Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy
Abstract
1. Introduction
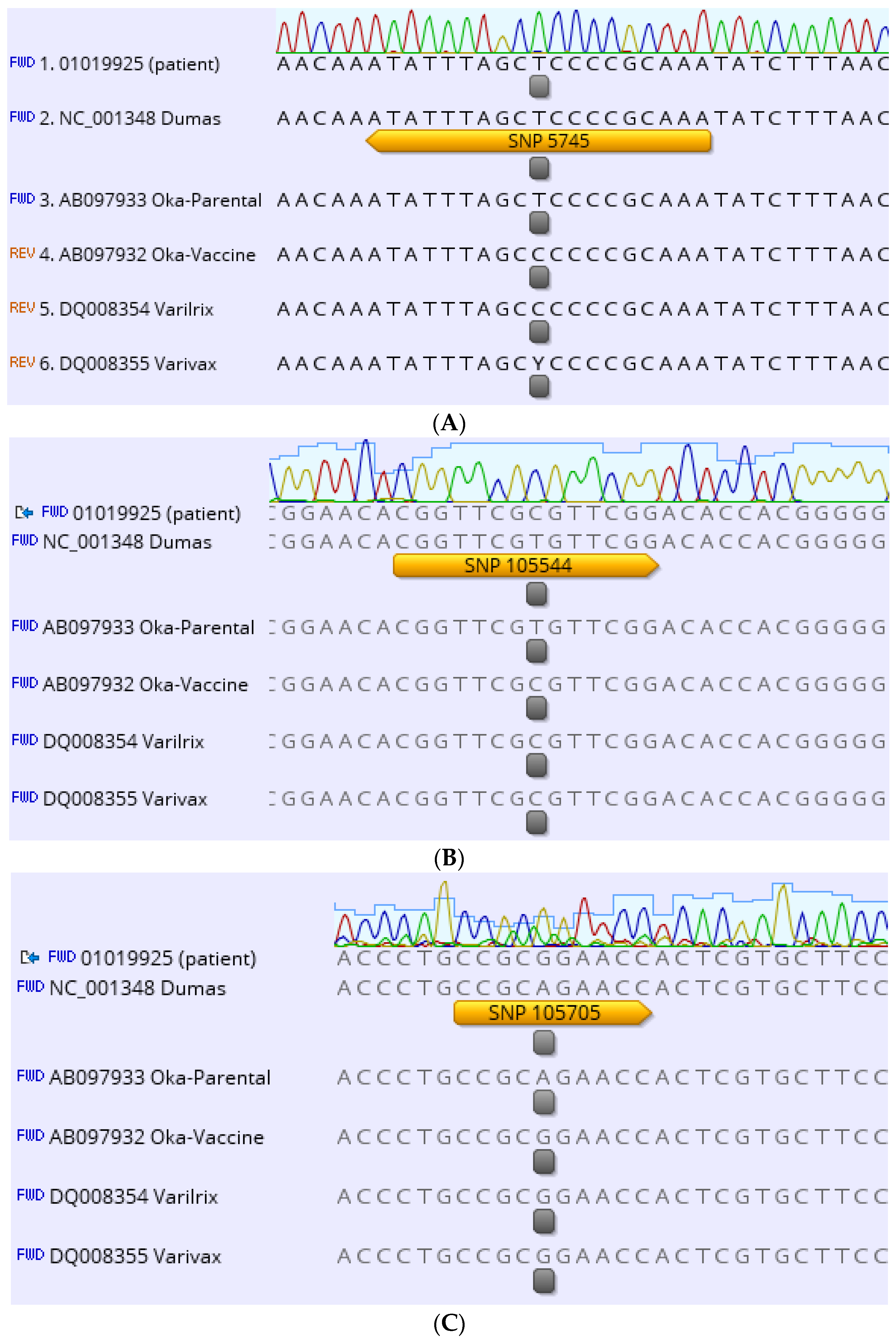
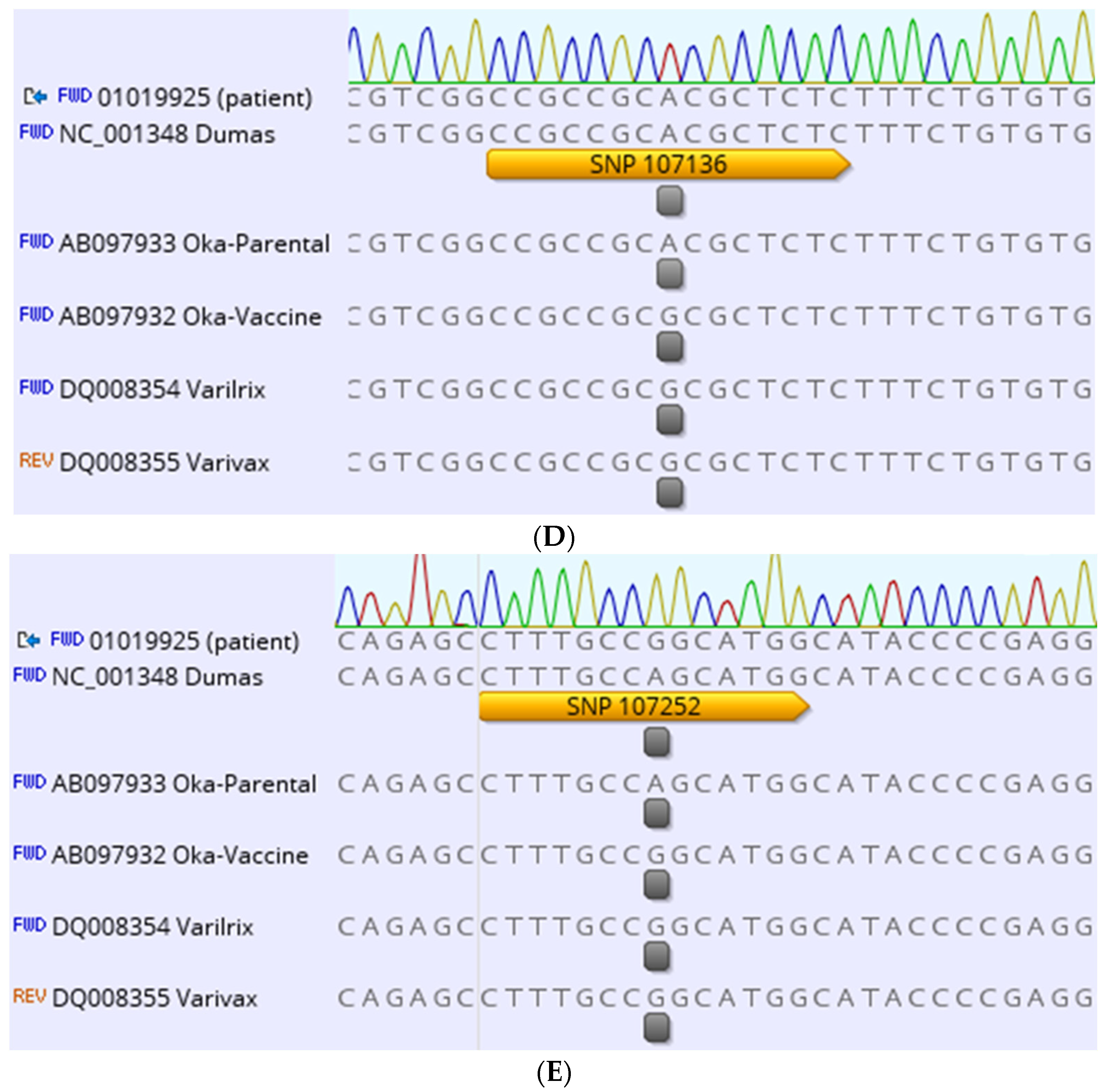
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VZV | Varicella-Zoster-Virus |
| CSF | Cerebrospinal fluid |
| SNP | single nucleotide polymorphism |
| ORF | open reading frame |
| wt | wildtype |
References
- Weibel, R.E.; Neff, B.J.; Kuter, B.J.; Guess, H.A.; Rothenberger, C.A.; Fitzgerald, A.J.; Connor, K.A.; McLean, A.A.; Hilleman, M.R.; Buynak, E.B.; et al. Live attenuated varicella virus vaccine. Efficacy trial in healthy children. N. Engl. J. Med. 1984, 310, 1409–1415. [Google Scholar] [CrossRef]
- Marin, M.; Meissner, H.C.; Seward, J.F. Varicella prevention in the United States: A review of successes and challenges. Pediatrics 2008, 122, e744–e751. [Google Scholar] [CrossRef]
- Bialek, S.R.; Perella, D.; Zhang, J.; Mascola, L.; Viner, K.; Jackson, C.; Lopez, A.S.; Watson, B.; Civen, R. Impact of a routine two-dose varicella vaccination program on varicella epidemiology. Pediatrics 2013, 132, e1134–e1140. [Google Scholar] [CrossRef] [PubMed]
- Heywood, A.E.; Wang, H.; Macartney, K.K.; McIntyre, P. Varicella and herpes zoster hospitalizations before and after implementation of one-dose varicella vaccination in Australia: An ecological study. Bull. World Health Organ. 2014, 92, 593–604. [Google Scholar] [CrossRef]
- Siedler, A.; Arndt, U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro Surveill. 2010, 15, 19530. [Google Scholar] [CrossRef]
- Bonanni, P.; Breuer, J.; Gershon, A.; Gershon, M.; Hryniewicz, W.; Papaevangelou, V.; Rentier, B.; Rumke, H.; Sadzot-Delvaux, C.; Senterre, J.; et al. Varicella vaccination in Europe—Taking the practical approach. BMC Med. 2009, 7, 26. [Google Scholar] [CrossRef]
- Dollard, S.; Chen, M.H.; Lindstrom, S.; Marin, M.; Rota, P.A. Diagnostic and Immunologic Testing for Varicella in the Era of High-Impact Varicella Vaccination: An Evolving Problem. J. Infect. Dis. 2022, 226, S450–S455. [Google Scholar] [CrossRef]
- Weinmann, S.; Chun, C.; Schmid, D.S.; Roberts, M.; Vandermeer, M.; Riedlinger, K.; Bialek, S.R.; Marin, M. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J. Infect. Dis. 2013, 208, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, S.; Naleway, A.L.; Koppolu, P.; Baxter, R.; Belongia, E.A.; Hambidge, S.J.; Irving, S.A.; Jackson, M.L.; Klein, N.P.; Lewin, B.; et al. Incidence of Herpes Zoster Among Children: 2003–2014. Pediatrics 2019, 144, e20182917. [Google Scholar] [CrossRef] [PubMed]
- Zoch-Lesniak, B.; Tolksdorf, K.; Siedler, A. Trends in herpes zoster epidemiology in Germany based on primary care sentinel surveillance data, 2005–2016. Hum. Vaccines Immunother. 2018, 14, 1807–1814. [Google Scholar] [CrossRef]
- Kleinschmidt-DeMasters, B.K.; Amlie-Lefond, C.; Gilden, D.H. The patterns of varicella zoster virus encephalitis. Hum. Pathol. 1996, 27, 927–938. [Google Scholar] [CrossRef]
- Chaves, S.S.; Haber, P.; Walton, K.; Wise, R.P.; Izurieta, H.S.; Schmid, D.S.; Seward, J.F. Safety of varicella vaccine after licensure in the United States: Experience from reports to the vaccine adverse event reporting system, 1995–2005. J. Infect. Dis. 2008, 197, S170–S177. [Google Scholar] [CrossRef]
- Levin, M.J.; DeBiasi, R.L.; Bostik, V.; Schmid, D.S. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J. Infect. Dis. 2008, 198, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Mittal, M.K.; Hodinka, R.L. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann. Emerg. Med. 2009, 53, 792–795. [Google Scholar] [CrossRef]
- Chen, Y.-C.; James, A.; Kung, E.; Madhavan, V. A case of herpes zoster and meningitis in a twice-vaccinated healthy adolescent. J. Pediatr. Infect. Dis. 2017, 12, 142–144. [Google Scholar] [CrossRef]
- Harrington, W.E.; Mato, S.; Burroughs, L.; Carpenter, P.A.; Gershon, A.; Schmid, D.S.; Englund, J.A. Vaccine Oka Varicella Meningitis in Two Adolescents. Pediatrics 2019, 144, e20191522. [Google Scholar] [CrossRef]
- Ramachandran, V.; Elliott, S.C.; Rogers, K.L.; Cohrs, R.J.; Weinberger, M.; Jackson, W.; Carpenter, J.E.; Grose, C.; Bonthius, D.J. Varicella Vaccine Meningitis as a Complication of Herpes Zoster in Twice-Immunized Immunocompetent Adolescents. J. Child Neurol. 2020, 35, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Loparev, V.N.; McCaustland, K.; Holloway, B.P.; Krause, P.R.; Takayama, M.; Schmid, D.S. Rapid genotyping of varicella-zoster virus vaccine and wild-type strains with fluorophore-labeled hybridization probes. J. Clin. Microbiol. 2000, 38, 4315–4319. [Google Scholar] [CrossRef]
- Jin, L.; Xu, S.; Maple, P.A.C.; Xu, W.; Brown, K.E. Differentiation between wild-type and vaccines strains of varicella zoster virus (VZV) based on four single nucleotide polymorphisms. Epidemiol. Infect. 2017, 145, 2618–2625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moro, P.L.; Leung, J.; Marquez, P.; Kim, Y.; Wei, S.; Su, J.R.; Marin, M. Safety Surveillance of Varicella Vaccines in the Vaccine Adverse Event Reporting System, United States, 2006–2020. J. Infect. Dis. 2022, 226, S431–S440. [Google Scholar] [CrossRef]
- Heusel, E.H.; Grose, C. Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus. Viruses 2020, 12, 1078. [Google Scholar] [CrossRef]
- Ramachandran, P.S.; Wilson, M.R.; Catho, G.; Blanchard-Rohner, G.; Schiess, N.; Cohrs, R.J.; Boutolleau, D.; Burrel, S.; Yoshikawa, T.; Wapniarski, A.; et al. Meningitis Caused by the Live Varicella Vaccine Virus: Metagenomic Next Generation Sequencing, Immunology Exome Sequencing and Cytokine Multiplex Profiling. Viruses 2021, 13, 2286. [Google Scholar] [CrossRef]
- Kawamura, Y.; Suzuki, D.; Kono, T.; Miura, H.; Kozawa, K.; Mizuno, H.; Yoshikawa, T. A Case of Aseptic Meningitis without Skin Rash Caused by Oka Varicella Vaccine. Pediatr. Infect. Dis. J. 2022, 41, 78–79. [Google Scholar] [CrossRef]
- Schmid, D.S.; Jumaan, A.O. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin. Microbiol. Rev. 2010, 23, 202–217. [Google Scholar] [CrossRef]
- Martin, J.H.; Dohner, D.E.; Wellinghoff, W.J.; Gelb, L.D. Restriction endonuclease analysis of varicella-zoster vaccine virus and wild-type DNAs. J. Med. Virol. 1982, 9, 69–76. [Google Scholar] [CrossRef]
- Adams, S.G.; Dohner, D.E.; Gelb, L.D. Restriction fragment differences between the genomes of the Oka varicella vaccine virus and American wild-type varicella-zoster virus. J. Med. Virol. 1989, 29, 38–45. [Google Scholar] [CrossRef]
- LaRussa, P.; Lungu, O.; Hardy, I.; Gershon, A.; Steinberg, S.P.; Silverstein, S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J. Virol. 1992, 66, 1016–1020. [Google Scholar] [CrossRef]
- LaRussa, P.; Steinberg, S.; Arvin, A.; Dwyer, D.; Burgess, M.; Menegus, M.; Rekrut, K.; Yamanishi, K.; Gershon, A. Polymerase chain reaction and restriction fragment length polymorphism analysis of varicella-zoster virus isolates from the United States and other parts of the world. J. Infect. Dis. 1998, 178, S64–S66. [Google Scholar] [CrossRef]
- Toi, C.S.; Dwyer, D.E. Differentiation between vaccine and wild-type varicella-zoster virus genotypes by high-resolution melt analysis of single nucleotide polymorphisms. J. Clin. Virol. 2008, 43, 18–24. [Google Scholar] [CrossRef]
- Takayama, M.; Takayama, N.; Inoue, N.; Kameoka, Y. Application of long PCR method of identification of variations in nucleotide sequences among varicella-zoster virus isolates. J. Clin. Microbiol. 1996, 34, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Mori, C.; Takahara, R.; Toriyama, T.; Nagai, T.; Takahashi, M.; Yamanishi, K. Identification of the Oka strain of the live attenuated varicella vaccine from other clinical isolates by molecular epidemiologic analysis. J. Infect. Dis. 1998, 178, 35–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hawrami, K.; Breuer, J. Development of a fluorogenic polymerase chain reaction assay (TaqMan) for the detection and quantitation of varicella zoster virus. J. Virol. Methods 1999, 79, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hawrami, K.; Breuer, J. Analysis of United Kingdom wild-type strains of varicella-zoster virus: Differentiation from the Oka vaccine strain. J. Med. Virol. 1997, 53, 60–62. [Google Scholar] [CrossRef]
- Hawrami, K.; Hart, I.J.; Pereira, F.; Argent, S.; Bannister, B.; Bovill, B.; Carrington, D.; Ogilvie, M.; Rawstorne, S.; Tryhorn, Y.; et al. Molecular epidemiology of varicella-zoster virus in East London, England, between 1971 and 1995. J. Clin. Microbiol. 1997, 35, 2807–2809. [Google Scholar] [CrossRef]
- Sauerbrei, A.; Rubtcova, E.; Wutzler, P.; Schmid, D.S.; Loparev, V.N. Genetic profile of an Oka varicella vaccine virus variant isolated from an infant with zoster. J. Clin. Microbiol. 2004, 42, 5604–5608. [Google Scholar] [CrossRef]
- Depledge, D.P.; Yamanishi, K.; Gomi, Y.; Gershon, A.A.; Breuer, J. Deep Sequencing of Distinct Preparations of the Live Attenuated Varicella-Zoster Virus Vaccine Reveals a Conserved Core of Attenuating Single-Nucleotide Polymorphisms. J. Virol. 2016, 90, 8698–8704. [Google Scholar] [CrossRef]
- Tillieux, S.L.; Halsey, W.S.; Thomas, E.S.; Voycik, J.J.; Sathe, G.M.; Vassilev, V. Complete DNA sequences of two oka strain varicella-zoster virus genomes. J. Virol. 2008, 82, 11023–11044. [Google Scholar] [CrossRef]
- Gilden, D.H.; Kleinschmidt-DeMasters, B.K.; LaGuardia, J.J.; Mahalingam, R.; Cohrs, R.J. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 2000, 342, 635–645. [Google Scholar] [CrossRef]
- Persson, A.; Bergstrom, T.; Lindh, M.; Namvar, L.; Studahl, M. Varicella-zoster virus CNS disease—Viral load, clinical manifestations and sequels. J. Clin. Virol. Off. Publ. Pan. Am. Soc. Clin. Virol. 2009, 46, 249–253. [Google Scholar] [CrossRef]
- Science, M.; MacGregor, D.; Richardson, S.E.; Mahant, S.; Tran, D.; Bitnun, A. Central nervous system complications of varicella-zoster virus. J. Pediatr. 2014, 165, 779–785. [Google Scholar] [CrossRef]
- Moffat, J.F.; Zerboni, L.; Kinchington, P.R.; Grose, C.; Kaneshima, H.; Arvin, A.M. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 1998, 72, 965–974. [Google Scholar] [CrossRef]
- Hambleton, S.; Steinberg, S.P.; Larussa, P.S.; Shapiro, E.D.; Gershon, A.A. Risk of herpes zoster in adults immunized with varicella vaccine. J. Infect. Dis. 2008, 197, S196–S199. [Google Scholar] [CrossRef]
- Civen, R.; Chaves, S.S.; Jumaan, A.; Wu, H.; Mascola, L.; Gargiullo, P.; Seward, J.F. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr. Infect. Dis. J. 2009, 28, 954–959. [Google Scholar] [CrossRef]
- Galil, K.; Brown, C.; Lin, F.; Seward, J. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 931–935. [Google Scholar] [CrossRef]
- Rawson, H.; Crampin, A.; Noah, N. Deaths from chickenpox in England and Wales 1995–1997: Analysis of routine mortality data. BMJ 2001, 323, 1091–1093. [Google Scholar] [CrossRef]
- Galea, S.A.; Sweet, A.; Beninger, P.; Steinberg, S.P.; Larussa, P.S.; Gershon, A.A.; Sharrar, R.G. The safety profile of varicella vaccine: A 10-year review. J. Infect. Dis. 2008, 197, S165–S169. [Google Scholar] [CrossRef]
- Jeon, J.S.; Won, Y.H.; Kim, I.K.; Ahn, J.H.; Shin, O.S.; Kim, J.H.; Lee, C.H. Analysis of single nucleotide polymorphism among Varicella-Zoster Virus and identification of vaccine-specific sites. Virology 2016, 496, 277–286. [Google Scholar] [CrossRef]
- Thiele, S.; Borschewski, A.; Kuchler, J.; Bieberbach, M.; Voigt, S.; Ehlers, B. Molecular analysis of varicella vaccines and varicella-zoster virus from vaccine-related skin lesions. Clin. Vaccine Immunol. CVI 2011, 18, 1058–1066. [Google Scholar] [CrossRef][Green Version]
- Kanda, R.K.; Quinlivan, M.L.; Gershon, A.A.; Nichols, R.A.; Breuer, J. Population diversity in batches of the varicella Oka vaccine. Vaccine 2011, 29, 3293–3298. [Google Scholar] [CrossRef]
- Spackova, M.; Wiese-Posselt, M.; Dehnert, M.; Matysiak-Klose, D.; Heininger, U.; Siedler, A. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine 2010, 28, 686–691. [Google Scholar] [CrossRef]
- Depledge, D.P.; Kundu, S.; Jensen, N.J.; Gray, E.R.; Jones, M.; Steinberg, S.; Gershon, A.; Kinchington, P.R.; Schmid, D.S.; Balloux, F.; et al. Deep sequencing of viral genomes provides insight into the evolution and pathogenesis of varicella zoster virus and its vaccine in humans. Mol. Biol. Evol. 2014, 31, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Heath, G.; Depledge, D.P.; Brown, J.R.; Hale, A.D.; Tutil, H.; Williams, R.; Breuer, J. Acute Retinal Necrosis Caused by the Zoster Vaccine Virus. Clin. Infect. Dis. 2017, 65, 2122–2125. [Google Scholar] [CrossRef]
| Parameter | Patient | Reference Range |
|---|---|---|
| White Cell Count | 5700 cells/µl | 4500–13,500 cells/µL |
| Lymphocytes | 32.3% | 20.4–51.1% |
| Monocytes | 9.1% | 1–14% |
| Neutrophil granulocytes | 50.1% | 42.2–75.2% |
| B-lymphocytes (CD19+) | 18.6% | 8–24% |
| T-lymphocytes (CD3+) | 73.1% | 52–78% |
| T-helper cells (CD3+CD4+) | 43.6% | 25–48% |
| Cytotoxic T cells (CD3+CD8+) | 24% | 9–35% |
| CD4/CD8 ratio | 1.8 | 0.9–3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bierbaum, S.; Fischer, V.; Briedigkeit, L.; Werner, C.; Hengel, H.; Huzly, D. Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy. Vaccines 2023, 11, 309. https://doi.org/10.3390/vaccines11020309
Bierbaum S, Fischer V, Briedigkeit L, Werner C, Hengel H, Huzly D. Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy. Vaccines. 2023; 11(2):309. https://doi.org/10.3390/vaccines11020309
Chicago/Turabian StyleBierbaum, Sibylle, Veronika Fischer, Lutz Briedigkeit, Claudius Werner, Hartmut Hengel, and Daniela Huzly. 2023. "Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy" Vaccines 11, no. 2: 309. https://doi.org/10.3390/vaccines11020309
APA StyleBierbaum, S., Fischer, V., Briedigkeit, L., Werner, C., Hengel, H., & Huzly, D. (2023). Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy. Vaccines, 11(2), 309. https://doi.org/10.3390/vaccines11020309





