Suitability of Polymyxin B as a Mucosal Adjuvant for Intranasal Influenza and COVID-19 Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Antigens and Adjuvant
2.3. Immunization of Mice
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Particle Diameter
2.6. Histopathological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Immunization of Mice with Influenza HA Split
3.2. Immunization of Mice with SARS-CoV-2 S1 Subunit
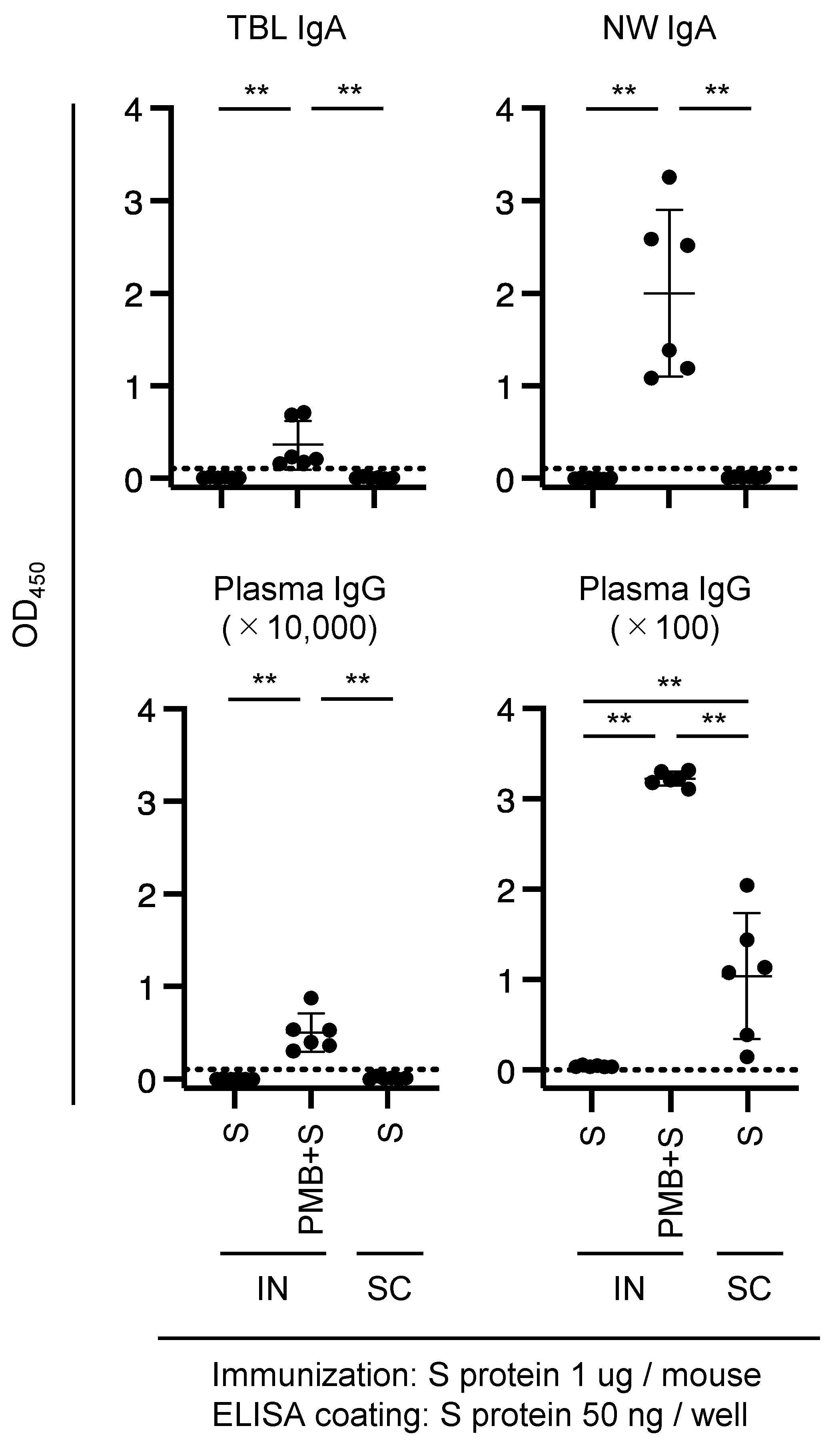
3.3. Immunization of Mice with SARS-CoV-2 S Protein
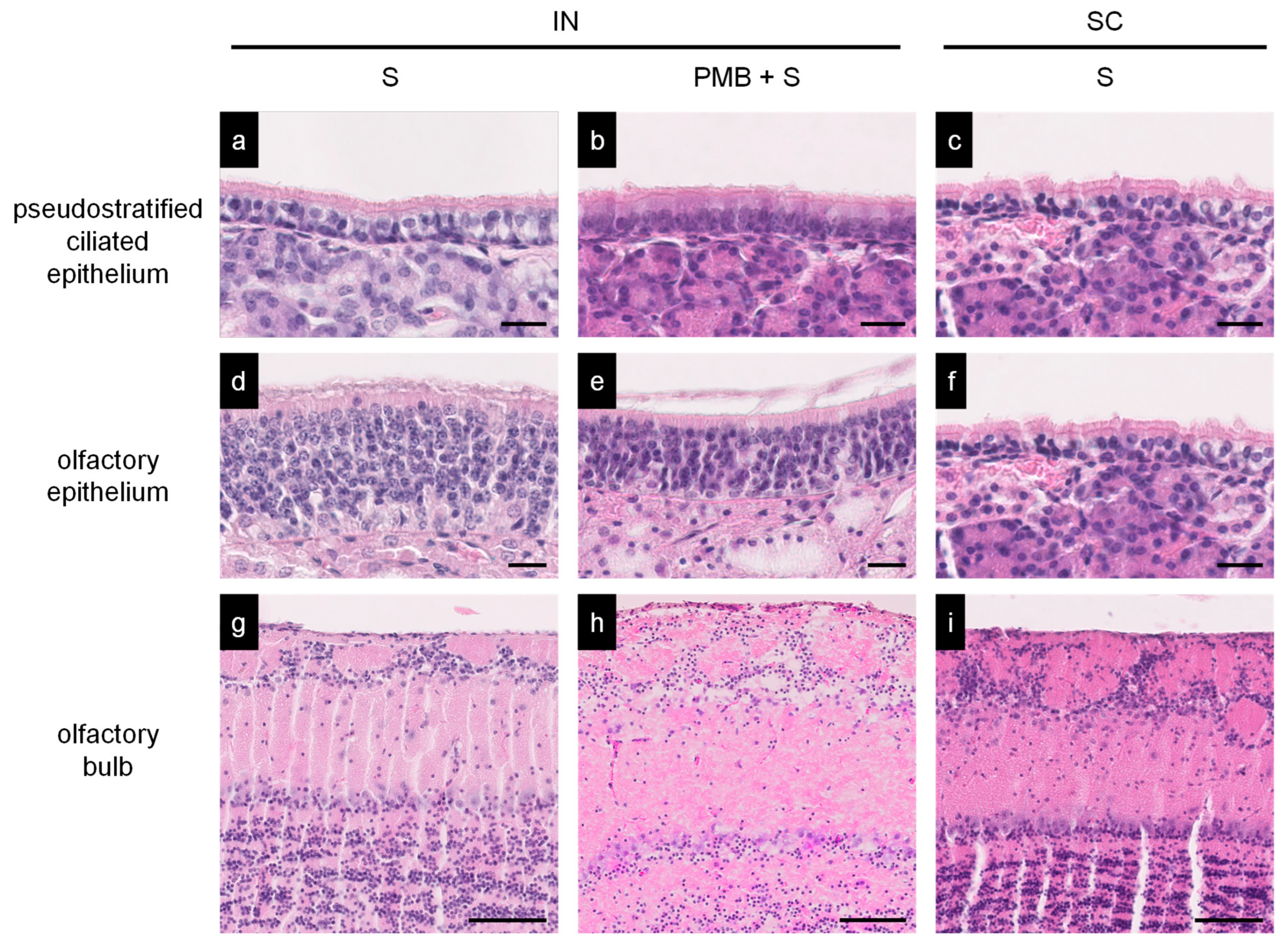
3.4. Histopathology of the Nasal Mucosa of Immunized Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, N.; Endo, M.; Kanno, H.; Matsukawa, N.; Tsutsumi, R.; Takeshita, R.; Sato, S. Polymyxins as novel and safe mucosal adjuvants to induce humoral immune responses in mice. PLoS ONE 2013, 8, e61643. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/ (accessed on 14 October 2023).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Shaw, R.H.; Supasa, P.; Liu, C.; Sv Stuart, A.; Pollard, A.J.; Liu, X.; Lambe, T.; Crook, D.; Stuart, D.I.; et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 2021, 399, 234–236. [Google Scholar] [CrossRef]
- Carreño, J.M.; Alshammary, H.; Tcheou, J.; Singh, G.; Raskin, A.J.; Kawabata, H.; Sominsky, L.A.; Clark, J.J.; Adelsberg, D.C.; Bielak, D.A.; et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 2022, 602, 682–688. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Lu, L.; Mok, B.W.-Y.; Chen, L.L.; Chan, J.M.-C.; Tsang, O.T.-Y.; Lam, B.H.-S.; Chuang, V.W.-M.; Chu, A.W.-H.; Chan, W.-M.; Ip, J.-D.; et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin. Infect. Dis. 2022, 75, e822–e826. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.; Matsuoka, O.; Inoue, S.; Inoue, T.; Meng, Y.; Nakama, T.; Kato, K.; Pandey, A.; Chang, L.J. Immunogenicity and safety of high-dose quadrivalent influenza vaccine in Japanese adults ≥65 years of age: A randomized controlled clinical trial. Hum. Vaccines Immunother. 2020, 16, 858–866. [Google Scholar] [CrossRef]
- Sekiya, T.; Ohno, M.; Nomura, N.; Handabile, C.; Shingai, M.; Jackson, D.C.; Brown, L.E.; Kida, H. Selecting and Using the Appropriate Influenza Vaccine for Each Individual. Viruses 2021, 13, 971. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Staats, H.F.; Jackson, R.J.; Marinaro, M.; Takahashi, I.; Kiyono, H.; McGhee, J.R. Mucosal immunity to infection with implications for vaccine development. Curr. Opin. Immunol. 1994, 6, 572–583. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Tokuhara, D.; Kataoka, K.; Gilbert, R.S.; McGhee, J.R.; Yuki, Y.; Kiyono, H.; Fujihashi, K. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev. Vaccines. 2012, 11, 367–379. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Aoshi, T. Modes of Action for Mucosal Vaccine Adjuvants. Viral Immunol. 2017, 30, 463–470. [Google Scholar] [CrossRef]
- Yoshino, N.; Takeshita, R.; Kawamura, H.; Murakami, K.; Sasaki, Y.; Sugiyama, I.; Sadzuka, Y.; Kagabu, M.; Sugiyama, T.; Muraki, Y.; et al. Critical micelle concentration and particle size determine adjuvanticity of cyclic lipopeptides. Scand. J. Immunol. 2018, 88, e12698. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, T.; Yoshino, N.; Sasaki, Y.; Muraki, Y. Division of Infectious Diseases and Immunology; Department of Microbiology, School of Medicine, Iwate Medical University: Yahaba, Japan, 2023; (manuscript in preparation). [Google Scholar]
- Tanimoto, T.; Nakatsu, R.; Fuke, I.; Ishikawa, T.; Ishibashi, M.; Yamanishi, K.; Takahashi, M.; Tamura, S. Estimation of the neuraminidase content of influenza viruses and split-product vaccines by immunochromatography. Vaccine 2005, 23, 4598–4609. [Google Scholar] [CrossRef]
- Yoshino, N.; Kawamura, H.; Sugiyama, I.; Sasaki, Y.; Odagiri, T.; Sadzuka, Y.; Muraki, Y. A systematic assessment of the relationship between synthetic surfactants and mucosal adjuvanticity. Eur. J. Pharm. Biopharm. 2021, 165, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, Z.; Fujihashi, K. Appendix II—Collection and processing of external secretions and tissues of mouse origin. In Mucosal Immunology, 3rd ed.; Lamm, M.E., McGhee, J.R., Bienenstock, J., Mayer, L., Strober, W., Eds.; Academic Press: Burlington, VT, USA, 2005; pp. 1841–1852. [Google Scholar] [CrossRef]
- Fukasaka, M.; Asari, D.; Kiyotoh, E.; Okazaki, A.; Gomi, Y.; Tanimoto, T.; Takeuchi, O.; Akira, S.; Hori, M. A Lipopolysaccharide from Pantoea Agglomerans is a Promising Adjuvant for Sublingual Vaccines to Induce Systemic and Mucosal Immune Responses in Mice via TLR4 Pathway. PLoS ONE 2015, 10, e0126849. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Russell, M.W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect. Immun. 1993, 61, 314–322. [Google Scholar] [CrossRef]
- Yoshino, N.; Fujihashi, K.; Hagiwara, Y.; Kanno, H.; Takahashi, K.; Kobayashi, R.; Inaba, N.; Noda, M.; Sato, S. Co-administration of cholera toxin and apple polyphenol extract as a novel and safe mucosal adjuvant strategy. Vaccine 2009, 27, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.A.; Florindo, H.F. Chapter 3.1: Nanocarriers Overcoming the Nasal Barriers: Physiological Considerations and Mechanistic Issues. In Nanostructures Biomaterials for Overcoming Biological Barriers; Alonso, M.J., Csaba, N.S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2012; pp. 117–132. [Google Scholar] [CrossRef]
- Johnson-Weaver, B.; Choi, H.W.; Abraham, S.N.; Staats, H.F. Mast cell activators as novel immune regulators. Curr. Opin. Pharmacol. 2018, 41, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 2023, 23, 189–199. [Google Scholar] [CrossRef]
- Tai, L.; Zhu, G.; Yang, M.; Cao, L.; Xing, X.; Yin, G.; Chan, C.; Qin, C.; Rao, Z.; Wang, X.; et al. Nanometer-resolution in situ structure of the SARS-CoV-2 postfusion spike protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2112703118. [Google Scholar] [CrossRef]
- Xia, S.; Li, Y.; Xia, Q.; Zhang, X.; Huang, Q. Glycosylation of bovine serum albumin via Maillard reaction prevents epigallocatechin-3-gallate-induced protein aggregation. Food Hydrocoll. 2015, 43, 228–235. [Google Scholar] [CrossRef]
- Ketas, T.J.; Chaturbhuj, D.; Portillo, V.M.C.; Francomano, E.; Golden, E.; Chandrasekhar, S.; Debnath, G.; Díaz-Tapia, R.; Yasmeen, A.; Kramer, K.D.; et al. Antibody Responses to SARS-CoV-2 mRNA Vaccines are Detectable in Saliva. Pathog. Immun. 2021, 6, 116–134. [Google Scholar] [CrossRef]
- Havervall, S.; Marking, U.; Svensson, J.; Greilert-Norin, N.; Bacchus, P.; Nilsson, P.; Hober, S.; Gordon, M.; Blom, K.; Klingström, J.; et al. Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal. Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef]
- Elson, C.O.; Ealding, W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 1984, 132, 2736–2741. [Google Scholar] [CrossRef]
- Valli, E.; Harriett, A.J.; Nowakowska, M.K.; Baudier, R.L.; Provosty, W.B.; McSween, Z.; Lawson, L.B.; Nakanishi, Y.; Norton, E.B. LTA1 is a safe, intranasal enterotoxin-based adjuvant that improves vaccine protection against influenza in young, old and B-cell-depleted (μMT) mice. Sci. Rep. 2019, 9, 15128. [Google Scholar] [CrossRef] [PubMed]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef]
- Boyce, T.G.; Gruber, W.C.; Coleman-Dockery, S.D.; Sannella, E.C.; Reed, G.W.; Wolff, M.; Wright, P.F. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine 1999, 18, 82–88. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, P.J.; Steele, A.D.; Hiemstra, L.A.; Rappaport, R.; Dunning, A.J.; Gruber, W.C.; Forrest, B.D.; LAIV Elderly Study Trial Network. Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine 2009, 28, 228–234. [Google Scholar] [CrossRef]
- Jensen, T.; Pedersen, S.S.; Garne, S.; Heilmann, C.; Høiby, N.; Koch, C. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J. Antimicrob. Chemother. 1987, 19, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Local administration of polymyxins into the respiratory tract for the prevention and treatment of pulmonary infections in patients without cystic fibrosis. Infection 2007, 35, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Quiding-Järbrink, M.; Lakew, M.; Nordström, I.; Banchereau, J.; Butcher, E.; Holmgren, J.; Czerkinsky, C. Human circulating specific antibody-forming cells after systemic and mucosal immunizations: Differential homing commitments and cell surface differentiation markers. Eur. J. Immunol. 1995, 25, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.L.; Wassén, L.; Holmgren, J.; Jertborn, M.; Rudin, A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 2001, 69, 7481–7486. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.L.; Bergquist, C.; Edebo, A.; Johansson, C.; Svennerholm, A.M. Comparison of different routes of vaccination for eliciting antibody responses in the human stomach. Vaccine 2004, 22, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Yoshino, N.; Murakami, K.; Kawamura, H.; Sugiyama, I.; Sasaki, Y.; Odagiri, T.; Sadzuka, Y.; Muraki, Y. The relationship between the chemical structure, physicochemical properties, and mucosal adjuvanticity of sugar-based surfactants. Eur. J. Pharm. Biopharm. 2023, 182, 1–11. [Google Scholar] [CrossRef] [PubMed]


| Antigen | PMB | Diameter Distribution by Intensity | PDI b | |||||
|---|---|---|---|---|---|---|---|---|
| Peak 1 a | Peak 2 | Peak 3 | ||||||
| D (nm) c | Int (%) d | D (nm) | Int (%) | D (nm) | Int (%) | |||
| HA | – | 202.4 ± 2.3 | 98.9 ± 1.0 | 39.2 ± 1.8 | 1.1 ± 1.0 | N/A | N/A | 0.207 ± 0.005 |
| + | 418.0 ± 16.9 | 97.7 ± 2.0 | 69.1 ± 2.5 | 2.3 ± 2.0 | 0.315 ± 0.025 | |||
| S1 | – | 391.4 ± 13.1 | 63.4 ± 1.8 | 16.3 ± 1.8 | 36.6 ± 1.8 | N/A | N/A | 0.737 ± 0.081 |
| + | 2209.3 ± 86.7 | 100.0 | N/A | N/A | 0.161 ± 0.051 | |||
| S | – | 246.6 ± 4.4 | 56.7 ± 1.9 | 24.5 ± 0.7 | 43.3 ± 1.9 | N/A | N/A | 0.610 ± 0.132 |
| + | 26.0 ± 1.7 | 48.6 ± 2.0 | 2.0 ± 0.0 | 34.3 ± 6.8 | 470.4 ± 79.0 | 17.0 ± 5.6 | 0.446 ± 0.139 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshino, N.; Yokoyama, T.; Sakai, H.; Sugiyama, I.; Odagiri, T.; Kimura, M.; Hojo, W.; Saino, T.; Muraki, Y. Suitability of Polymyxin B as a Mucosal Adjuvant for Intranasal Influenza and COVID-19 Vaccines. Vaccines 2023, 11, 1727. https://doi.org/10.3390/vaccines11111727
Yoshino N, Yokoyama T, Sakai H, Sugiyama I, Odagiri T, Kimura M, Hojo W, Saino T, Muraki Y. Suitability of Polymyxin B as a Mucosal Adjuvant for Intranasal Influenza and COVID-19 Vaccines. Vaccines. 2023; 11(11):1727. https://doi.org/10.3390/vaccines11111727
Chicago/Turabian StyleYoshino, Naoto, Takuya Yokoyama, Hironori Sakai, Ikumi Sugiyama, Takashi Odagiri, Masahiro Kimura, Wataru Hojo, Tomoyuki Saino, and Yasushi Muraki. 2023. "Suitability of Polymyxin B as a Mucosal Adjuvant for Intranasal Influenza and COVID-19 Vaccines" Vaccines 11, no. 11: 1727. https://doi.org/10.3390/vaccines11111727
APA StyleYoshino, N., Yokoyama, T., Sakai, H., Sugiyama, I., Odagiri, T., Kimura, M., Hojo, W., Saino, T., & Muraki, Y. (2023). Suitability of Polymyxin B as a Mucosal Adjuvant for Intranasal Influenza and COVID-19 Vaccines. Vaccines, 11(11), 1727. https://doi.org/10.3390/vaccines11111727






