W.; Rosenberg, E.S.; et al. Phenotypic signatures of immune selection in HIV-1 reservoir cells. Nature 2023. online ahead of print. [Google Scholar] [CrossRef]
- Duraiswamy, J.; Ibegbu, C.C.; Masopust, D.; Miller, J.D.; Araki, K.; Doho, G.H.; Tata, P.; Gupta, S.; Zilliox, M.J.; Nakaya, H.I.; et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J. Immunol. 2011, 186, 4200–4212. [Google Scholar] [CrossRef]
- Quigley, M.; Pereyra, F.; Nilsson, B.; Porichis, F.; Fonseca, C.; Eichbaum, Q.; Julg, B.; Jesneck, J.L.; Brosnahan, K.; Imam, S.; et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 2010, 16, 1147–1151. [Google Scholar] [CrossRef]
- Chen, K.-C.; Wang, T.-Y.; Chan, C.-H. Associations between HIV and human pathways revealed by protein-protein interactions and correlated gene expression profiles. PLoS ONE 2012, 7, e34240. [Google Scholar] [CrossRef]
- Besnard, E.; Hakre, S.; Kampmann, M.; Lim, H.W.; Hosmane, N.N.; Martin, A.; Bassik, M.C.; Verschueren, E.; Battivelli, E.; Chan, J.; et al. The mTOR Complex Controls HIV Latency. Cell Host Microbe 2016, 20, 785–797. [Google Scholar] [CrossRef]
- Crater, J.M.; Nixon, D.F.; O’Brien, R.L.F. HIV-1 replication and latency are balanced by mTOR-driven cell metabolism. Front. Cell Infect. Microbiol. 2022, 12, 1068436. [Google Scholar] [CrossRef]
- Valle-Casuso, J.C.; Angin, M.; Volant, S.; Passaes, C.; Monceaux, V.; Mikhailova, A.; Bourdic, K.; Avettand-Fenoel, V.; Boufassa, F.; Sitbon, M.; et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4 T Cells and Offers an Opportunity to Tackle Infection. Cell Metab. 2019, 29, 611–626.e5. [Google Scholar] [CrossRef]
- Rebhun, J.F.; Castro, A.F.; Quilliam, L.A. Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction. J. Biol. Chem. 2000, 275, 34901–34908. [Google Scholar] [CrossRef]
- Folgueira, L.; Algeciras, A.; MacMorran, W.S.; Bren, G.D.; Paya, C.V. The Ras-Raf pathway is activated in human immunodeficiency virus-infected monocytes and particpates in the activation of NF-kappa B. J. Virol. 1996, 70, 2332–2338. [Google Scholar] [CrossRef]
- Gerritsen, M.E.; Williams, A.J.; Neish, A.S.; Moore, S.; Shi, Y.; Collins, T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. USA 1997, 94, 2927–2932. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.-D.; Zhou, M.-M.; Ozato, K.; Chen, L.-F. Brd4 Coactivates Transcriptional Activation of NF-κB via Specific Binding to Acetylated RelA. Mol. Cell. Biol. 2009, 29, 1375–1387. [Google Scholar] [CrossRef]
- Brogdon, J.; Ziani, W.; Wang, X.; Veazey, R.S.; Xu, H. In Vitro effects of the small-molecule protein kinase C agonists on HIV latency reactivation. Sci. Rep. 2016, 6, 39032. [Google Scholar] [CrossRef]
- Kabi, M.; Filion, G.J. Chromatin and viral integration in immunity: The challenge of silencing non-self genes. Trends Immunol. 2022, 43, 449–458. [Google Scholar] [CrossRef]
- Qu, D.; Sun, W.-W.; Li, L.; Ma, L.; Sun, L.; Jin, X.; Li, T.; Hou, W.; Wang, J.-H. Long noncoding RNA MALAT1 releases epigenetic silencing of HIV-1 replication by displacing the polycomb repressive complex 2 from binding to the LTR promoter. Nucleic Acids Res. 2019, 47, 3013–3027. [Google Scholar] [CrossRef]
- Quaranta, M.G.; Mattioli, B.; Spadaro, F.; Straface, E.; Giordani, L.; Ramoni, C.; Malorni, W.; Viora, M. HIV-1 Nef triggers Vav-mediated signaling pathway leading to functional and morphological differentiation of dendritic cells. FASEB J. 2003, 17, 2025–2036. [Google Scholar] [CrossRef]
- Rauch, S.; Pulkkinen, K.; Saksela, K.; Fackler, O.T. Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vav1 via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity. J. Virol. 2008, 82, 2918–2929. [Google Scholar] [CrossRef]
- Sarmady, M.; Dampier, W.; Tozeren, A. Sequence- and interactome-based prediction of viral protein hotspots targeting host proteins: A case study for HIV Nef. PLoS ONE 2011, 6, e20735. [Google Scholar] [CrossRef]
- Jang, M.K.; Mochizuki, K.; Zhou, M.; Jeong, H.-S.; Brady, J.N.; Ozato, K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 2005, 19, 523–534. [Google Scholar] [CrossRef]
- Yang, Z.; Yik, J.H.N.; Chen, R.; He, N.; Jang, M.K.; Ozato, K.; Zhou, Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 2005, 19, 535–545. [Google Scholar] [CrossRef]
- Bisgrove, D.A.; Mahmoudi, T.; Henklein, P.; Verdin, E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc. Natl. Acad. Sci. USA 2007, 104, 13690–13695. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Wu, Y.; Zhou, Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 2013, 41, 277–287. [Google Scholar] [CrossRef]
- Ladha, J.S.; Tripathy, M.K.; Mitra, D. Mitochondrial complex I activity is impaired during HIV-1-induced T-cell apoptosis. Cell Death Differ. 2005, 12, 1417–1428. [Google Scholar] [CrossRef]
- Chaussabel, D.; Baldwin, N. Democratizing systems immunology with modular transcriptional repertoire analyses. Nat. Rev. Immunol. 2014, 14, 271–280. [Google Scholar] [CrossRef]
- Li, S.; Rouphael, N.; Duraisingham, S.; Romero-Steiner, S.; Presnell, S.; Davis, C.; Schmidt, D.S.; Johnson, S.E.; Milton, A.; Rajam, G.; et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. 2014, 15, 195–204. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Dunn, P.; Thomas, C.G.; Smith, B.; Schaefer, H.; Chen, J.; Hu, Z.; Zalocusky, K.A.; Shankar, R.D.; Shen-Orr, S.S.; et al. ImmPort, toward repurposing of open access immunological assay data for translational and clinical research. Sci. Data 2018, 5, 180015. [Google Scholar] [CrossRef]
- Xie, D.; Han, L.; Luo, Y.; Liu, Y.; He, S.; Bai, H.; Wang, S.; Bo, X. Exploring the associations of host genes for viral infection revealed by genome-wide RNAi and virus-host protein interactions. Mol. Biosyst. 2015, 11, 2511–2519. [Google Scholar] [CrossRef]
- Bermejo, M.; López-Huertas, M.R.; Hedgpeth, J.; Mateos, E.; Rodríguez-Mora, S.; Maleno, M.J.; Plana, M.; Swindle, J.; Alcamí, J.; Coiras, M. Analysis of protein kinase C theta inhibitors for the control of HIV-1 replication in human CD4+ T cells reveals an effect on retrotranscription in addition to viral transcription. Biochem. Pharmacol. 2015, 94, 241–256. [Google Scholar] [CrossRef]
- López-Huertas, M.R.; Li, J.; Zafar, A.; Rodríguez-Mora, S.; García-Domínguez, C.; Mateos, E.; Alcamí, J.; Rao, S.; Coiras, M. PKCθ and HIV-1 Transcriptional Regulator Tat Co-exist at the LTR Promoter in CD4(+) T Cells. Front. Immunol. 2016, 7, 69. [Google Scholar] [CrossRef]
- Smith, B.L.; Krushelnycky, B.W.; Mochly-Rosen, D.; Berg, P. The HIV Nef Protein Associates with Protein Kinase C Theta. J. Biol. Chem. 1996, 271, 16753–16757. [Google Scholar] [CrossRef]
- Witte, V.; Laffert, B.; Gintschel, P.; Krautkrämer, E.; Blume, K.; Fackler, O.T.; Baur, A.S. Induction of HIV transcription by Nef involves Lck activation and protein kinase Cθ raft recruitment leading to activation of ERK1/2 but not NFκB. J. Immunol. 2009, 181, 3327. [Google Scholar] [CrossRef]
- Janardhan, A.; Swigut, T.; Hill, B.; Myers, M.P.; Skowronski, J. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2004, 2, E6. [Google Scholar] [CrossRef]
- Olivetta, E.; Pietraforte, D.; Schiavoni, I.; Minetti, M.; Federico, M.; Sanchez, M. HIV-1 Nef regulates the release of superoxide anions from human macrophages. Biochem. J. 2005, 390, 591–602. [Google Scholar] [CrossRef]
- Turner, A.-M.W.; Dronamraju, R.; Potjewyd, F.; James, K.S.; Winecoff, D.K.; Kirchherr, J.L.; Archin, N.M.; Browne, E.P.; Strahl, B.D.; Margolis, D.M.; et al. Evaluation of EED Inhibitors as a Class of PRC2-Targeted Small Molecules for HIV Latency Reversal. ACS Infect. Dis. 2020, 6, 1719–1733. [Google Scholar] [CrossRef]
- Ruiz-Riol, M.; Berdnik, D.; Llano, A.; Mothe, B.; Gálvez, C.; Pérez-Álvarez, S.; Oriol-Tordera, B.; Olvera, A.; Silva-Arrieta, S.; Meulbroek, M.; et al. Identification of Interleukin-27 (IL-27)/IL-27 Receptor Subunit Alpha as a Critical Immune Axis for HIV Control. J. Virol. 2017, 91, e00441-17. [Google Scholar] [CrossRef]
- Zheng, Y.-H.; Xiao, S.-L.; He, B.; He, Y.; Zhou, H.-Y.; Chen, Z.; Zheng, L.-W.; He, M.; Wang, H.-Y.; Lin, Y.-H.; et al. The Role of IL-27 and its Receptor in the Pathogenesis of HIV/AIDS and Anti-viral Immune Response. Curr. HIV Res. 2017, 15, 279–284. [Google Scholar] [CrossRef]
- Zheng, C.F.; Jones, G.J.; Shi, M.; Wiseman, J.C.D.; Marr, K.J.; Berenger, B.M.; Huston, S.M.; John Gill, M.; Krensky, A.M.; Kubes, P.; et al. Late expression of granulysin by microbicidal CD4+ T cells requires PI3K- and STAT5-dependent expression of IL-2Rbeta that is defective in HIV-infected patients. J. Immunol. 2008, 180, 7221–7229. [Google Scholar] [CrossRef]
- De Armas, L.R.; Gavegnano, C.; Pallikkuth, S.; Rinaldi, S.; Pan, L.; Battivelli, E.; Verdin, E.; Younis, R.T.; Pahwa, R.; Williams, S.L.; et al. The Effect of JAK1/2 Inhibitors on HIV Reservoir Using Primary Lymphoid Cell Model of HIV Latency. Front. Immunol. 2021, 12, 720697. [Google Scholar] [CrossRef]
- Matusali, G.; Tchidjou, H.K.; Pontrelli, G.; Bernardi, S.; D’Ettorre, G.; Vullo, V.; Buonomini, A.R.; Andreoni, M.; Santoni, A.; Cerboni, C.; et al. Soluble ligands for the NKG2D receptor are released during HIV-1 infection and impair NKG2D expression and cytotoxicity of NK cells. FASEB J. 2013, 27, 2440–2450. [Google Scholar] [CrossRef]
- Abitew, A.M.; Sobti, R.C.; Sharma, V.L.; Wanchu, A. Analysis of transporter associated with antigen presentation (TAP) genes polymorphisms with HIV-1 infection. Mol. Cell Biochem. 2020, 464, 65–71. [Google Scholar] [CrossRef]
- Rom, S.; Pacifici, M.; Passiatore, G.; Aprea, S.; Waligorska, A.; Del Valle, L.; Peruzzi, F. HIV-1 Tat binds to SH3 domains: Cellular and viral outcome of Tat/Grb2 interaction. Biochim. Biophys. Acta. 2011, 1813, 1836–1844. [Google Scholar] [CrossRef]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A whole-genome association study of major determinants for host control of HIV-1. Science 2007, 317, 944–947. [Google Scholar] [CrossRef]
- Lee, G.Q.; Orlova-Fink, N.; Einkauf, K.; Chowdhury, F.Z.; Sun, X.; Harrington, S.; Kuo, H.-H.; Hua, S.; Chen, H.-R.; Ouyang, Z.; et al. Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J. Clin. Invest. 2017, 127, 2689–2696. [Google Scholar] [CrossRef]
- Einkauf, K.B.; Lee, G.Q.; Gao, C.; Sharaf, R.; Sun, X.; Hua, S.; Chen, S.M.Y.; Jiang, C.; Lian, X.; Chowdhury, F.Z.; et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J. Clin. Invest. 2019, 129, 988–998. [Google Scholar] [CrossRef]

| Pretreatment (ut) | Short Period of ART (st) | Long Period of ART (lt) | Elite Controllers |
|---|---|---|---|
| ACOX1 | AAK1 | ABCC1 | ABCA11P |
| ACSF3 | AC004791.2 | ACTR3 | AGPAT3 |
| ADARB1 | ACAP2 | ADGRE5 | ANKRD11 |
| ADTRP | ADD3 | AHR | APBA2 |
| AIM1 | AJ003147.9 | ANKRD11 | ARHGAP17 |
| ANKLE2 | ANKRD11 | APC | ARHGAP31 |
| ANKRD31 | ARHGAP24 | ARHGEF12 | ARHGEF18 |
| ARHGAP35 | ARIH2 | ARHGEF3 | ASXL1 |
| ARID4B | ASAP1 | ARID1A | ATR |
| ASNA1 | ASH1L | ARNT | BACH2 |
| BRD1 | ATXN2L | ASH1L | CEACAM21 |
| BUB1B | BOLA2 | ASPSCR1 | COX10-AS1 |
| C10orf76 | BRD4 | ATN1 | CREBBP |
| CCDC26 | C16orf52 | AUH | DCLRE1C |
| CCDC57 | C17orf62 | B3GALT4 | DECR1 |
| CD58 | C4B | BACH2 | DHTKD1 |
| CEP85 | C6orf89 | BCL7C | DIP2A |
| CHAF1A | CHD6 | BRD4 | DIP2A-IT1 |
| CLIP1 | CLN5 | C16orf72 | DLGAP1 |
| CNN2 | CLUAP1 | C4orf22 | ELMO1 |
| CNOT1 | CNOT6L | CAMK2D | ENSG00000231731 |
| CNOT4 | CNTRL | CCDC30 | ENSG00000260989 |
| CRTC3 | COL5A1 | CCDC57 | ENSG00000265987 |
| CSNK1G1 | COPB1 | CD84 | ENSG00000267179 |
| CSNK1G2 | CPNE1 | CENPC | ENSG00000267552 |
| CTCF | CREBBP | CHMP4B | ENSG00000281016 |
| CTSA | CTSS | CLIP4 | ENSG00000283515 |
| CYTH1 | CYLD | CNKSR2 | ENSG00000285816 |
| DAB1 | DARS-AS1 | COTL1 | ENSG00000288715 |
| DAZAP1 | DGKA | CRKL | ENTR1 |
| DNM2 | DIAPH1 | CUL5 | ERC1 |
| EHMT1 | DNAJC18 | CYTH1 | EVL |
| ETFA | DPF2 | DAP3 | FCHSD2 |
| FANCA | DYNC1H1 | DAPK2 | GRB2 |
| FBXL12 | EHD4 | DAZAP1 | HLA-DQA1 |
| FGD3 | EP400 | DCLRE1C | HSF5 |
| FOXK2 | ERCC6L2 | DCUN1D1 | IFT140 |
| FRMD4B | FAM102A | DLG1 | IWS1 |
| FUS | FAM102B | DNAH1 | JMJD1C |
| GBF1 | FAM134B | DNAJC18 | KANSL2 |
| GRSF1 | FANCA | DNMT1 | KDM2A |
| HSPBAP1 | FBXL20 | DPP8 | KIAA0319L |
| IARS | FGD3 | E2F3 | KMT2C |
| IFNAR1 | FOXK2 | EED | LHPP |
| IGSF6 | FUT8 | EIF4G3 | LINC00298 |
| IL12RB1 | GIMAP8 | EPB41 | LMTK2 |
| IP6K1 | GPR89A | ERCC6L2 | MGMT |
| KIAA2026 | GRB2 | ERMP1 | MYDGF |
| KIF21A | GTF2E2 | ESCO1 | MYO1F |
| KMT2A | HIST1H2AC | EVI5 | NELL2 |
| LINC | HMGCS1 | EXOC7 | NF1 |
| LINC00499 | HN1L | FAM13A | NPHP3 |
| LINC00536 | HNRNPC | FAM214A | NPHP3-ACAD11 |
| MACF1 | HTT | FNBP1 | NUMA1 |
| MALAT1 | IKZF3 | FRY | P4HB |
| METTL15 | IRF2 | GATAD2A | PACS1 |
| METTL8 | IRGQ | GGA3 | PARP4 |
| MFSD12 | ITPKB | GON4L | PCNT |
| MROH1 | ITPR3 | GRAP2 | PIK3IP1 |
| MTG1 | KIAA0753 | HBS1L | POLA2 |
| NAA38 | KXD1 | HECTD4 | PPM1G |
| NCOA7 | LCOR | HFE | PPP4R3A |
| NCOR1 | LINC00869 | HIVEP3 | PRDM2 |
| NLRC5 | MACF1 | IL27RA | RAB14 |
| NPLOC4 | MALT1 | IL2RB | RABEP1 |
| NT5C2 | MAN1A2 | IQGAP1 | RAD54L2 |
| NUDT3 | MAPK14 | IQGAP2 | RAI1 |
| NUP98 | MARS | ITGA6 | RAP1GDS1 |
| PARPBP | MAST2 | ITGAL | RNF157 |
| PBX3 | MCF2L2 | JAK1 | RPL15P4 |
| PDK3 | MDS2 | KANSL3 | RPTOR |
| PDXDC2P | MED15 | KDM2A | SMG7 |
| PGM2L1 | METTL15 | KDM3A | SPEN |
| PPFIA1 | MKL1 | KIAA0430 | SPPL3 |
| PPP2CA | MORC3 | KIAA0753 | STK38 |
| PRDM2 | MRPS6 | KIF13B | STX16 |
| PRKAG1 | MYH9 | LDLRAD4 | STX16-NPEPL1 |
| PRRC1 | NACC2 | LEF1 | TBCD |
| PSMB2 | NCAPD2 | LPP | TMEM245 |
| PTPN1 | NCBP3 | LSM14A | TNRC6A |
| RAPGEF2 | NDC80 | LUZP1 | TPTE2P6 |
| RBX1 | NDUFA6 | MALAT1 | TRAPPC14 |
| RN7SKP127 | NDUFAF2 | MAP3K3 | TRIM37 |
| RNF214 | NMD3 | MCOLN2 | TRIT1 |
| RNF216 | NPEPPS | MEGF8 | UBE2O |
| RNF34 | NR2C2 | MICB | UBE3C |
| RPTOR | OS9 | MIR3681HG | UBR2 |
| RRN3 | PACS1 | MROH1 | UTP20 |
| RUNX2 | PIBF1 | MYCBP2 | ZMYM4 |
| SARS | PIK3R1 | NDUFA6 | ZNF160 |
| SCMH1 | PLEC | NFKBIL1 | ZNF180 |
| SECISBP2L | PPP3CC | NSD1 | ZNF225 |
| SEPT9 | PPP6R3 | NUDT3 | ZNF274 |
| SETD3 | PRKCA | NUDT4 | ZNF350 |
| SMARCC1 | PRKCQ | NVL | ZNF350-AS1 |
| SPG11 | PRR19 | OGDH | ZNF407 |
| STAU1 | PSME4 | PATL1 | ZNF430 |
| TAF1 | PSMF1 | PBRM1 | ZNF544 |
| TBC1D4 | PTPN4 | PCBP2 | ZNF607 |
| TGFB1 | QRICH1 | PHACTR4 | ZNF700 |
| THRAP3 | RAB11FIP3 | PHC1 | ZNF720 |
| TMEM87A | RAC2 | PHF12 | ZNF721 |
| UBAP1 | RAD51 | PIP4K2A | ZSCAN16 |
| UBE2D2 | RALGDS | PITPNC1 | ZSCAN16-AS1 |
| UBE2G1 | RAPGEF2 | PLA2G16 | |
| UMAD1 | RB1 | PPP3CC | |
| UNC13B | RBL1 | PPP6R1 | |
| USP3 | RCOR1 | PRR19 | |
| VAV1 | REV1 | PUM1 | |
| VDR | RNF157 | PXK | |
| VPS13B | RPRD2 | RAD18 | |
| WARS2 | RPTOR | RANBP9 | |
| ZAN | SACM1L | RAPGEF2 | |
| ZBED5 | SAE1 | RAPGEF6 | |
| ZNF226 | SCYL1 | RBM15 | |
| ZNF557 | SGMS1 | RFFL | |
| ZNF701 | SKAP1 | RIMKLB | |
| ZNF737 | SMCHD1 | RPS19 | |
| ZNF850 | SMG6 | RPS6KA3 | |
| ZNF91 | SNX25 | RPTOR | |
| ZSCAN22 | SPECC1L | RSRC2 | |
| STK11 | SAE1 | ||
| TARBP1 | SCNN1A | ||
| TATDN2 | SERPINH1 | ||
| TBK1 | SETX | ||
| TEX2 | SF3B2 | ||
| TMEM65 | SFMBT1 | ||
| TNRC6C | SHCBP1 | ||
| TOM1L2 | SLC23A2 | ||
| TPM3 | SLC25A40 | ||
| TRAF3 | SLC30A7 | ||
| TRPC4AP | SLC35A3 | ||
| TUBA8 | SLC44A2 | ||
| TYW1 | SLC9A6 | ||
| UBOX5 | SLX4IP | ||
| USP25 | SNTB2 | ||
| USP49 | SPEN | ||
| USP6NL | ST13 | ||
| USP9Y | ST7 | ||
| VCL | STAT5B | ||
| VRK1 | SUMO2 | ||
| WASH1 | TAB1 | ||
| WBP1L | TAF2 | ||
| WHSC1L1 | TANGO6 | ||
| WNK1 | TAP2 | ||
| ZBED4 | TBC1D2B | ||
| ZC3H18 | TCF3 | ||
| ZNF564 | TMBIM6 | ||
| ZNF609 | TNFSF12 | ||
| TNRC6B | |||
| TNRC6C | |||
| TOM1L2 | |||
| TP53TG5 | |||
| TRAT1 | |||
| TSEN54 | |||
| TSG101 | |||
| UBA6 | |||
| UBE2Q2 | |||
| UBTD2 | |||
| UPF1 | |||
| USP34 | |||
| VARS | |||
| VAV1 | |||
| VPS13D | |||
| VPS8 | |||
| WASF2 | |||
| XPO6 | |||
| Y_RNA | |||
| YWHAE | |||
| ZCCHC11 | |||
| ZCCHC7 | |||
| ZKSCAN8 | |||
| ZNF140 | |||
| ZNF451 | |||
| ZNF529 | |||
| ZNRF1 |
| Patient Sample | Number of Provirus-Targeted Genes (Number of Enriched Immunologic Signatures) | |
|---|---|---|
| Immune Cell Types | Proinflammatory Soluble Factors | |
| Pretreatment (ut) | 35 (6) | 7 (1) |
| Short period of ART (st) | 101 (37) | 37 (7) |
| Long period of ART (lt) | 164 (121) | 109 (33) |
| Elite controllers | 48 (14) | 25 (6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-C. The Dynamic Linkage between Provirus Integration Sites and the Host Functional Genome Property Alongside HIV-1 Infections Associated with Antiretroviral Therapy. Vaccines 2023, 11, 402. https://doi.org/10.3390/vaccines11020402
Chen H-C. The Dynamic Linkage between Provirus Integration Sites and the Host Functional Genome Property Alongside HIV-1 Infections Associated with Antiretroviral Therapy. Vaccines. 2023; 11(2):402. https://doi.org/10.3390/vaccines11020402
Chicago/Turabian StyleChen, Heng-Chang. 2023. "The Dynamic Linkage between Provirus Integration Sites and the Host Functional Genome Property Alongside HIV-1 Infections Associated with Antiretroviral Therapy" Vaccines 11, no. 2: 402. https://doi.org/10.3390/vaccines11020402
APA StyleChen, H.-C. (2023). The Dynamic Linkage between Provirus Integration Sites and the Host Functional Genome Property Alongside HIV-1 Infections Associated with Antiretroviral Therapy. Vaccines, 11(2), 402. https://doi.org/10.3390/vaccines11020402






