Effect of COVID-19 Vaccination on the In-Hospital Prognosis of Patients Admitted during Delta and Omicron Waves in Italy
Abstract
:1. Introduction
2. Materials
Statistical Analysis
3. Results
3.1. Patients during Delta and Omicron Variant Waves
3.2. Vaccination Status
3.3. Vaccinated and Unvaccinated Patients
3.4. Different Vaccination Regimens
3.5. Clinical Severity at Emergency Room Presentation by Vaccination Status
3.6. Outcomes by Vaccination Status
3.7. Impact of Vaccination on Outcomes
3.8. Impact of Variant on Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Liu, B.; Zhang, S.; Huang, N.; Zhao, T.; Lu, Q.B.; Cui, F. Differences in incidence and fatality of COVID-19 by SARS-CoV-2 Omicron variant versus Delta variant in relation to vaccine coverage: A world-wide review. J. Med. Virol. 2023, 95, e28118. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Lim, S.R.; Yau, C.E.; Liew, T.M. Examining the Prevailing Negative Sentiments Related to COVID-19 Vaccination: Unsupervised Deep Learning of Twitter Posts over a 16 Month Period. Vaccines 2022, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Piccini, G.; Pierleoni, G.; Leonardi, M.; Dapporto, F.; Marchi, S.; Andreano, E.; Paciello, I.; Benincasa, L.; Lovreglio, P.; et al. Immune response to SARS-CoV-2 Omicron variant in patients and vaccinees following homologous and heterologous vaccinations. Commun. Biol. 2022, 5, 903. [Google Scholar] [CrossRef]
- Gram, M.A.; Emborg, H.D.; Schelde, A.B.; Friis, N.U.; Nielsen, K.F.; Moustsen-Helms, I.R.; Legarth, R.; Lam, J.U.H.; Chaine, M.; Malik, A.Z.; et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022, 19, e1003992. [Google Scholar] [CrossRef]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Passariello, L.; Albanese, L.; Molinari, A.M.; Angelillo, I.F. Antibody levels after BNT162b2 vaccine booster and SARS-CoV-2 Omicron infection. Vaccine 2022, 40, 5726–5731. [Google Scholar] [CrossRef]
- Keyel, A.C.; Russell, A.; Plitnick, J.; Rowlands, J.V.; Lamson, D.M.; Rosenberg, E.; St George, K. SARS-CoV-2 Vaccine Breakthrough by Omicron and Delta Variants, New York, USA. Emerg. Infect. Dis. 2022, 28, 1990–1998. [Google Scholar] [CrossRef]
- AIFA. Trattamenti Utilizzabili nei Pazienti COVID-19 nel Setting Ospedaliero. Available online: https://www.aifa.gov.it/documents/20142/1269602/SOC_ospedaliera_09.12.2020.pdf (accessed on 11 November 2022).
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- Troiano, G.; Nardi, A. Vaccine hesitancy in the era of COVID-19. Public Health 2021, 194, 245–251. [Google Scholar] [CrossRef]
- Papini, F.; Grassi, N.; Guglielmi, G.; Gattini, V.; Rago, L.; Bisordi, C.; Scateni, M.; Totaro, M.; Tulipani, A.; Porretta, A.; et al. COVID-19 vaccine management (Comirnaty and mrna-1273 Moderna) in a teaching hospital in Italy: A short report on the vaccination campaign. Environ. Health Prev. Med. 2021, 26, 99. [Google Scholar] [CrossRef]
- Franchina, V.; Bonfanti, R.C.; Lo Coco, G.; Salerno, L. The Role of Existential Concerns in the Individual’s Decisions regarding COVID-19 Vaccine Uptake: A Survey among Non-Vaccinated Italian Adults during the Third Wave of the Pandemic. Vaccines 2022, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Zarbo, C.; Candini, V.; Ferrari, C.; d’Addazio, M.; Calamandrei, G.; Starace, F.; Caserotti, M.; Gavaruzzi, T.; Lotto, L.; Tasso, A.; et al. COVID-19 Vaccine Hesitancy in Italy: Predictors of Acceptance, Fence Sitting and Refusal of the COVID-19 Vaccination. Front. Public Health 2022, 10, 873098. [Google Scholar] [CrossRef] [PubMed]
- Borga, L.G.; Clark, A.E.; D’Ambrosio, C.; Lepinteur, A. Characteristics associated with COVID-19 vaccine hesitancy. Sci. Rep. 2022, 12, 12435. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Franza, L.; Todi, L.; Carusi, V.; Centrone, M.; Buonomo, A.; Chini, R.; Newton, E.E.; Schiavino, D.; Nucera, E. The Importance of Complying with Vaccination Protocols in Developed Countries: “Anti-Vax” Hysteria and the Spread of Severe Preventable Diseases. Curr. Med. Chem. 2018, 25, 6070–6081. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Natesan, S.; Vora, K.S.; Yatoo, M.I.; Tiwari, R.; Saxena, S.K.; Singh, K.P.; Singh, R.; Malik, Y.S. COVID-19 in the elderly people and advances in vaccination approaches. Hum. Vaccines Immunother. 2020, 16, 2938–2943. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef]
- Sultan, H.; Mansour, R.; Shamieh, O.; Al-Tabba, A.; Al-Hussaini, M. DNR and COVID-19: The Ethical Dilemma and Suggested Solutions. Front. Public Health 2021, 9, 560405. [Google Scholar] [CrossRef]
- Cianci, R.; Franza, L.; Massaro, M.G.; Borriello, R.; De Vito, F.; Gambassi, G. The Interplay between Immunosenescence and Microbiota in the Efficacy of Vaccines. Vaccines 2020, 8, 636. [Google Scholar] [CrossRef]
- DeSilva, M.B.; Mitchell, P.K.; Klein, N.P.; Dixon, B.E.; Tenforde, M.W.; Thompson, M.G.; Naleway, A.L.; Grannis, S.J.; Ong, T.C.; Natarajan, K.; et al. Protection of 2 and 3 mRNA Vaccine Doses against Severe Outcomes among Adults Hospitalized with COVID-19—VISION Network, August 2021–March 2022. J. Infect. Dis. 2022, jiac458. [Google Scholar] [CrossRef]
- Chatzilena, A.; Hyams, C.; Challen, R.; Marlow, R.; King, J.; Adegbite, D.; Kinney, J.; Clout, M.; Maskell, N.; Oliver, J.; et al. Effectiveness of BNT162b2 COVID-19 vaccination in prevention of hospitalisations and severe disease in adults with SARS-CoV-2 Delta (B.1.617.2) and Omicron (B.1.1.529) variant between June 2021 and July 2022: A prospective test negative case-control study. Lancet Reg. Health Eur. 2022, 25, 100552. [Google Scholar] [CrossRef]
- Powell, A.A.; Kirsebom, F.; Stowe, J.; Ramsay, M.E.; Lopez-Bernal, J.; Andrews, N.; Ladhani, S.N. Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021–March, 2022: A national, observational, test-negative, case-control study. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Intawong, K.; Chariyalertsak, S.; Chalom, K.; Wonghirundecha, T.; Kowatcharakul, W.; Ayood, P.; Thongprachum, A.; Chotirosniramit, N.; Noppakun, K.; Khwanngern, K.; et al. Reduction in severity and mortality in COVID-19 patients owing to heterologous third and fourth-dose vaccines during the periods of delta and omicron predominance in Thailand. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2023, 126, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Pignataro, G.; Torelli, E.; Gullì, A.; Nista, E.C.; Petrucci, M.; Saviano, A.; Marchesini, D.; Covino, M.; Ojetti, V.; et al. Effect of influenza vaccine on COVID-19 mortality: A retrospective study. Intern. Emerg. Med. 2021, 16, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Ohler, A. The Flu Vaccination May Have a Protective Effect on the Course of COVID-19 in the Pediatric Population: When Does Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Meet Influenza? Cureus 2021, 13, e12533. [Google Scholar] [CrossRef]
- Cowling, B.J.; Fang, V.J.; Nishiura, H.; Chan, K.-H.; Ng, S.; Ip, D.K.M.; Chiu, S.S.; Leung, G.M.; Peiris, J.S.M. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 1778–1783. [Google Scholar] [CrossRef]
- Piedra, P.A.; Gaglani, M.J.; Riggs, M.; Herschler, G.; Fewlass, C.; Watts, M.; Kozinetz, C.; Hessel, C.; Glezen, W.P. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community-based, nonrandomized, open-label trial. Pediatrics 2005, 116, e397–e407. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Hofmann Bowman, M.A. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am. J. Infect. Control 2021, 49, 694–700. [Google Scholar] [CrossRef]
- Gostin, L.O.; Salmon, D.A. The Dual Epidemics of COVID-19 and Influenza: Vaccine Acceptance, Coverage, and Mandates. JAMA 2020, 324, 335–336. [Google Scholar] [CrossRef]
- AIFA. Varianti del Virus. Available online: https://www.iss.it/en/cov19-cosa-fa-iss-varianti?p_p_id=com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_delta=20&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_year=2023&p_r_p_resetCur=false&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_cur=2 (accessed on 12 December 2022).
- Ng, Q.X.; Lim, Y.L.; Han, M.X.; Teoh, S.E.; Thumboo, J.; Tan, B.H. The Performance of Lateral Flow Tests in the Age of the Omicron: A Rapid Systematic Review. Life 2022, 12, 1941. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, R.; Wang, Y.; Zhao, M.; Liu, N.; Li, S.; Huang, H.; Yang, D.; Au, K.K.; Wang, H.; et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine 2022, 77, 103904. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944, Erratum in Lancet 2022. [Google Scholar] [CrossRef] [PubMed]



| All Patients (N = 821) | Unvaccinated (N = 463) | Vaccinated (N = 358) | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (years, mean value ± SD) | 62 ± 18 | 57 ± 17 | 68 ± 18 | <0.0001 |
| Males N (%) | 485 (59) | 276 (59) | 209 (58) | 0.1270 |
| BMI (mean value ± SD) | 28 ± 6 | 28 ± 6 | 27 ± 6 | 0.5325 |
| Delta variant N (%) | 545 (66) | 345 (75) | 200 (56) | <0.0001 |
| Omicron variant N (%) | 276 (33) | 118 (25) | 158 (44) | <0.0001 |
| Comorbidities N (%) | ||||
| Diabetes | 116 (14) | 54 (12) | 62 (17) | 0.0211 |
| Hypertension | 324 (40) | 140 (30) | 184 (51) | <0.0001 |
| Coronary Heart Disease | 76 (9) | 19 (4) | 57 (16) | <0.0001 |
| Congestive Heart Failure | 55 (7) | 16 (3) | 39 (11) | <0.0001 |
| Cardiac Valve Disease | 14 (2) | 6 (1) | 8 (2) | 0.3029 |
| Atrial Fibrillation | 83 (10) | 29 (6) | 54 (15) | <0.0001 |
| COPD | 77 (9) | 23 (5) | 54 (15) | <0.0001 |
| Active cancer | 99 (12) | 32 (7) | 67 (19) | <0.0001 |
| Other lung conditions * | 56 (7) | 36 (8) | 20 (6) | 0.2173 |
| Chronic Kidney Disease | 50 (6) | 14 (3) | 36 (10) | <0.0001 |
| Parkinson disease | 13 (2) | 3 (1) | 10 (3) | 0.0213 |
| Alzheimer’s disease | 38 (5) | 12 (3) | 26 (7) | 0.0016 |
| Obesity | 341 (41) | 187 (41) | 154 (43) | 0.4486 |
| Other Chronic Diseases ** | 41 (5) | 17 (4) | 24 (7) | 0.0479 |
| N. of comorbidities > 1 | 361(44) | 144 (31) | 217 (62) | <0.0001 |
| At-home treatment N (%) | ||||
| Anticoagulants | 112 (14) | 54 (12) | 58 (16) | 0.0603 |
| ACEi/ARB | 184 (22) | 81 (17) | 103 (29) | 0.0001 |
| Laboratory Values (Mean value ± SD) | ||||
| BUN (mg/dL) | 26 ± 24 | 24 ± 25 | 29 ± 24 | 0.0042 |
| LDH (UI/L) | 333 ± 299 | 365 ± 219 | 292 ±160 | <0.0001 |
| CRP (mg/L) | 70 ± 70 | 66 ± 67 | 75 ± 75 | 0.0689 |
| Procalcitonin (ng/mL) | 3 ± 24 | 3 ± 31 | 2 ± 11 | 0.2739 |
| Neutrophils (×109/L) | 6326 ± 4267 | 6110 ± 3610 | 6310 ± 3270 | 0.7472 |
| Eosinophils (×107/L) | 72 ± 265 | 58 ± 199 | 95 ± 329 | 0.0269 |
| Lymphocytes (×109/L) | 1222 ± 757 | 1157 ± 629 | 1305 ± 890 | 0.0081 |
| D-dimer (ng/mL) | 2330 ± 4995 | 2185 ± 4925 | 2524 ± 5088 | 0.3608 |
| Fibrinogen (mg/dL) | 493 ± 164 | 497 ± 159 | 488 ± 171 | 0.4349 |
| PaO2/FiO2 | 302 ± 106 | 287 ± 102 | 320 ± 107 | <0.0001 |
| In-hospital treatment N (%) | ||||
| Anticoagulants | 568 (69) | 335 (72) | 229 (64) | 0.0102 |
| Corticosteroids | 568 (69) | 346 (74) | 222 (62) | <0.0001 |
| Remdesivir | 221 (27) | 147 (32) | 74 (21) | 0.0004 |
| Monoclonal Antibodies *** | 70 (8) | 20 (4) | 50 (14) | <0.0001 |
| Tocilizumab | 134 (16) | 106 (23) | 28 (8) | <0.0001 |
| Other anti-IL-6 | 41 (5) | 23 (5) | 18 (5) | 0.9686 |
| All Patients (N = 821) | Delta Variant (N = 545) | Omicron Variant (N = 276) | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (years, mean value ± DS) | 62 ± 18 | 59 ± 19 | 67 ± 17 | <0.0001 |
| Male sex N (%) | 485 (59) | 319 (59) | 166 (60) | 0.6571 |
| BMI (mean value ± DS) | 28 ± 6 | 28 ± 6 | 28 ± 7 | 0.9614 |
| Comorbidities N (%) | ||||
| Diabetes | 116 (14) | 74 (14) | 42 (15) | 0.5241 |
| Hypertension | 324 (40) | 184 (34) | 140 (51) | <0.0001 |
| Coronary Heart Disease | 76 (9) | 44 (8) | 32 (12) | 0.1001 |
| Chronic Heart Failure | 55 (7) | 32 (6) | 23 (8) | 0.1826 |
| Cardiac Valve Disease | 14 (2) | 9 (2) | 5 (2) | 0.8670 |
| Atrial Fibrillation | 83 (10) | 59 (11) | 24 (9) | 0.3389 |
| COPD | 77 (9) | 43 (8) | 34 (12) | 0.0398 |
| Active cancer | 99 (12) | 57 (10) | 42 (15) | 0.0479 |
| Other lung conditions * | 56 (7) | 39 (7) | 17 (6) | 0.5926 |
| Chronic Kidney Disease | 50 (6) | 27 (5) | 23 (8) | 0.0558 |
| Parkinson disease | 13 (2) | 8 (2) | 5 (2) | 0.7697 |
| Alzheimer disease | 38 (5) | 21 (4) | 17 (6) | 0.1373 |
| Obesity | 341 (41) | 240 (44) | 101 (37) | 0.0409 |
| Other Chronic Diseases ** | 41 (5) | 25 (5) | 16 (6) | 0.4521 |
| N. of comorbidities > 1 | 361(44) | 213 (39) | 148 (54) | <0.0001 |
| At-home treatment N (%) | ||||
| Anticoagulants | 112 (14) | 78 (14) | 34 (12) | 0.4319 |
| ACEi/ARB | 184 (22) | 105 (19) | 79 (29) | 0.0024 |
| Vaccine status N (%) | ||||
| No vaccine | 463 (56) | 345 (63) | 118 (43) | <0.0001 |
| One shot | 56 (7) | 46 (8) | 10 (4) | 0.0011 |
| Two shots | 226 (28) | 153 (28) | 73 (26) | 0.6226 |
| Three shots | 76 (9) | 1 (1) | 75 (27) | <0.0001 |
| At least 1 shot | 358 (44) | 200 (37) | 158 (57) | <0.0001 |
| Laboratory Values (Mean value ± DS) | ||||
| BUN (mg/dL) | 26 ± 24 | 24 ± 22 | 31 ± 29 | 0.0005 |
| LDH (UI/L) | 333 ± 299 | 346 ± 193 | 308 ± 208 | 0.0119 |
| CRP (mg/L) | 70 ± 70 | 69 ± 68 | 72 ± 74 | 0.6142 |
| Procalcitonin (ng/mL) | 3 ± 24 | 3 ± 28 | 2 ± 13 | 0.6812 |
| Eosinophils (×107/L) | 72 ± 265 | 81 ± 317 | 54 ± 91 | 0.0671 |
| Lymphocytes (×109/L) | 1222 ± 757 | 1216 ± 658 | 1232 ± 922 | 0.8030 |
| D-dimer (ng/mL) | 2330 ± 4995 | 2046 ± 4803 | 2883 ± 5315 | 0.0407 |
| Fibrinogen (mg/dL) | 493 ± 164 | 521 ± 167 | 438 ± 140 | <0.0001 |
| PaO2/FiO2 | 302 ± 106 | 304 ± 100 | 298 ± 117 | 0.4739 |
| In-hospital treatment N (%) | ||||
| Anticoagulants | 568 (69) | 465 (85) | 215 (78) | 0.0077 |
| Corticosteroids | 568 (69) | 401 (74) | 167 (61) | <0.0001 |
| Remdesivir | 221 (27) | 156 (29) | 65 (24) | 0.1180 |
| Monoclonal Antibodies *** | 70 (8) | 43 (8) | 27 (10) | 0.3590 |
| Tocilizumab | 134 (16) | 130 (24) | 4 (1) | <0.0001 |
| Other anti-IL-6 | 41 (5) | 16 (3) | 25 (10) | <0.0001 |
| Unvaccinated (N = 463) | One Dose (N = 56) | Two Doses (N = 226) | Three Doses (N = 76) | p | |
| Demographic data | |||||
| Age (years, mean value ± SD) | 57 ± 17 | 52 ± 18 | 71 ± 16 | 74 ± 15 | <0.0001 |
| Male sex N (%) | 276 (59) | 35 (63) | 127 (56) | 47 (62) | 0.6931 |
| BMI (mean value ± SD) | 28 ± 6 | 28 ± 5 | 27 ± 5 | 28 ± 8 | 0.5694 |
| Delta variant N (%) | 345 (75) | 46 (88) | 153 (68) | 1 (1) | <0.0001 |
| Omicron variant N (%) | 118 (25) | 10 (18) | 73 (32) | 75 (99) | <0.0001 |
| Comorbidities N (%) | |||||
| Diabetes | 54 (12) | 4 (7) | 45 (20) | 13 (17) | 0.0081 |
| Hypertension | 140 (30) | 16 (28) | 125 (55) | 43 (57) | <0.0001 |
| Coronary Heart Disease | 19 (4) | 4 (7) | 39 (27) | 14 (18) | <0.0001 |
| Chronic Heart Failure | 16 (3) | 0 (0) | 30 (13) | 9 (12) | <0.0001 |
| Cardiac Valve Disease | 6 (1) | 0 (0) | 6 (3) | 2 (3) | 0.6317 |
| Atrial Fibrillation | 29 (6) | 4 (7) | 39 (17) | 111 (14) | <0.0001 |
| COPD | 23 (5) | 3 (5) | 39 (17) | 12 (16) | <0.0001 |
| Active cancer | 32 (7) | 4 (7) | 49 (22) | 14 (18) | <0.0001 |
| Other lung conditions * | 36 (8) | 3 (5) | 14 (6) | 3 (4) | 0.0586 |
| Chronic Kidney Disease | 14 (3) | 1 (2) | 23 (10) | 12 (16) | <0.0001 |
| Parkinson disease | 3 (1) | 1 (2) | 6 (3) | 3 (4) | 0.0674 |
| Alzheimer disease | 12 (3) | 4 (7) | 11 (5) | 11 (14) | <0.0001 |
| Obesity | 187 (41) | 27 (45) | 98 (43) | 29 (38) | 0.3854 |
| Other Chronic Disease ** | 17 (4) | 4 (7) | 15 (7) | 5 (7) | 0.8533 |
| N. of comorbidities > 1 | 144 (31) | 17 (30) | 147 (65) | 53 (70) | <0.0001 |
| At-home treatment N (%) | |||||
| Anticoagulants | 54 (12) | 3 (5) | 44 (19) | 11 (14) | 0.0080 |
| ACEi/ARB | 81 (17) | 13 (23) | 65 (29) | 25 (33) | 0.0021 |
| Laboratory Values (Mean value ± Sd) | |||||
| BUN (mg/dL) | 24 ± 25 | 20 ± 12 | 29 ± 25 | 36 ± 24 | <0.0001 |
| LDH (UI/L) | 365 ± 219 | 327 ± 164 | 298 ± 169 | 269 ± 122 | <0.0001 |
| CRP (mg/L) | 66 ± 67 | 77 ± 86 | 80 ± 74 | 64 ± 69 | 0.1428 |
| Procalcitonin (ng/mL) | 3 ± 31 | 1 ± 2 | 1 ± 3 | 5 ± 22 | 0.4610 |
| Eosinophils (×107/L) | 58 ± 199 | 181 ± 729 | 109 ± 186 | 73 ± 126 | 0.0091 |
| Lymphocytes (×109/L) | 1157 ± 629 | 1463 ± 867 | 1290 ± 861 | 1315 ± 985 | 0.0174 |
| D-dimer (ng/mL) | 2185 ± 4925 | 2076 ± 5212 | 2746 ± 5449 | 2137 ± 3140 | 0.6138 |
| Fibrinogen (mg/dL) | 497 ± 159 | 513 ± 212 | 505 ± 168 | 426 ± 133 | 0.0033 |
| PaO2/FiO2 | 287 ± 102 | 330 ± 88 | 314 ± 110 | 329 ± 113 | <0.0001 |
| In-hospital treatment N (%) | |||||
| Anticoagulants | 390 (84) | 40 (70) | 190 (84) | 60 (79) | 0.1732 |
| Corticosteroids | 346 (74) | 35 (61) | 144 (64) | 43 (57) | 0.0009 |
| Remdesivir | 147 (32) | 13 (23) | 47 (21) | 14 (18) | 0.0047 |
| Monoclonal Antibodies *** | 20 (4) | 1 (2) | 35 (15) | 14 (18) | <0.0001 |
| Tocilizumab | 106 (23) | 12 (21) | 15 (7) | 1 (1) | <0.0001 |
| Other anti-IL-6 | 23 (5) | 5 (9) | 10 (4) | 3 (4) | 0.5954 |
| Unvaccinated | One Dose | p | Odds Ratio | 95%CI | |
|---|---|---|---|---|---|
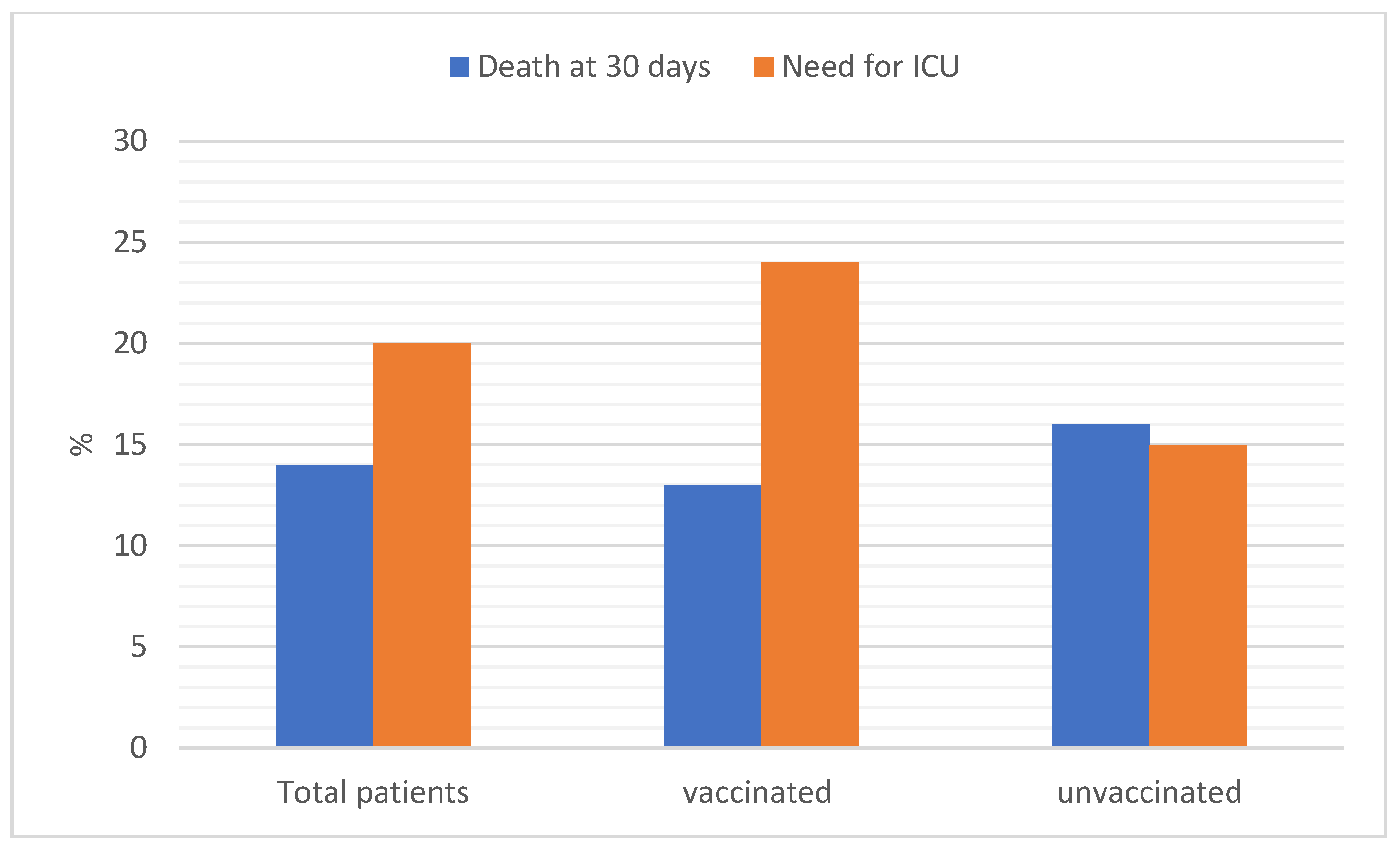
| Need for ICU admission | 113/463 (24) | 7/56 (12) | 0.153 | 1.9 | 0.8–4.4 |
| All-cause mortality at 30 days | 58/463 (13) | 1/56 (1) | 0.048 | 3.9 | 1.8–19.0 |
| Unvaccinated | two doses | ||||
| Need for ICU admission | 113/463 (24) | 39/226 (17) | 0.012 | 1.9 | 1.1–3.0 |
| All-cause mortality at 30 days | 58/463 (13) | 38/226 (17) | 0.049 | 1.9 | 1.5–2.9 |
| Unvaccinated | three doses | ||||
| Need for ICU admission | 113/463 (24) | 8/76 (11) | 0.004 | 3.5 | 1.5–8.5 |
| All-cause mortality at 30 days | 58/463 (13) | 17/76 (22) | 0.489 | 1.3 | 0.6–2.8 |
| Unvaccinated | At least one dose | ||||
| Need for ICU admission | 113/463 (24) | 54/358 (15) | 0.002 | 2.0 | 1.3–3.1 |
| All-cause mortality at 30 days | 58/463 (13) | 56/358 (16) | 0.047 | 1.7 | 1.3–2.7 |
| Delta | Omicron | p | Odds Ratio | 95%IC | |
|---|---|---|---|---|---|
| Need for ICU admission | 111 | 56 | 0.007 | 1.9 | 1.2–3.1 |
| All-cause mortality at 30 days | 74 | 40 | 0.064 | 0.6 | 0.3–1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianci, R.; Franza, L.; Pignataro, G.; Massaro, M.G.; Rio, P.; Tota, A.; Ocarino, F.; Sacco Fernandez, M.; Franceschi, F.; Gasbarrini, A.; et al. Effect of COVID-19 Vaccination on the In-Hospital Prognosis of Patients Admitted during Delta and Omicron Waves in Italy. Vaccines 2023, 11, 373. https://doi.org/10.3390/vaccines11020373
Cianci R, Franza L, Pignataro G, Massaro MG, Rio P, Tota A, Ocarino F, Sacco Fernandez M, Franceschi F, Gasbarrini A, et al. Effect of COVID-19 Vaccination on the In-Hospital Prognosis of Patients Admitted during Delta and Omicron Waves in Italy. Vaccines. 2023; 11(2):373. https://doi.org/10.3390/vaccines11020373
Chicago/Turabian StyleCianci, Rossella, Laura Franza, Giulia Pignataro, Maria Grazia Massaro, Pierluigi Rio, Antonio Tota, Francesca Ocarino, Marta Sacco Fernandez, Francesco Franceschi, Antonio Gasbarrini, and et al. 2023. "Effect of COVID-19 Vaccination on the In-Hospital Prognosis of Patients Admitted during Delta and Omicron Waves in Italy" Vaccines 11, no. 2: 373. https://doi.org/10.3390/vaccines11020373
APA StyleCianci, R., Franza, L., Pignataro, G., Massaro, M. G., Rio, P., Tota, A., Ocarino, F., Sacco Fernandez, M., Franceschi, F., Gasbarrini, A., Gambassi, G., & Candelli, M. (2023). Effect of COVID-19 Vaccination on the In-Hospital Prognosis of Patients Admitted during Delta and Omicron Waves in Italy. Vaccines, 11(2), 373. https://doi.org/10.3390/vaccines11020373











