Qualitative Conceptual Content Analysis of COVID-19 Vaccine Administration Error Inquiries
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
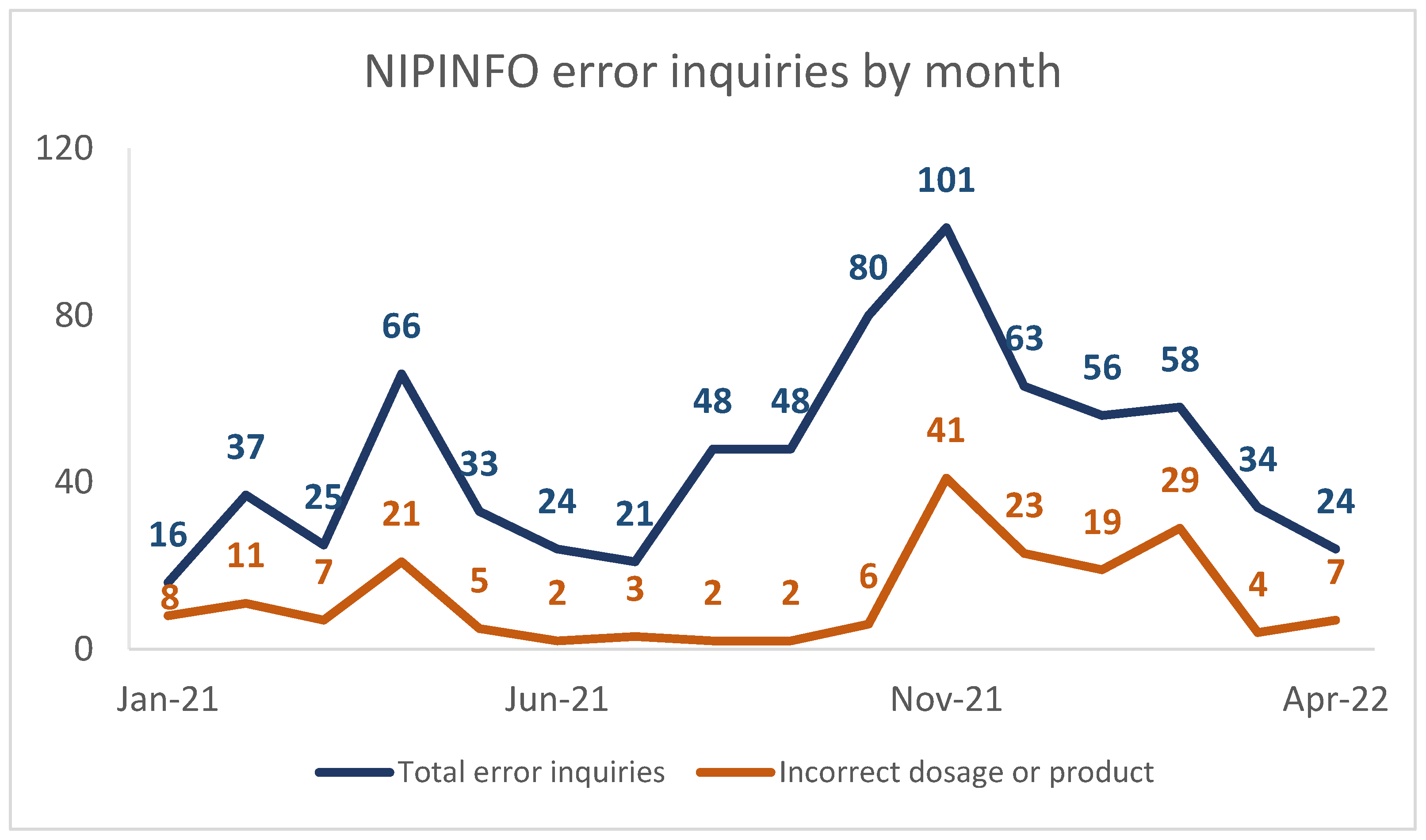
2.3. Data Visualization
3. Results
3.1. Storage
3.2. Incorrect Dosage or Product
“A nurse was administering a Moderna COVID vaccine. As they were injecting the vaccine into the muscle, they noticed liquid coming out of the injection site, while the needle was still inserted in the patient’s muscle. They checked to see if the liquid was coming from the needle/hub area and found nothing coming from that area. It is unknown exactly how much of the vaccine was administered and how much was running down the patient’s arm”.
“If [an] immunocompromised patient received the wrong dosage when given their 3rd Moderna vaccine should they repeat the 3rd dose? Patient received 0.25 mL Moderna as the 3rd dose but is immunocompromised and should have received 0.5 mL”.
3.3. Unauthorized Age Group
“We had a 16-year-old female who inadvertently received the Moderna vaccine for her first COVID-19 shot. Would this be considered a valid dose? Would we give her the second shot with the Moderna vaccine or should we restart the series with the Pfizer vaccine? If we use Pfizer, how long should we wait and would she need two doses?”
3.4. Schedule
3.5. Preparation and Administration
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimers
References
- U.S. Food and Drug Administration. FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020. [Google Scholar]
- U.S. Food and Drug Administration. FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020. [Google Scholar]
- U.S. Food and Drug Administration. FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2021. [Google Scholar]
- U.S. Department of Health and Human Services. Eighth Amendment to Declaration Under the Public Readiness and Emergency Preparedness Act for Medical Countermeasures Against COVID-19; U.S. Department of Health and Human Services: Washington, DC, USA, 2021; Volume 86, pp. 41977–41982. [Google Scholar]
- U.S. Department of Health and Human Services. Ninth Amendment to Declaration Under the Public Readiness and Emergency Preparedness Act for Medical Countermeasures Against COVID-19; U.S. Department of Health and Human Services: Washington, DC, USA, 2021; Volume 86, pp. 51160–51166. [Google Scholar]
- National Coordinating Council for Medication Error Reporting and Prevention. About Medication Errors. Available online: https://www.nccmerp.org/about-medication-errors (accessed on 21 July 2022).
- World Health Organization. Patient Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/patient-safety (accessed on 21 July 2022).
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Gee, J.; Su, J.R.; Zhang, B.; Thompson, D.; Shimabukuro, T.T.; Shay, D.K. COVID-19 Vaccine Safety in Children Aged 5–11 Years—United States, November 3–December 19, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1755–1760. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Abara, W.E.; Olubajo, B.; Myers, T.R.; Su, J.R.; Thompson, D.; Gee, J.; Shimabukuro, T.T.; et al. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12–17 Years—United States, December 9, 2021–February 20, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 347–351. [Google Scholar] [CrossRef]
- Bengtsson, M. How to plan and perform a qualitative study using content analysis. Nurs. Open 2016, 2, 8–14. [Google Scholar] [CrossRef]
- Kleinheksel, A.J.; Rockich-Winston, N.; Tawfik, H.; Wyatt, T.R. Demystifying Content Analysis. Am. J. Pharm. Educ. 2020, 84, 7113. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 21 November 2022).
- Dooling, K.; McClung, N.; Chamberland, M.; Marin, M.; Wallace, M.; Bell, B.P.; Lee, G.M.; Talbot, H.K.; Romero, J.R.; Oliver, S.E. The Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine—United States, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1857–1859. [Google Scholar]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine—United States, December 2020. Morb. Mortal. Wkly. Rep. 2021, 69, 1653–1656. [Google Scholar]
- Oliver, S.E.; Gargano, J.W.; Scobie, H.; Wallace, M.; Hadler, S.C.; Leung, J.; Blain, A.E.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Janssen COVID-19 Vaccine—United States, February 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 329–332. [Google Scholar]
- MacNeil, J.R.; Su, J.R.; Broder, K.R.; Guh, A.Y.; Gargano, J.W.; Wallace, M.; Hadler, S.C.; Scobie, H.M.; Blain, A.E.; Moulia, D.; et al. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients—United States, April 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 651–656. [Google Scholar]
- Wallace, M.; Woodworth, K.R.; Gargano, J.W.; Scobie, H.M.; Blain, A.E.; Moulia, D.; Chamberland, M.; Reisman, N.; Hadler, S.C.; MacNeil, J.R.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12–15 Years—United States, May 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 749–752. [Google Scholar]
- Mbaeyi, S.; Oliver, S.E.; Collins, J.P.; Godfrey, M.; Goswami, N.D.; Hadler, S.C.; Jones, J.; Moline, H.; Moulia, D.; Reddy, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines—United States, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1545–1552. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Moulia, D.; Collins, J.P.; Hadler, S.C.; Jones, J.; Reddy, S.; Chamberland, M.; Campos-Outcalt, D.; Morgan, R.L.; Brooks, O.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Children Aged 5–11 Years—United States, November 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1579–1583. [Google Scholar] [CrossRef]
- Oliver, S.E.; Wallace, M.; See, I.; Mbaeyi, S.; Godfrey, M.; Hadler, S.C.; Jatlaoui, T.C.; Twentyman, E.; Hughes, M.M.; Rao, A.K.; et al. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices—United States, December 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 90–95. [Google Scholar]
- Kim, H.; Kim, H.S.; Kim, H.M.; Kim, M.J.; Kwon, K.T.; Cha, H.H.; Seong, W.J. Impact of vaccination and the omicron variant on COVID-19 severity in pregnant women. Am. J. Infect. Control 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Kirson, N.; Swallow, E.; Lu, J.; Foroughi, C.; Bookhart, B.; DeMartino, J.K.; Maynard, J.; Shivdasani, Y.; Eid, D.; Lefebvre, P. Increasing COVID-19 vaccination in the United States: Projected impact on cases, hospitalizations, and deaths by age and racial group. Public Health 2022, 210, 99–106. [Google Scholar] [CrossRef]
- Orangi, S.; Ojal, J.; Brand, S.P.; Orlendo, C.; Kairu, A.; Aziza, R.; Ogero, M.; Agweyu, A.; Warimwe, G.M.; Uyoga, S.; et al. Epidemiological impact and cost-effectiveness analysis of COVID-19 vaccination in Kenya. BMJ Glob. Health 2022, 7, e009430. [Google Scholar] [CrossRef]
- Sanz-Leon, P.; Hamilton, L.H.W.; Raison, S.J.; Pan, A.J.X.; Stevenson, N.J.; Stuart, R.M.; Abeysuriya, R.G.; Kerr, C.C.; Lambert, S.B.; Roberts, J.A. Modelling herd immunity requirements in Queensland: Impact of vaccination effectiveness, hesitancy and variants of SARS-CoV-2. Philos. Trans. R. Soc. A 2022, 380, 20210311. [Google Scholar] [CrossRef]
- Stepanova, M.; Lam, B.; Younossi, E.; Felix, S.; Ziayee, M.; Price, J.; Pham, H.; de Avila, L.; Terra, K.; Austin, P.; et al. The impact of variants and vaccination on the mortality and resource utilization of hospitalized patients with COVID-19. BMC Infect Dis. 2022, 22, 702. [Google Scholar] [CrossRef]
- Thielmann, A.; Viehmann, A.; Weltermann, B.M. Effectiveness of a web-based education program to improve vaccine storage conditions in primary care (Keep Cool): Study protocol for a randomized controlled trial. Trials 2015, 16, 301. [Google Scholar] [CrossRef]
- Hampton, L.M. Vaccine handling and administration errors should be addressed to improve vaccine program safety. Vaccine 2020, 38, 4933–4934. [Google Scholar] [CrossRef]
- CDC. Storage and Handling. Available online: https://www2.cdc.gov/vaccines/ed/covid19/SHVA/20010.asp (accessed on 5 September 2022).
- CDC. Vaccine Storage and Handling Toolkit; CDC: Atlanta, GA, USA, 2022; p. 19. [Google Scholar]
- CDC. Moderna COVID-19 Vaccine, Storage and Handling Summary; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- CDC. Storage and Handling of Pfizer-BioNTech COVID-19 Vaccines. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/storage.html (accessed on 5 September 2022).
- CDC. Storage and Handling of Novavax COVID-19 Vaccines. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/novavax/storage.html (accessed on 21 November 2022).
- Friedensohn, L.; Zur, M.; Timofeyev, M.; Burshtein, S.; Michael, Y.B.; Fink, N.; Glassberg, E. Sub-cutaneous Pfizer/BioNTech COVID-19 vaccine administration results in seroconversion among young adults. Vaccine 2021, 39, 6210–6212. [Google Scholar] [CrossRef]
- Buchan, S.A.; Seo, C.Y.; Johnson, C.; Alley, S.; Kwong, J.C.; Nasreen, S.; Calzavara, A.; Lu, D.; Harris, T.M.; Yu, K.; et al. Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. JAMA Netw. Open 2022, 5, e2218505. [Google Scholar] [CrossRef]
- Pillay, J.; Gaudet, L.; Wingert, A.; Bialy, L.; Mackie, A.S.; Paterson, D.I.; Hartling, L. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: Living evidence syntheses and review. Bmj 2022, 378, e069445. [Google Scholar] [CrossRef]
- Bassi, S.; Begum, R.; Onatade, R.; Olie, C. Analysis of clinical enquiries received by five COVID-19 vaccination centres in the UK. Eur. J. Hosp. Pharm. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Institute for Safe Medication Practices (ISMP). Any New Process Poses a Risk for Errors: Learning from 4 Months of Coronavirus Disease 2019 (COVID-19) Vaccinations. Available online: https://www.ismp.org/resources/any-new-process-poses-risk-errors-learning-4-months-coronavirus-disease-2019-covid-19 (accessed on 5 September 2022).
- Chiodini, J. Vaccine storage: A hot topic for cool practice. Travel Med. Infect. Dis. 2019, 31, 101477. [Google Scholar] [CrossRef]
- Bulula, N.; Mwiru, D.P.; Swalehe, O.; Thomas Mori, A. Vaccine storage and distribution between expanded program on immunization and medical store department in Tanzania: A cost-minimization analysis. Vaccine 2020, 38, 8130–8135. [Google Scholar] [CrossRef]
- Leidner, A.J.; Lee, C.E.; Tippins, A.; Messonnier, M.L.; Stevenson, J.M. Evaluation of non-continuous temperature monitoring practices for vaccine storage units: A Monte Carlo simulation study. J. Public Health 2020, 29, 1253–1260. [Google Scholar] [CrossRef]
- Thielmann, A.; Puth, M.T.; Weltermann, B. Improving knowledge on vaccine storage management in general practices: Learning effectiveness of an online-based program. Vaccine 2020, 38, 7551–7557. [Google Scholar] [CrossRef]
- Reed, L.; Tarini, B.A.; Andreae, M.C. Vaccine administration error rates at a large academic medical center and its affiliated clinics-Familiarity matters. Vaccine 2019, 37, 5390–5396. [Google Scholar] [CrossRef]
- Lang, S.; Ford, K.J.; John, T.; Pollard, A.J.; McCarthy, N.D. Immunisation errors reported to a vaccine advice service: Intelligence to improve practice. Qual. Prim. Care 2014, 22, 139–146. [Google Scholar]
- Hibbs, B.F.; Miller, E.R.; Shimabukuro, T.; Centers for Disease, C. Prevention. Notes from the field: Rotavirus vaccine administration errors—United States, 2006–2013. Morb. Mortal. Wkly. Rep. 2014, 63, 81. [Google Scholar]
- CDC. Vaccine Administration: Preventing Vaccine Administration Errors. Available online: https://www.cdc.gov/vaccines/hcp/admin/downloads/vaccine-administration-preventing-errors.pdf (accessed on 4 September 2022).
- Paparella, S.F. Vaccine Errors: Understanding the Risks and the Responsibilities for Public Safety. J. Emerg. Nurs. 2015, 41, 428–430. [Google Scholar] [CrossRef]
| Month, Year | CDC Recommendation |
|---|---|
| December 2020 | |
| February 2021 | |
| March 2021 |
|
| April 2021 |
|
| May 2021 |
|
| August 2021 |
|
| September 2021 |
|
| October 2021 | |
| November 2021 |
|
| December 2021 |
|
| January 2022 |
|
| March 2022 |
|
| Error Categories | Error Subcategories | Frequency n (%) |
|---|---|---|
| Storage | Expired by expiration date Past beyond-use date Temperature excursion | 191 (26.0%) |
| Incorrect dosage or product | Higher-than-authorized dosage Lower-than-authorized dosage | 190 (25.9%) |
| Unauthorized age group | Younger than age 5 years Age 5 through 11 years Age 12 through 15 years Age 16 through 17 years | 108 (14.7%) |
| Schedule | Interval error Mixed primary series More doses than authorized Other incorrect product error | 105 (14.3%) |
| Preparation and administration | Incorrect site or route Incorrect diluent Other preparation or administration error | 71 (9.7%) |
| Multiple errors | N/A | 69 (9.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, E.; Odafe, S.; Madden, J.; Schillie, S. Qualitative Conceptual Content Analysis of COVID-19 Vaccine Administration Error Inquiries. Vaccines 2023, 11, 254. https://doi.org/10.3390/vaccines11020254
Hall E, Odafe S, Madden J, Schillie S. Qualitative Conceptual Content Analysis of COVID-19 Vaccine Administration Error Inquiries. Vaccines. 2023; 11(2):254. https://doi.org/10.3390/vaccines11020254
Chicago/Turabian StyleHall, Elisha, Solomon Odafe, Joseph Madden, and Sarah Schillie. 2023. "Qualitative Conceptual Content Analysis of COVID-19 Vaccine Administration Error Inquiries" Vaccines 11, no. 2: 254. https://doi.org/10.3390/vaccines11020254
APA StyleHall, E., Odafe, S., Madden, J., & Schillie, S. (2023). Qualitative Conceptual Content Analysis of COVID-19 Vaccine Administration Error Inquiries. Vaccines, 11(2), 254. https://doi.org/10.3390/vaccines11020254





