Safety, Tolerability, and Immunogenicity of Measles and Rubella Vaccine Delivered with a High-Density Microarray Patch: Results from a Randomized, Partially Double-Blinded, Placebo-Controlled Phase I Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Participants and Study Design
2.2. Vaccines
2.3. HD-MAP Manufacture
2.4. Vaccination Procedure
2.5. Immunogenicity Assays
2.6. Thermostability
2.7. Statistical Analyses (Immunogenicity)
3. Results
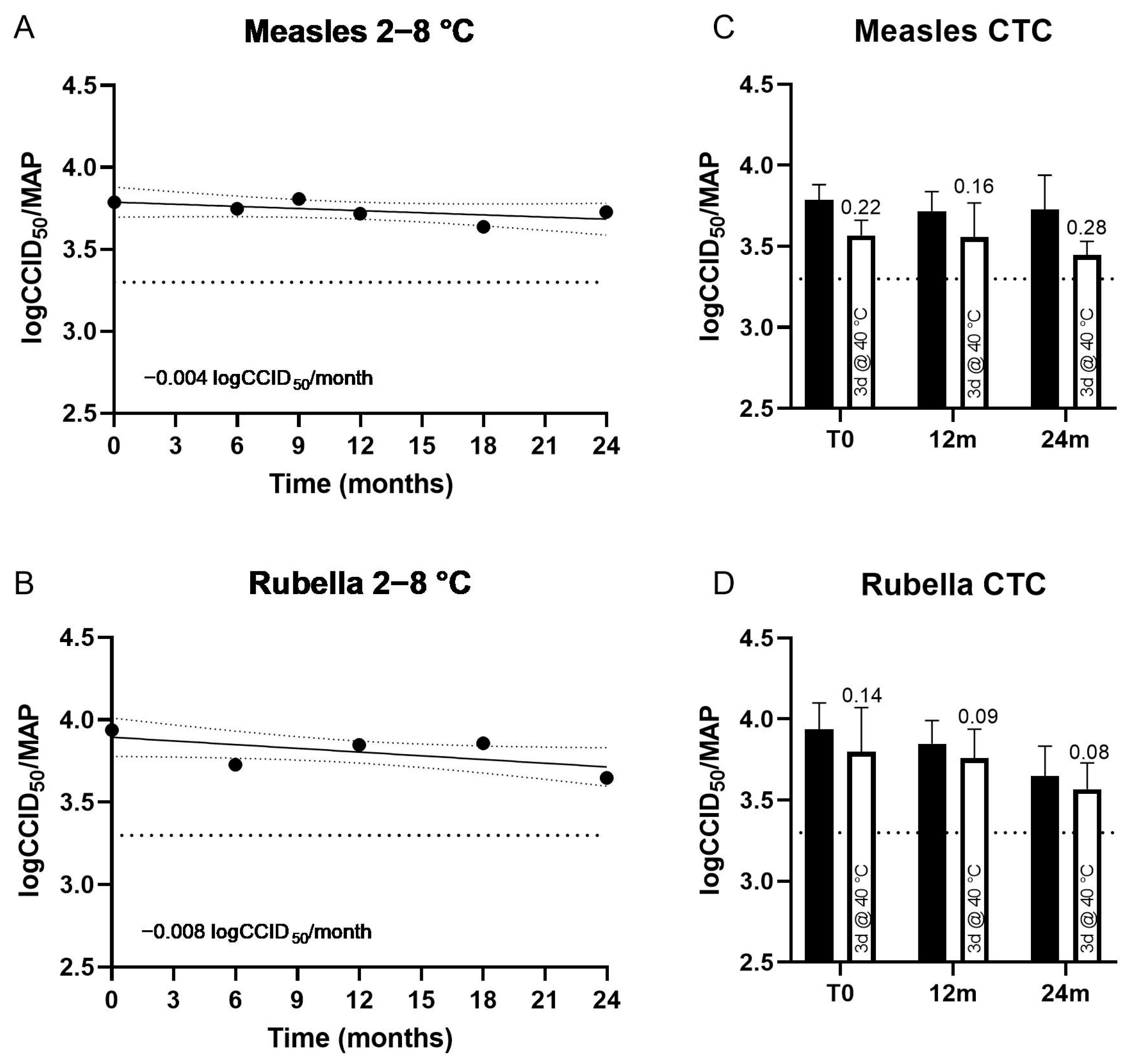
3.1. Thermostability of MR HD-MAPs
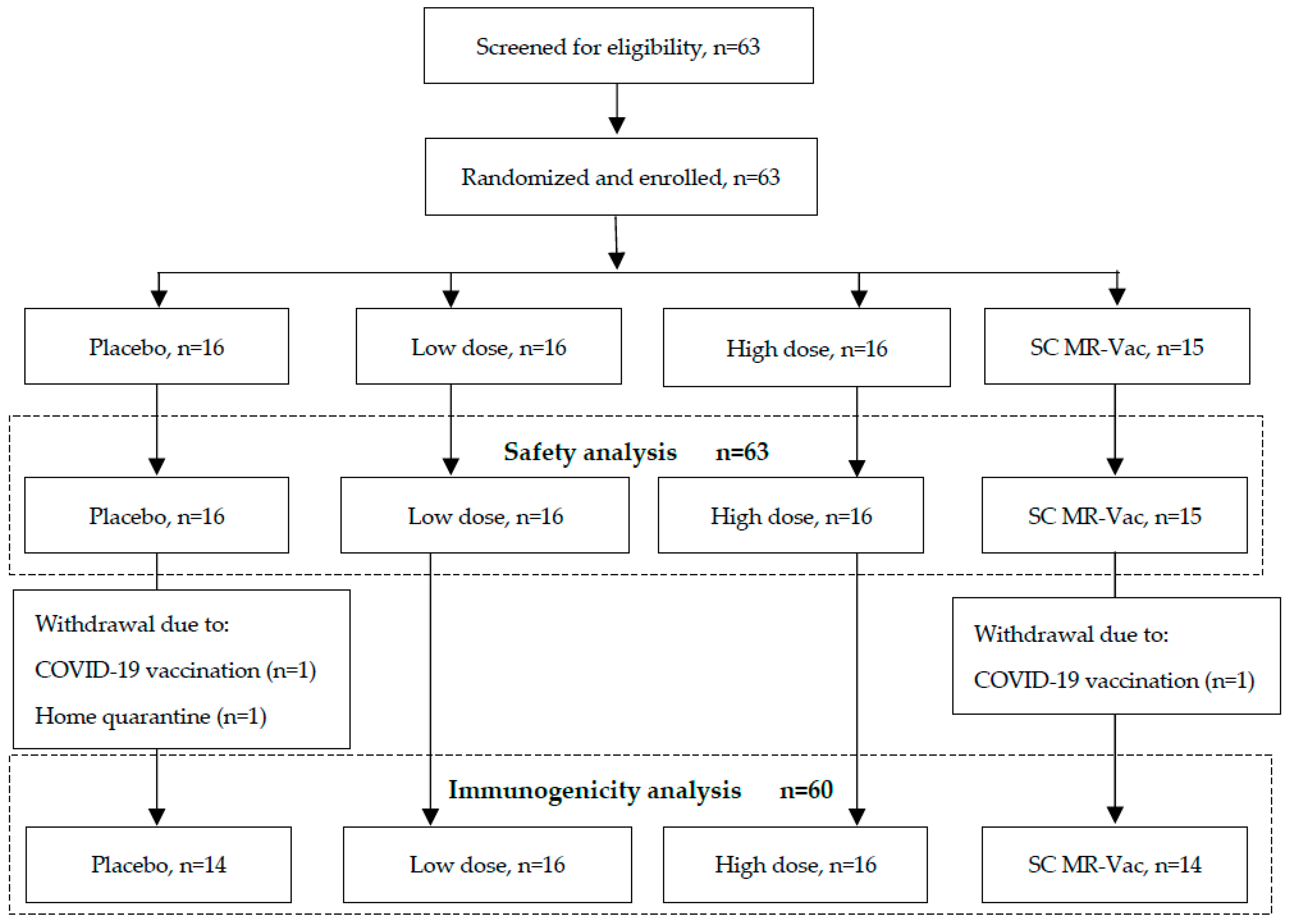
3.2. Particpants and Study Procedures
3.3. Summary of Adverse Events
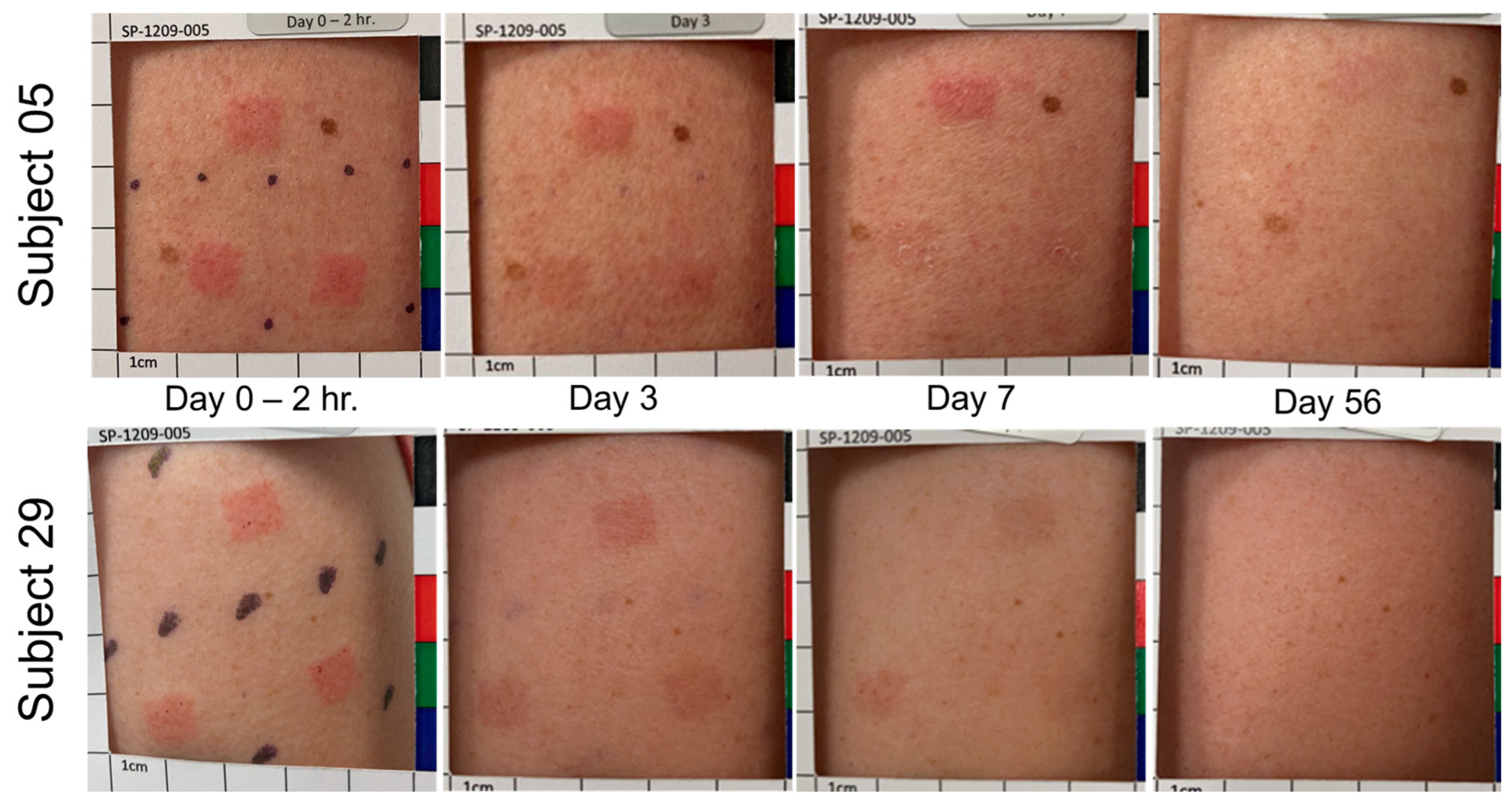
3.4. Treatment Site Reactions and Resolution
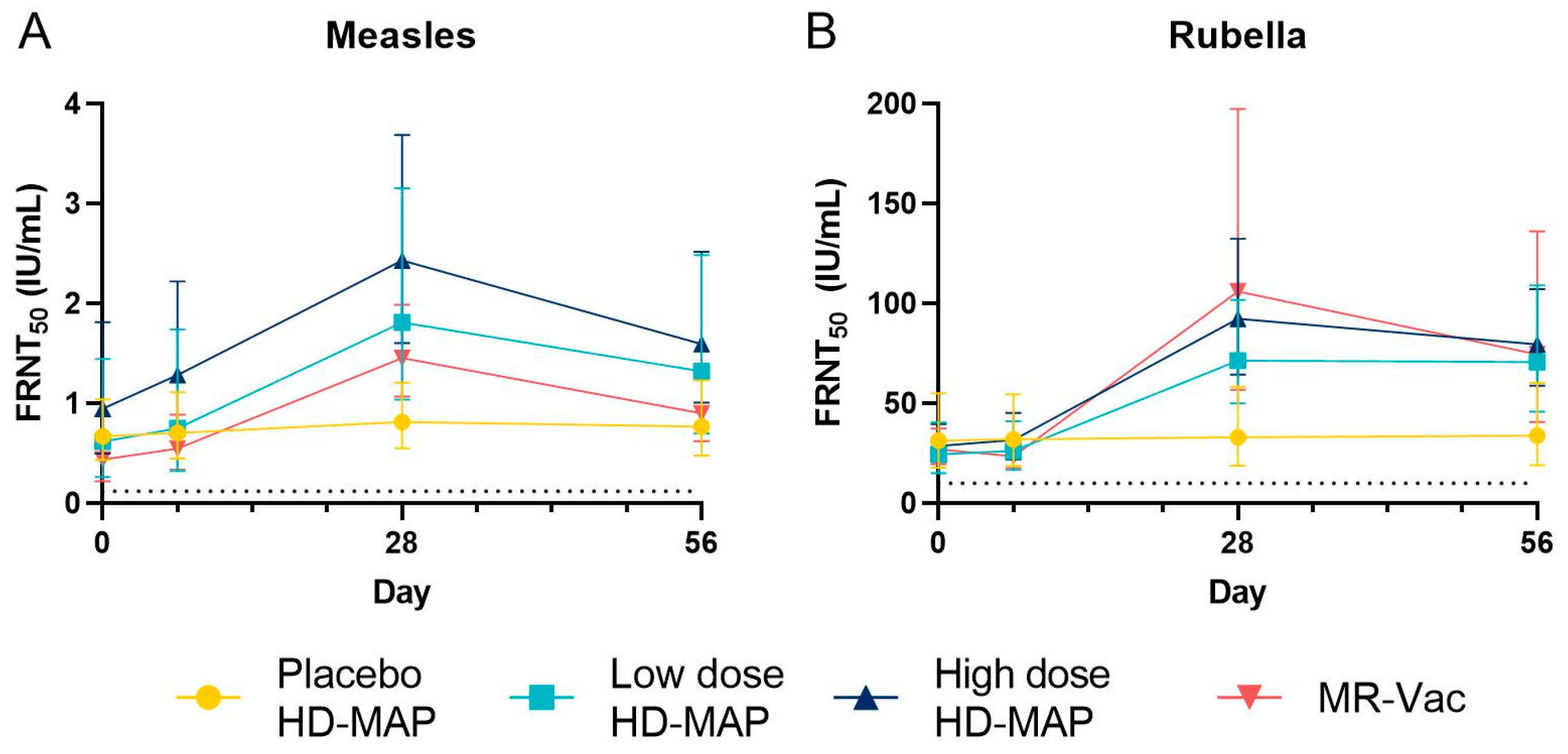
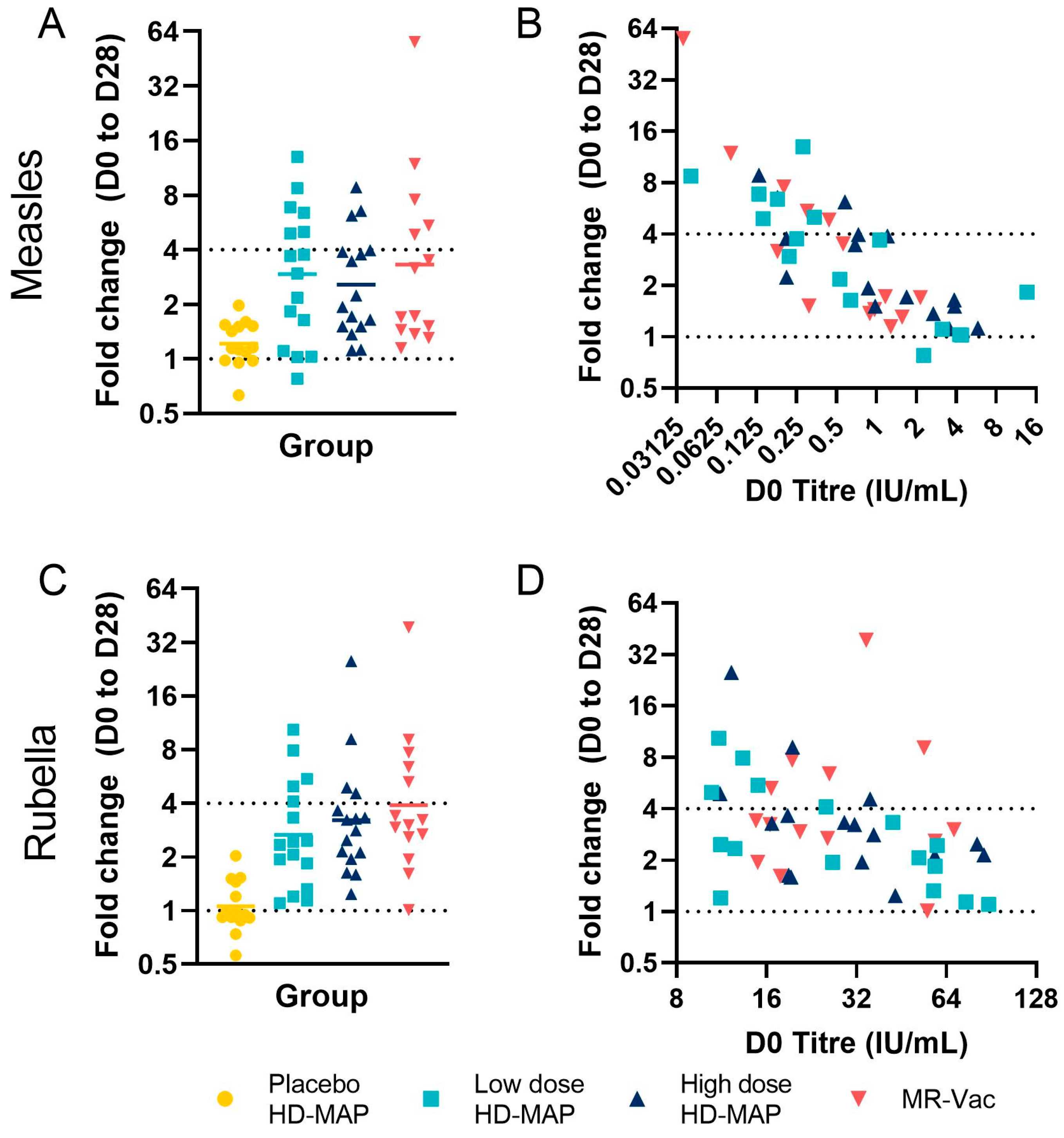
3.5. Serum Antibody Responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orenstein, W.A.; Cairns, L.; Hinman, A.; Nkowane, B.; Olivé, J.-M.; Reingold, A.L. Measles and Rubella Global Strategic Plan 2012-2020 midterm review report: Background and summary. Vaccine 2018, 36 (Suppl. 1), A35–A42. [Google Scholar] [CrossRef]
- Gastañaduy, P.A.; Goodson, J.L.; Panagiotakopoulos, L.; Rota, P.A.; Orenstein, W.A.; Patel, M. Measles in the 21st Century: Progress Toward Achieving and Sustaining Elimination. J. Infect. Dis. 2021, 224, S420–S428. [Google Scholar] [CrossRef]
- Shet, A.; Carr, K.; Danovaro-Holliday, M.C.; Sodha, S.V.; Prosperi, C.; Wunderlich, J.; Wonodi, C.; Reynolds, H.W.; Mirza, I.; Gacic-Dobo, M.; et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: Evidence of disruption and recovery from 170 countries and territories. Lancet Glob. Health 2022, 10, e186–e194. [Google Scholar] [CrossRef]
- Grant, G.B.; Masresha, B.G.; Moss, W.J.; Mulders, M.N.; Rota, P.A.; Omer, S.B.; Shefer, A.; Kriss, J.L.; Hanson, M.; Durrheim, D.N.; et al. Accelerating measles and rubella elimination through research and innovation—Findings from the Measles & Rubella Initiative research prioritization process, 2016. Vaccine 2019, 37, 5754–5761. [Google Scholar] [CrossRef]
- Kriss, J.L.; Grant, G.B.; Moss, W.J.; Durrheim, D.N.; Shefer, A.; Rota, P.A.; Omer, S.B.; Masresha, B.G.; Mulders, M.N.; Hanson, M.; et al. Research priorities for accelerating progress toward measles and rubella elimination identified by a cross-sectional web-based survey. Vaccine 2019, 37, 5745–5753. [Google Scholar] [CrossRef]
- Fernando, G.J.; Hickling, J.; Flores, C.M.J.; Griffin, P.; Anderson, C.D.; Skinner, S.R.; Davies, C.; Witham, K.; Pryor, M.; Bodle, J.; et al. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (NanopatchTM). Vaccine 2018, 36, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Azukizawa, H.; Hanafusa, T.; Matsuo, K.; Quan, Y.-S.; Kamiyama, F.; Katayama, I.; Okada, N.; Nakagawa, S. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 2015, 57, 50–58. [Google Scholar] [CrossRef]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- Iwata, H.; Kakita, K.; Imafuku, K.; Takashima, S.; Haga, N.; Yamaguchi, Y.; Taguchi, K.; Oyamada, T. Safety and dose-sparing effect of Japanese encephalitis vaccine administered by microneedle patch in uninfected, healthy adults (MNA-J): A randomised, partly blinded, active-controlled, phase 1 trial. Lancet Microbe 2022, 3, e96–e104. [Google Scholar] [CrossRef]
- Forster, A.H.; Witham, K.; Depelsenaire, A.C.I.; Veitch, M.; Wells, J.W.; Wheatley, A.; Pryor, M.; Lickliter, J.D.; Francis, B.; Rockman, S.; et al. Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: Results from a randomized, controlled phase I clinical trial. PLoS Med. 2020, 17, e1003024. [Google Scholar] [CrossRef]
- Joyce, J.C.; Carroll, T.D.; Collins, M.L.; Chen, M.-H.; Fritts, L.; Dutra, J.C.; Rourke, T.L.; Goodson, J.L.; McChesney, M.B.; Prausnitz, M.R.; et al. A Microneedle Patch for Measles and Rubella Vaccination Is Immunogenic and Protective in Infant Rhesus Macaques. J. Infect. Dis. 2018, 218, 124–132. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Goodson, J.L.; Rota, P.A.; Orenstein, W.A. A microneedle patch for measles and rubella vaccination: A game changer for achieving elimination. Curr. Opin. Virol. 2020, 41, 68–76. [Google Scholar] [CrossRef]
- Mvundura, M.; Frivold, C.; Osborne, A.J.; Soni, P.; Robertson, J.; Kumar, S.; Anena, J.; Gueye, A.; Menozzi-Arnaud, M.; Giersing, B.; et al. Vaccine innovation prioritisation strategy: Findings from three country-stakeholder consultations on vaccine product innovations. Vaccine 2021, 39, 7195–7207. [Google Scholar] [CrossRef]
- Wan, Y.; Hickey, J.M.; Bird, C.; Witham, K.; Fahey, P.; Forster, A.; Joshi, S.B.; Volkin, D.B. Development of Stabilizing Formulations of a Trivalent Inactivated Poliovirus Vaccine in a Dried State for Delivery in the NanopatchTM Microprojection Array. J. Pharm. Sci. 2018, 107, 1540–1551. [Google Scholar] [CrossRef]
- Wan, Y.; Gupta, V.; Bird, C.; Pullagurla, S.R.; Fahey, P.; Forster, A.; Volkin, D.B.; Joshi, S.B. Formulation Development and Improved Stability of a Combination Measles and Rubella Live-Viral Vaccine Dried for Use in the NanopatchTM Microneedle Delivery System. Hum. Vaccin. Immunother. 2021, 17, 2501–2516. [Google Scholar] [CrossRef]
- Bolotin, S.; Hughes, S.L.; Gul, N.; Khan, S.; Rota, P.; Severini, A.; Hahné, S.; Tricco, A.; Moss, W.J.; Orenstein, W.; et al. What Is the Evidence to Support a Correlate of Protection for Measles? A Systematic Review. J. Infect. Dis. 2020, 221, 1576–1583. [Google Scholar] [CrossRef]
- NCCLS. Detection and Quantitation of Rubella IgG Antibody: Evaluation and Performance Criteria for Multiple Component Test Products, Specimen Handling, and Use of Test Products in the Clinical Laboratory; Approved Guideline; NCCLS document I/LA6-A (ISBN) 1-56238-335-3; NCCLS: Wayne, PA, USA, 1997. [Google Scholar]
- Dadari, I.K.; Zgibor, J.C. How the use of vaccines outside the cold chain or in controlled temperature chain contributes to improving immunization coverage in low- and middle-income countries (LMICs): A scoping review of the literature. J. Glob. Health 2021, 11, 04004. [Google Scholar] [CrossRef]
- MVDP Author Group; Agarkhedkar, S.; Kulkarni, P.S.; Winston, S.; Sievers, R.; Dhere, R.M.; Gunale, B.; Powell, K.; Rota, P.A.; Papania, M.; et al. Safety and immunogenicity of dry powder measles vaccine administered by inhalation: A randomized controlled Phase I clinical trial. Vaccine 2014, 32, 6791–6797. [Google Scholar] [CrossRef]
- Simon, J.K.; Pasetti, M.F.; Viret, J.F.; Mischler, R.; Muñoz, A.; Lagos, R.; Levine, M.M.; Campbell, J.D. A clinical study to assess the safety and immunogenicity of attenuated measles vaccine administered intranasally to healthy adults. Hum. Vaccines 2007, 3, 54–58. [Google Scholar] [CrossRef]
- Etchart, N.; Hennino, A.; Friede, M.; Dahel, K.; Dupouy, M.; Goujonhenry, C.; Nicolas, J.-F.; Kaiserlian, D. Safety and efficacy of transcutaneous vaccination using a patch with the live-attenuated measles vaccine in humans. Vaccine 2007, 25, 6891–6899. [Google Scholar] [CrossRef]
- Cutts, F.T.; Clements, C.J.; Bennett, J.V. Alternative routes of measles immunization: A review. Biologicals 1997, 25, 323–338. [Google Scholar] [CrossRef]
- Depelsenaire, A.C.I.; Witham, K.; Veitch, M.; Wells, J.W.; Anderson, C.D.; Lickliter, J.D.; Rockman, S.; Bodle, J.; Treasure, P.; Hickling, J.; et al. Cellular responses at the application site of a high-density microarray patch delivering an influenza vaccine in a randomized, controlled phase I clinical trial. PLoS ONE 2021, 16, e0255282. [Google Scholar] [CrossRef]
- Kristensen, D.; Giersing, B.; Hickling, J.; Kazi, F.; Scarna, T.; Kahn, A.-L.; Hsu, V.; Gandrup-Marino, K.; Menozzi-Arnaud, M. A global collaboration to advance vaccine product innovations—The Vaccine Innovation Prioritisation Strategy. Vaccine 2021, 39, 7191–7194. [Google Scholar] [CrossRef]
- Joyce, J.C.; Collins, M.L.; Rota, P.A.; Prausnitz, M.R. Thermostability of Measles and Rubella Vaccines in a Microneedle Patch. Adv. Ther. (Weinh) 2021, 4, 2100095. [Google Scholar] [CrossRef]
- Guillermet, E.; Alfa, D.A.; Mai, L.T.P.; Subedi, M.; Demolis, R.; Giersing, B.; Jaillard, P. End-user acceptability study of the nanopatchTM; a microarray patch (MAP) for child immunization in low and middle-income countries. Vaccine 2019, 37, 4435–4443. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.B.; Goodson, J.L.; Chu, S.Y.; Rota, P.A.; Meltzer, M.I. Assessing the Potential Cost-Effectiveness of Microneedle Patches in Childhood Measles Vaccination Programs: The Case for Further Research and Development. Drugs R&D 2016, 16, 327–338. [Google Scholar] [CrossRef]
- Hasso-Agopsowicz, M.; Crowcroft, N.; Biellik, R.; Gregory, C.J.; Menozzi-Arnaud, M.; Amorij, J.-P.; Gilbert, P.-A.; Earle, K.; Frivold, C.; Jarrahian, C.; et al. Accelerating the Development of Measles and Rubella Microarray Patches to Eliminate Measles and Rubella: Recent Progress, Remaining Challenges. Front. Public Health 2022, 10, 809675. [Google Scholar] [CrossRef]
- PATH Measles-Rubella Microarray Patch Vaccines: A Business Case Analysis. Available online: https://media.path.org/documents/PATH_MR_MAP_business_case_report_2021March.pdf?_gl=1*1veo2ap*_gcl_au*MTMwOTMzODgzMS4xNjkyMjQzMTQx*_ga*MTEzNjUzMzUwNi4xNjkyMjQzMTQx*_ga_YBSE7ZKDQM*MTY5MjI0MzE0MS4xLjAuMTY5MjI0MzE0OS41Mi4wLjA. (accessed on 11 August 2023).

| Parameter | Value | Uncoated HD-MAP n = 16 | Low-Dose HD-MAP n = 16 | High-Dose HD-MAP n = 16 | MR-Vac n = 15 | Overall n = 63 |
|---|---|---|---|---|---|---|
| Age (year) | n | 16 | 16 | 16 | 15 | 63 |
| Mean (SD) | 27.8 (7.4) | 33.2 (9.9) | 32.5 (9.3) | 27.1 (10.3) | 30.2 (9.4) | |
| Range | 19–44 | 18–48 | 19–49 | 18–49 | 18–49 | |
| Sex, n (%) | Male | 9 (56.3) | 6 (37.5) | 8 (50.0) | 8 (53.3) | 31 (49.2) |
| Female | 7 (43.8) | 10 (62.5) | 8 (50.0) | 7 (46.7) | 32 (50.8) | |
| BMI (kg/m2) | n | 16 | 16 | 16 | 15 | 63 |
| Mean (SD) | 22.7 (2.096) | 25.4 (3.7) | 25.6 (3.8) | 25.8 (3.914) | 24.8 (3.6) | |
| Range | 20.3–26.5 | 19.4–32.0 | 18.9–32.0 | 20.9–31.7 | 18.9–32.0 | |
| Race, n (%) | Aboriginal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 1 (1.6) |
| Asian | 3 (18.8) | 0 (0.0) | 2 (12.5) | 2 (13.3) | 7 (11.1) | |
| Caucasian | 12 (75.0) | 14 (87.5) | 14 (87.5) | 12 (80.0) | 52 (82.5) | |
| European and Filipino | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 1 (1.6) | |
| Hispanic | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 1 (1.6) | |
| Middle Eastern | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | |
| Ethnicity, n (%) | Aboriginal/Torres Strait Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 1 (1.6) |
| Jewish | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | |
| Latin, Central and South American | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 1 (1.6) | |
| North-West European and Mediterranean | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 1 (1.6) | |
| North-East Asian | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 1 (1.6) | |
| North-West European | 11 (68.8) | 15 (93.8) | 14 (87.5) | 10 (66.7) | 50 (79.4) | |
| South-East Asian | 3 (18.8) | 0 (0.0) | 1 (6.3) | 2 (13.3) | 6 (9.5) | |
| Southern and Eastern European | 1 (6.3) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 2 (3.2) |
| Group | Time and Condition | LogCCID50 Loss (95% CI) | |||
|---|---|---|---|---|---|
| Measles | Rubella | ||||
| MR HD-MAP | MR-Vac | MR HD-MAP | MR-Vac | ||
| Accelerated 1 | 3 days, 40 °C | 0.39 (0.29–0.49) | 0.37 (0.17–0.55) | 0.16 (−0.06–0.38) | 0.00 (−0.19–0.19) |
| 7 days, 37 °C | 0.43 (0.33–0.53) | 0.50 (0.31–0.69) | 0.07 (−0.01–0.15) | 0.33 (0.00–0.66) | |
| 14 days, 37 °C | 0.35 (0.26–0.44) | 0.55 (0.55–0.55) | 0.17 (−0.02–0.36) | 0.05 (−0.35–0.45) | |
| Long-term 2 | 6 months, 25 °C | 0.67 (0.55–0.79) | 0.75 (0.28–1.22) | 0.24 (0.07–0.41) | 0.23 (0.16–0.31) |
| 30 months, 2–8 °C | 0.38 (0.21–0.55) | 0.47 (0.35–0.59) | 0.18 (0.13–0.23) | −0.03 (−0.25–0.19) | |
| Long-term (clinical) 2 | 24 months, 2–8 °C | 0.06 (−0.15–0.27) | Not carried out | 0.29 (0.11–0.47) | Not carried out |
| Low-Dose HD-MAP n = 16 n (%) [e] | High-Dose HD-MAP n = 16 n (%) [e] | Uncoated HD-MAP n = 16 n (%) [e] | MR-Vac n = 15 n (%) [e] | |
|---|---|---|---|---|
| Systemic | ||||
| Fatigue | 0 | 1 (6.3) [1] | 0 | 0 |
| Arthralgia | 0 | 0 | 0 | 1 (6.7) [1] |
| Myalgia | 0 | 0 | 0 | 1 (6.7) [1] |
| Headache | 0 | 2 (12.5) [2] | 2 (12.5) [2] | 1 (6.7) [2] |
| Local | ||||
| Application site exfoliation | 1 (6.3) [1] | 0 | 1 (6.3) [1] | 0 |
| Injection site pain | 1 (6.3) [1] | 2 (12.5) [2] | 1 (6.3) [1] | 3 (20.0) [3] |
| Injection site pruritus | 3 (18.8) [3] | 6 (37.5) [7] | 0 | 0 |
| Parameter | Timepoint | Low-Dose HD-MAP n = 16 | High-Dose HD-MAP n = 16 | Uncoated HD-MAP n = 16 | MR-Vac n = 15 |
|---|---|---|---|---|---|
| Visible, No. (%) | Day 0 (10-Min PT) | 48 (100.0) | 48 (100.0) | 48 (100.0) | 13 (86.7) |
| Day 0 (1-Hr PT) | 48 (100.0) | 48 (100.0) | 48 (100.0) | 11 (73.3) | |
| Day 0 (2-Hr PT) | 48 (100.0) | 48 (100.0) | 48 (100.0) | 11 (73.3) | |
| Day 3 | 48 (100.0) | 48 (100.0) | 45 (100.0) | 3 (20.0) | |
| Day 7 | 46 (95.8) | 48 (100.0) | 48 (100.0) | 0 (0.0) | |
| Day 28 | 16 (33.3) | 46 (95.8) | 10 (23.8) | 0 (0.0) | |
| Day 56 or Early term. | 7 (14.6) | 26 (54.2) | 3 (6.3) | 0 (0.0) | |
| Erythema, No. (%) | Day 0 (10-Min PT) | 44 (91.7) | 45 (93.8) | 42 (87.5) | 9 (60.0) |
| Day 0 (1-Hr PT) | 45 (93.8) | 45 (93.8) | 42 (87.5) | 5 (33.3) | |
| Day 0 (2-Hr PT) | 45 (93.8) | 45 (93.8) | 42 (87.5) | 3 (20.0) | |
| Day 3 | 44 (91.7) | 45 (93.8) | 36 (80.0) | 1 (6.7) | |
| Day 7 | 22 (45.8) | 44 (91.7) | 21 (43.8) | 0 (0.0) | |
| Day 28 | 5 (10.4) | 18 (37.5) | 2 (4.8) | 0 (0.0) | |
| Day 56 or Early term. | 2 (4.2) | 4 (8.3) | 0 (0.0) | 0 (0.0) | |
| Swelling, No. (%) | Day 0 (10-Min PT) | 42 (87.5) | 39 (81.3) | 41 (85.4) | 7 (46.7) |
| Day 0 (1-Hr PT) | 42 (87.5) | 42 (87.5) | 39 (81.3) | 1 (6.7) | |
| Day 0 (2-Hr PT) | 43 (89.6) | 41 (85.4) | 33 (68.8) | 0 (0.0) | |
| Day 3 | 8 (16.7) | 31 (64.6) | 0 (0.0) | 0 (0.0) | |
| Day 7 | 10 (20.8) | 32 (66.7) | 0 (0.0) | 0 (0.0) | |
| Day 28 | 1 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Day 56 or Early term. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Oedema, No. (%) | Day 0 (10-Min PT) | 42 (87.5) | 39 (81.3) | 41 (85.4) | 7 (46.7) |
| Day 0 (1-Hr PT) | 42 (87.5) | 42 (87.5) | 39 (81.3) | 1 (6.7) | |
| Day 0 (2-Hr PT) | 43 (89.6) | 41 (85.4) | 32 (66.7) | 0 (0.0) | |
| Day 3 | 7 (14.6) | 28 (58.3) | 0 (0.0) | 0 (0.0) | |
| Day 7 | 6 (12.5) | 27 (56.3) | 0 (0.0) | 0 (0.0) | |
| Day 28 Day 56 or Early term. | 0 (0.0) 0 (0.0) | 0 (0.0) 0 (0.0) | 0 (0.0) 0 (0.0) | 0 (0.0) 0 (0.0) | |
| Induration, No. (%) | Day 0 (10-Min PT) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Day 0 (1-Hr PT) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Day 0 (2-Hr PT) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0 (0.0) | |
| Day 3 | 1 (2.1) | 3 (6.3) | 0 (0.0) | 0 (0.0) | |
| Day 7 | 4 (8.3) | 5 (10.4) | 0 (0.0) | 0 (0.0) | |
| Day 28 | 1 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Day 56 or Early term. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Skin flaking, No. (%) | Day 0 (10-Min PT) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0 (0.0) |
| Day 0 (1-Hr PT) | 0 (0.0) | 1 (2.1) | 1 (2.1) | 0 (0.0) | |
| Day 0 (2-Hr PT) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Day 3 | 4 (8.3) | 3 (6.3) | 1 (2.2) | 0 (0.0) | |
| Day 7 | 26 (54.2) | 31 (64.6) | 19 (39.6) | 0 (0.0) | |
| Day 28 | 3 (6.3) | 13 (27.1) | 1 (2.4) | 0 (0.0) | |
| Day 56 or Early term. | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Uncoated HD-MAP n = 14 | Low-Dose HD-MAP n = 16 | High-Dose HD-MAP n = 16 | MR-Vac n = 14 | |
|---|---|---|---|---|
| Measles | ||||
| Day 0 | ||||
| GMT IU/mL (95% CI) | 0.673 (0.434–1.042) | 0.617 (0.264–1.447) | 0.949 (0.497–1.813) | 0.439 (0.221–0.873) |
| Day 7 | ||||
| GMT IU/mL (95% CI) | 0.705 (0.447–1.113) | 0.751 (0.323–1.742) | 1.284 (0.742–2.222) | 0.547 (0.337–0.889) |
| Seroconversion, No. (%) | 0 (0) | 0 (0) | 1 (6.3) | 2 (14.3) |
| Day 28 | ||||
| GMT IU/mL (95% CI) | 0.816 (0.55–1.209) | 1.811 (1.039–3.156) | 2.431 (1.603–3.687) | 1.456 (1.067–1.987) |
| Seroconversion, No. (%) | 0 (0) | 6 (37.5) | 3 (18.8) | 5 (35.7) |
| Day 56 | ||||
| GMT IU/mL (95% CI) | 0.768 (0.478–1.233) | 1.321 (0.702–2.484) | 1.595 (1.01–2.518) | 0.902 (0.623–1.306) |
| Seroconversion, No. (%) | 0 (0) | 3 (18.8) | 1 (6.3) | 3 (21.4) |
| Rubella | ||||
| Day 0 | ||||
| GMT IU/mL (95% CI) | 31.385 (17.875–55.104) | 24.609 (15.005–40.358) | 28.71 (20.697–39.826) | 27.137 (19.651–37.475) |
| Day 7 | ||||
| GMT IU/mL (95% CI) | 32.025 (18.762–54.662) | 26.214 (16.727–41.082) | 31.675 (22.149–45.298) | 23.527 (17.588–31.471) |
| Seroconversion, No. (%) | 0 (0) | 1 (6.3) | 0 (0) | 0 (0) |
| Day 28 | ||||
| GMT IU/mL (95% CI) | 33.164 (18.85–58.346) | 71.38 (50.062–101.774) | 92.385 (64.458–132.412) | 106.053 (56.96–197.458) |
| Seroconversion, No. (%) | 0 (0) | 6 (37.5) | 4 (25.0) | 5 (35.7) |
| Day 56 | ||||
| GMT IU/mL (95% CI) | 33.894 (19.073–60.233) | 70.821 (45.997–109.044) | 79.572 (58.992–107.329) | 74.518 (40.821–136.032) |
| Seroconversion, No. (%) | 0 (0) | 6 (37.5) | 3 (18.8) | 4 (28.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, B.; Bermingham, I.M.; Leelasena, I.; Hickling, J.; Young, P.R.; Muller, D.A.; Forster, A.H. Safety, Tolerability, and Immunogenicity of Measles and Rubella Vaccine Delivered with a High-Density Microarray Patch: Results from a Randomized, Partially Double-Blinded, Placebo-Controlled Phase I Clinical Trial. Vaccines 2023, 11, 1725. https://doi.org/10.3390/vaccines11111725
Baker B, Bermingham IM, Leelasena I, Hickling J, Young PR, Muller DA, Forster AH. Safety, Tolerability, and Immunogenicity of Measles and Rubella Vaccine Delivered with a High-Density Microarray Patch: Results from a Randomized, Partially Double-Blinded, Placebo-Controlled Phase I Clinical Trial. Vaccines. 2023; 11(11):1725. https://doi.org/10.3390/vaccines11111725
Chicago/Turabian StyleBaker, Ben, Imogen M. Bermingham, Indika Leelasena, Julian Hickling, Paul R. Young, David A. Muller, and Angus H. Forster. 2023. "Safety, Tolerability, and Immunogenicity of Measles and Rubella Vaccine Delivered with a High-Density Microarray Patch: Results from a Randomized, Partially Double-Blinded, Placebo-Controlled Phase I Clinical Trial" Vaccines 11, no. 11: 1725. https://doi.org/10.3390/vaccines11111725
APA StyleBaker, B., Bermingham, I. M., Leelasena, I., Hickling, J., Young, P. R., Muller, D. A., & Forster, A. H. (2023). Safety, Tolerability, and Immunogenicity of Measles and Rubella Vaccine Delivered with a High-Density Microarray Patch: Results from a Randomized, Partially Double-Blinded, Placebo-Controlled Phase I Clinical Trial. Vaccines, 11(11), 1725. https://doi.org/10.3390/vaccines11111725






