Immunotherapeutic Agents for Intratumoral Immunotherapy
Abstract
:1. Introduction
1.1. Systemic Cancer Immunotherapy
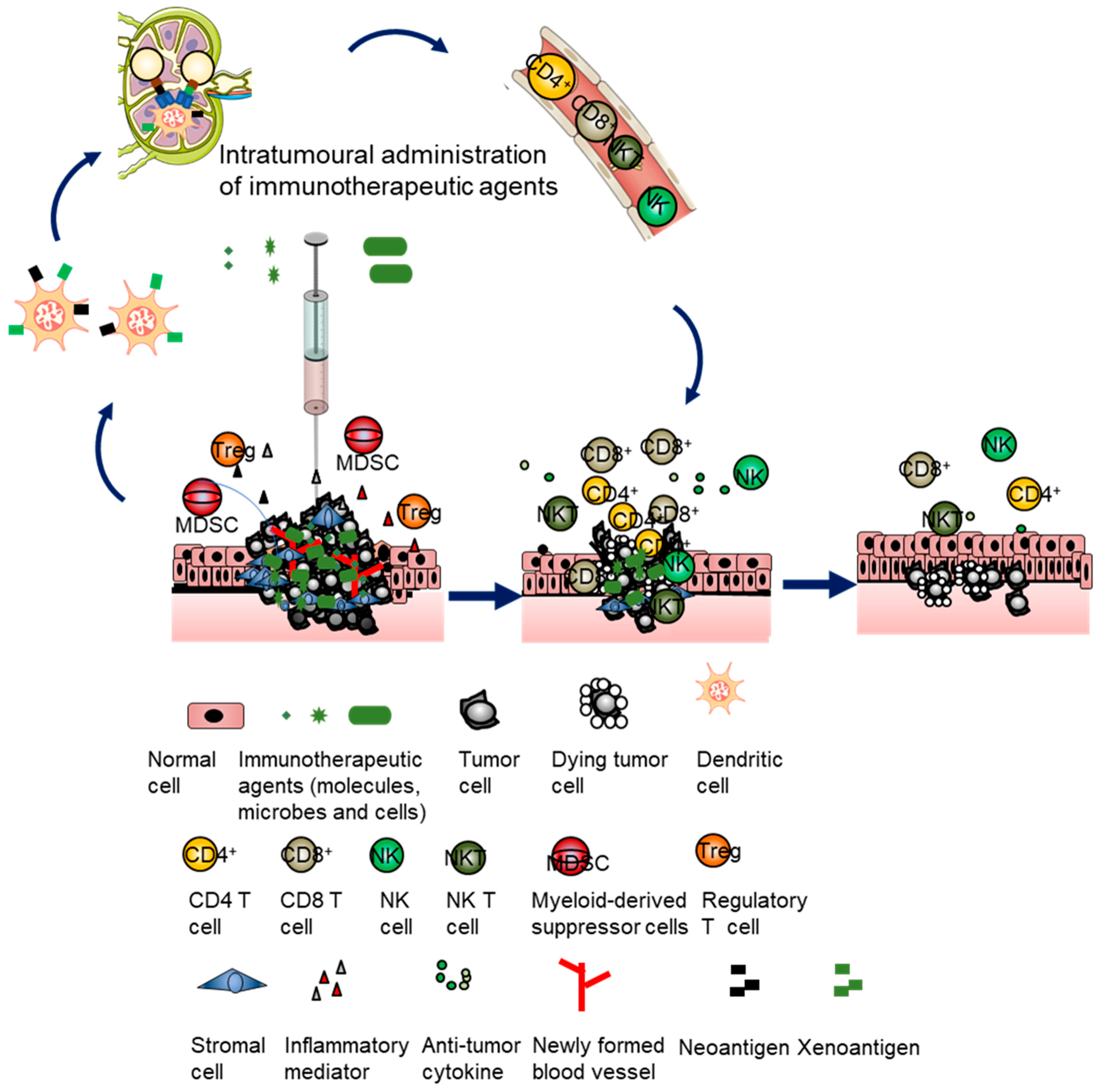
1.2. Locoregional Intratumoral Immunotherapy
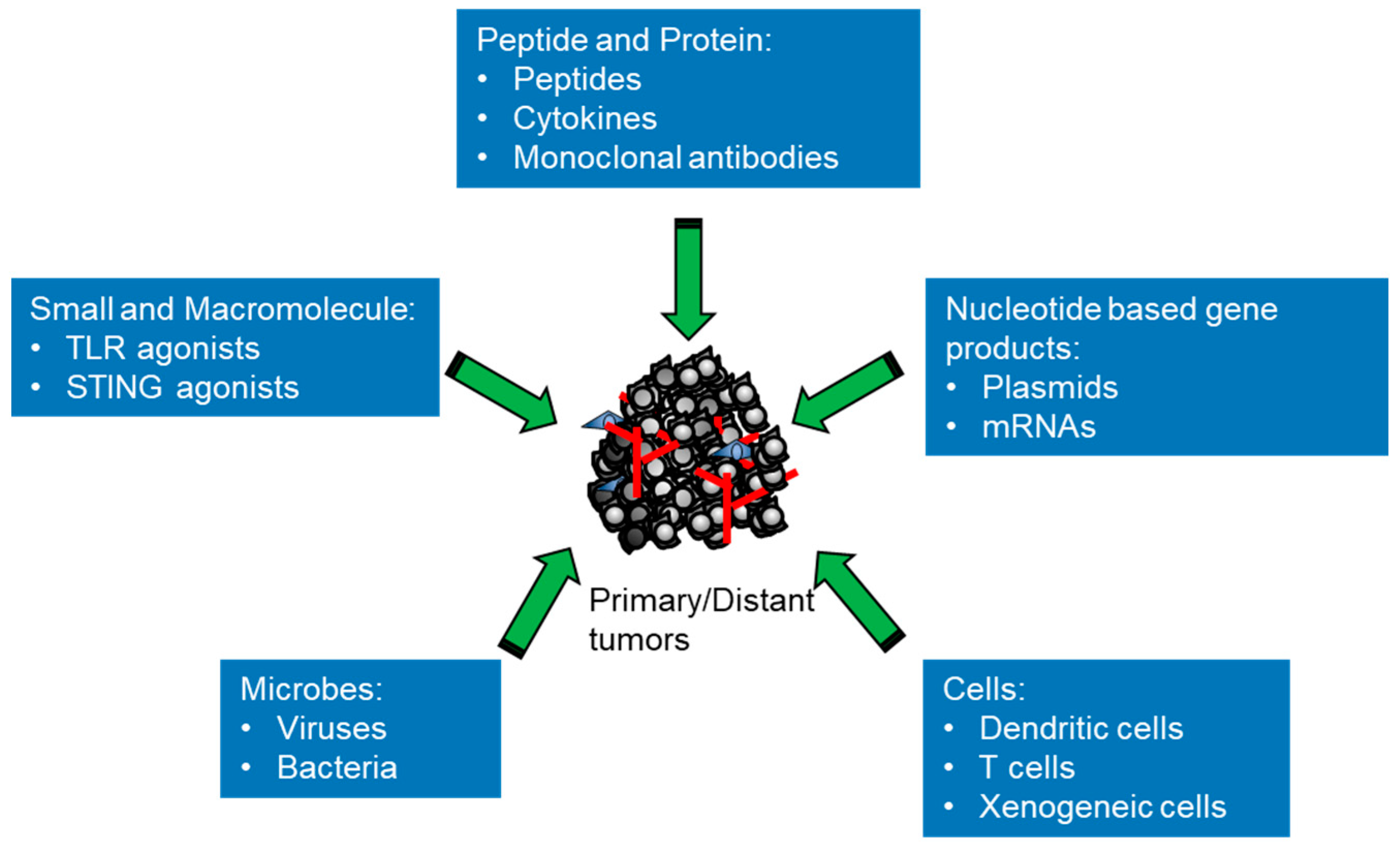
2. Classes of Intratumoral Immunotherapeutic Agents in Preclinical and Clinical Studies
2.1. Small and Macromolecules
2.2. Peptides and Proteins
2.3. Nucleic Acid-Based Gene Products
2.4. Microbes
2.4.1. Viruses
2.4.2. Bacteria
2.5. Cells
3. Clinical Challenges for Intratumoral Immunotherapy with Immune Modulators
3.1. Feasibility of Intratumoral Injection Tumor Size and Location Limits
3.2. Intratumoral Immunotherapy Pharmacology and Toxicology
3.3. Clinical Outcome Assessment and Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of death among cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, J.L.; Ambrus, C.M.; Mink, I.B.; Pickren, J.W. Causes of death in cancer patients. J. Med. 1975, 6, 61–64. [Google Scholar] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Publisher Correction: Harnessing innate immunity in cancer therapy. Nature 2019, 576, E3. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, Y.P.; Du, X.J.; Liu, J.Q.; Huang, C.L.; Chen, L.; Zhou, G.Q.; Li, W.F.; Mao, Y.P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ (Clin. Res. Ed.) 2018, 363, k4226. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 38. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Kichloo, A.; Albosta, M.; Dahiya, D.; Guidi, J.C.; Aljadah, M.; Singh, J.; Shaka, H.; Wani, F.; Kumar, A.; Lekkala, M. Systemic adverse effects and toxicities associated with immunotherapy: A review. World J. Clin. Oncol. 2021, 12, 150–163. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Coley, W.B. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat. Res. 1991, 262, 3–11. [Google Scholar]
- Hoption Cann, S.A.; van Netten, J.P.; van Netten, C. Dr William Coley and tumour regression: A place in history or in the future. Postgrad. Med. J. 2003, 79, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.X.; Haebe, S.; Lee, A.S.; Westphalen, C.B.; Norton, J.A.; Jiang, W.; Levy, R. Intratumoral Immunotherapy for Early-stage Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 3091–3099. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Andtbacka, R.; Harrington, K.; Melero, I.; Leidner, R.; de Baere, T.; Robert, C.; Ascierto, P.A.; Baurain, J.F.; Imperiale, M.; et al. Starting the fight in the tumor: Expert recommendations for the development of human intratumoral immunotherapy (HIT-IT). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Ismail, R.; Puzanov, I. Intratumoral Immunotherapy-Update 2019. Oncol. 2020, 25, e423–e438. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Tselikas, L.; de Baere, T.; Houot, R. Intratumoral immunotherapy: Using the tumor as the remedy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, xii33–xii43. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, S.A.; van der Most, R.G.; Prosser, A.C.; Mahendran, S.; Tovey, M.G.; Smyth, M.J.; Robinson, B.W.; Currie, A.J. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J. Immunol. 2009, 182, 5217–5224. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Sweis, R.F.; Kasper, S.; Hamid, O.; Bhatia, S.; Dummer, R.; Stradella, A.; Long, G.V.; Spreafico, A.; Shimizu, T.; et al. Combination of the STING Agonist MIW815 (ADU-S100) and PD-1 Inhibitor Spartalizumab in Advanced/Metastatic Solid Tumors or Lymphomas: An Open-Label, Multicenter, Phase Ib Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 110–121. [Google Scholar] [CrossRef]
- Halwani, A.S.; Panizo, C.; Isufi, I.; Herrera, A.F.; Okada, C.Y.; Cull, E.H.; Kis, B.; Chaves, J.M.; Bartlett, N.L.; Ai, W.; et al. Phase 1/2 study of intratumoral G100 (TLR4 agonist) with or without pembrolizumab in follicular lymphoma. Leuk. Lymphoma 2022, 63, 821–833. [Google Scholar] [CrossRef]
- Hong, W.X.; Sagiv-Barfi, I.; Czerwinski, D.K.; Sallets, A.; Levy, R. Neoadjuvant Intratumoral Immunotherapy with TLR9 Activation and Anti-OX40 Antibody Eradicates Metastatic Cancer. Cancer Res. 2022, 82, 1396–1408. [Google Scholar] [CrossRef]
- Negrao, M.V.; Papadimitrakopoulou, V.A.; Price, A.C.; Tam, A.L.; Furqan, M.; Laroia, S.T.; Massarelli, E.; Pacheco, J.; Heymach, J.V.; Tsao, A.S.; et al. Vidutolimod in Combination With Atezolizumab With and Without Radiation Therapy in Patients With Programmed Cell Death Protein 1 or Programmed Death-Ligand 1 Blockade-Resistant Advanced NSCLC. JTO Clin. Res. Rep. 2023, 4, 100423. [Google Scholar] [CrossRef]
- Whalen, G.F.; Sullivan, M.; Piperdi, B.; Wasseff, W.; Galili, U. Cancer immunotherapy by intratumoral injection of α-gal glycolipids. Anticancer Res. 2012, 32, 3861–3868. [Google Scholar] [PubMed]
- Spicer, J.; Marabelle, A.; Baurain, J.F.; Jebsen, N.L.; Jøssang, D.E.; Awada, A.; Kristeleit, R.; Loirat, D.; Lazaridis, G.; Jungels, C.; et al. Safety, Antitumor Activity, and T-cell Responses in a Dose-Ranging Phase I Trial of the Oncolytic Peptide LTX-315 in Patients with Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Eigentler, T.K.; Pflugfelder, A.; Leiter, U.; Meier, F.; Bauer, J.; Schmidt, D.; Radny, P.; Pföhler, C.; Garbe, C. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol. Immunother. CII 2011, 60, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, S.; Kocyigit, P.; Alp, A.; Erdem, C.; Gürgey, E. Treatment of Basal Cell Carcinoma Located in the Head and Neck Region with Intralesional Interferon alpha-2a: Evaluation of Long-Term Follow-Up Results. Clin. Drug Investig. 2005, 25, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.; Tucker, S.B.; Perednia, D.; Smiles, K.A.; Taylor, E.L.; Tanner, D.J.; Peets, E. The effect of an intralesional sustained-release formulation of interferon alfa-2b on basal cell carcinomas. Arch. Dermatol. 1990, 126, 1029–1032. [Google Scholar] [CrossRef]
- Tran, C.A.; Lynch, K.T.; Meneveau, M.O.; Katyal, P.; Olson, W.C.; Slingluff, C.L., Jr. Intratumoral IFN-γ or topical TLR7 agonist promotes infiltration of melanoma metastases by T lymphocytes expanded in the blood after cancer vaccine. J. Immunother. Cancer 2023, 11, e005952. [Google Scholar] [CrossRef]
- Si, Z.; Hersey, P.; Coates, A.S. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res. 1996, 6, 247–255. [Google Scholar] [CrossRef]
- Danielli, R.; Patuzzo, R.; Di Giacomo, A.M.; Gallino, G.; Maurichi, A.; Di Florio, A.; Cutaia, O.; Lazzeri, A.; Fazio, C.; Miracco, C.; et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: Results of a phase II study. Cancer Immunol. Immunother. CII 2015, 64, 999–1009. [Google Scholar] [CrossRef]
- Schwarze, J.K.; Duerinck, J.; Dufait, I.; Awada, G.; Klein, S.; Fischbuch, L.; Seynaeve, L.; Vaeyens, F.; Rogiers, A.; Everaert, H.; et al. A phase I clinical trial on intratumoral and intracavitary administration of ipilimumab and nivolumab in patients with recurrent glioblastoma. J. Clin. Oncol. 2020, 38, 2534. [Google Scholar] [CrossRef]
- Omland, S.H.; Ejlertsen, J.S.; Krustrup, D.; Christensen, R.L.; Svane, I.M.; Olesen, U.H.; Hædersdal, M. Feasibility of Intratumoral Anti-PD1 as Treatment of Human Basal Cell Carcinoma: An Explorative Study with Adjuvant Ablative Fractional Laser. Cancers 2022, 14, 5815. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.; Ravetch, J.; D’Andrea, G.; Vahdat, L.; Klebanoff, C.; Robson, M. 496 Toxicity of an Fc engineered anti-CD40 antibody is abrogated by intratumoral injection and results in durable anti-tumor immunity in patients. J. ImmunoTherapy Cancer 2021, 9, A528. [Google Scholar] [CrossRef]
- Irenaeus, S.M.M.; Nielsen, D.; Ellmark, P.; Yachnin, J.; Deronic, A.; Nilsson, A.; Norlén, P.; Veitonmäki, N.; Wennersten, C.S.; Ullenhag, G.J. First-in-human study with intratumoral administration of a CD40 agonistic antibody, ADC-1013, in advanced solid malignancies. Int. J. Cancer 2019, 145, 1189–1199. [Google Scholar] [CrossRef]
- Chang, H.-P.; Le, H.K.; Shah, D.K. Pharmacokinetics and Pharmacodynamics of Antibody-Drug Conjugates Administered via Subcutaneous and Intratumoral Routes. Pharmaceutics 2023, 15, 1132. [Google Scholar] [CrossRef] [PubMed]
- Algazi, A.; Bhatia, S.; Agarwala, S.; Molina, M.; Lewis, K.; Faries, M.; Fong, L.; Levine, L.P.; Franco, M.; Oglesby, A.; et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 532–540. [Google Scholar] [CrossRef]
- Algazi, A.P.; Twitty, C.G.; Tsai, K.K.; Le, M.; Pierce, R.; Browning, E.; Hermiz, R.; Canton, D.A.; Bannavong, D.; Oglesby, A.; et al. Phase II Trial of IL-12 Plasmid Transfection and PD-1 Blockade in Immunologically Quiescent Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2827–2837. [Google Scholar] [CrossRef]
- Hamid, O.; Hellman, M.; Carneiro, B.; Marron, T.; Subbiah, V.; Mehmi, I.; Eyles, J.; Dubois, V.; Ridgway, B.; Hamid, O.; et al. 19O Preliminary safety, antitumor activity and pharmacodynamics results of HIT-IT MEDI1191 (mRNA IL-12) in patients with advanced solid tumours and superficial lesions. Ann. Oncol. 2021, 32, S9. [Google Scholar] [CrossRef]
- Patel, M.R.; Bauer, T.M.; Jimeno, A.; Wang, D.; LoRusso, P.; Do, K.T.; Stemmer, S.M.; Maurice-Dror, C.; Geva, R.; Zacharek, S.; et al. A phase I study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ, for intratumoral (iTu) injection alone and in combination with durvalumab. J. Clin. Oncol. 2020, 38, 3092. [Google Scholar] [CrossRef]
- Bechter, O.; Utikal, J.; Baurain, J.-F.; Massard, C.; Sahin, U.; Derhovanessian, E.; Ozoux, M.-L.; Marpadga, R.; Imedio, E.-R.; Acquavella, N.; et al. 391 A first-in-human study of intratumoral SAR441000, an mRNA mixture encoding IL-12sc, interferon alpha2b, GM-CSF and IL-15sushi as monotherapy and in combination with cemiplimab in advanced solid tumors. J. ImmunoTherapy Cancer 2020, 8, A237–A238. [Google Scholar] [CrossRef]
- Van Lint, S.; Renmans, D.; Broos, K.; Goethals, L.; Maenhout, S.; Benteyn, D.; Goyvaerts, C.; Du Four, S.; Van der Jeught, K.; Bialkowski, L.; et al. Intratumoral Delivery of TriMix mRNA Results in T-cell Activation by Cross-Presenting Dendritic Cells. Cancer Immunol. Res. 2016, 4, 146–156. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, J.; Sun, J.; Chen, Y.; Du, Y.; Tan, Y.; Wu, L.; Zhai, M.; Wei, L.; Li, N.; et al. Intratumoral delivered novel circular mRNA encoding cytokines for immune modulation and cancer therapy. Mol. Ther. Nucleic Acids 2022, 30, 184–197. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Collichio, F.; Harrington, K.J.; Middleton, M.R.; Downey, G.; Ӧhrling, K.; Kaufman, H.L. Final analyses of OPTiM: A randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J. Immunother. Cancer 2019, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Marx, A.N.; Liu, D.D.; Shen, Y.; Gao, H.; Reuben, J.M.; Whitman, G.; Krishnamurthy, S.; Ross, M.I.; Litton, J.K.; et al. A phase II study of talimogene laherparepvec for patients with inoperable locoregional recurrence of breast cancer. Sci. Rep. 2021, 11, 22242. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Curti, B.D.; Kaufman, H.; Daniels, G.A.; Nemunaitis, J.J.; Spitler, L.E.; Hallmeyer, S.; Lutzky, J.; Schultz, S.M.; Whitman, E.D.; et al. Final data from CALM: A phase II study of Coxsackievirus A21 (CVA21) oncolytic virus immunotherapy in patients with advanced melanoma. J. Clin. Oncol. 2015, 33, 9030. [Google Scholar] [CrossRef]
- Kimata, H.; Imai, T.; Kikumori, T.; Teshigahara, O.; Nagasaka, T.; Goshima, F.; Nishiyama, Y.; Nakao, A. Pilot study of oncolytic viral therapy using mutant herpes simplex virus (HF10) against recurrent metastatic breast cancer. Ann. Surg. Oncol. 2006, 13, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Ross, M.I.; Agarwala, S.S.; Taylor, M.H.; Vetto, J.T.; Neves, R.I.; Daud, A.; Khong, H.T.; Ungerleider, R.S.; Tanaka, M.; et al. Final results of a phase II multicenter trial of HF10, a replication-competent HSV-1 oncolytic virus, and ipilimumab combination treatment in patients with stage IIIB-IV unresectable or metastatic melanoma. J. Clin. Oncol. 2017, 35, 9510. [Google Scholar] [CrossRef]
- Hirooka, Y.; Kasuya, H.; Ishikawa, T.; Kawashima, H.; Ohno, E.; Villalobos, I.B.; Naoe, Y.; Ichinose, T.; Koyama, N.; Tanaka, M.; et al. A Phase I clinical trial of EUS-guided intratumoral injection of the oncolytic virus, HF10 for unresectable locally advanced pancreatic cancer. BMC Cancer 2018, 18, 596. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Brown, K.T.; Heo, J.; Borad, M.J.; Luca, A.; Pelusio, A.; Agathon, D.; Lusky, M.; et al. PHOCUS: A phase 3 randomized, open-label study comparing the oncolytic immunotherapy Pexa-Vec followed by sorafenib (SOR) vs SOR in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy. J. Clin. Oncol. 2016, 34, TPS4146. [Google Scholar] [CrossRef]
- Rallis, K.S.; Makrakis, D.; Ziogas, I.A.; Tsoulfas, G. Immunotherapy for advanced hepatocellular carcinoma: From clinical trials to real-world data and future advances. World J. Clin. Oncol. 2022, 13, 448–472. [Google Scholar] [CrossRef]
- Moehler, M.; Heo, J.; Lee, H.C.; Tak, W.Y.; Chao, Y.; Paik, S.W.; Yim, H.J.; Byun, K.S.; Baron, A.; Ungerechts, G.; et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: A randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology 2019, 8, 1615817. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. ImmunoTherapy Cancer 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Noonan, A.M.; Farren, M.R.; Geyer, S.M.; Huang, Y.; Tahiri, S.; Ahn, D.; Mikhail, S.; Ciombor, K.K.; Pant, S.; Aparo, S.; et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1150–1158. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- De Jager, R.; Guinan, P.; Lamm, D.; Khanna, O.; Brosman, S.; De Kernion, J.; Williams, R.; Richardson, C.; Muenz, L.; Reitsma, D.; et al. Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette Guerin. Overview analysis of six phase II clinical trials. Urology 1991, 38, 507–513. [Google Scholar] [CrossRef]
- Cunningham, C.; Nemunaitis, J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum. Gene Ther. 2001, 12, 1594–1596. [Google Scholar]
- Janku, F.; Zhang, H.H.; Pezeshki, A.; Goel, S.; Murthy, R.; Wang-Gillam, A.; Shepard, D.R.; Helgason, T.; Masters, T.; Hong, D.S.; et al. Intratumoral Injection of Clostridium novyi-NT Spores in Patients with Treatment-refractory Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 96–106. [Google Scholar] [CrossRef]
- Triozzi, P.L.; Khurram, R.; Aldrich, W.A.; Walker, M.J.; Kim, J.A.; Jaynes, S. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer 2000, 89, 2646–2654. [Google Scholar] [CrossRef]
- Jin, C.; Ali, A.; Iskantar, A.; Fotaki, G.; Wang, H.; Essand, M.; Karlsson-Parra, A.; Yu, D. Intratumoral administration of pro-inflammatory allogeneic dendritic cells improved the anti-tumor response of systemic anti-CTLA-4 treatment via unleashing a T cell-dependent response. Oncoimmunology 2022, 11, 2099642. [Google Scholar] [CrossRef]
- Möller, P.; Wittig, B.; Schadendorf, D. Intratumoral adoptive immunotherapy with tumor infiltrating lymphocytes (TIL) in a melanoma patient leading to regression of local tumor mass. A case report. Anticancer Res. 1998, 18, 1237–1241. [Google Scholar]
- Papa, S.; Adami, A.; Metoudi, M.; Beatson, R.; George, M.S.; Achkova, D.; Williams, E.; Arif, S.; Reid, F.; Elstad, M.; et al. Intratumoral pan-ErbB targeted CAR-T for head and neck squamous cell carcinoma: Interim analysis of the T4 immunotherapy study. J. Immunother. Cancer 2023, 11, e007162. [Google Scholar] [CrossRef]
- Tchou, J.; Zhao, Y.; Levine, B.L.; Zhang, P.J.; Davis, M.M.; Melenhorst, J.J.; Kulikovskaya, I.; Brennan, A.L.; Liu, X.; Lacey, S.F.; et al. Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol. Res. 2017, 5, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.P.; Liu, L.C.; Chang, C.C.; Wu, C.C.; Shyr, C.R. Intratumoral xenogeneic tissue-specific cell immunotherapy inhibits tumor growth by increasing antitumor immunity in murine triple negative breast and pancreatic tumor models. Cancer Lett. 2022, 545, 115478. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.P.; Lu, H.L.; Shyr, C.R. Anti-tumor activity of intratumoral xenogeneic urothelial cell monotherapy or in combination with chemotherapy in syngeneic murine models of bladder cancer. Am. J. Cancer Res. 2023, 13, 2285–2306. [Google Scholar] [PubMed]
- Andón, F.T.; Leon, S.; Ummarino, A.; Redin, E.; Allavena, P.; Serrano, D.; Anfray, C.; Calvo, A. Innate and Adaptive Responses of Intratumoral Immunotherapy with Endosomal Toll-Like Receptor Agonists. Biomedicines 2022, 10, 1590. [Google Scholar] [CrossRef]
- Chen, C.; Xu, P. Cellular functions of cGAS-STING signaling. Trends Cell Biol. 2023, 33, 630–648. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Bhatia, S.; Miller, N.J.; Lu, H.; Longino, N.V.; Ibrani, D.; Shinohara, M.M.; Byrd, D.R.; Parvathaneni, U.; Kulikauskas, R.; Ter Meulen, J.; et al. Intratumoral G100, a TLR4 Agonist, Induces Antitumor Immune Responses and Tumor Regression in Patients with Merkel Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1185–1195. [Google Scholar] [CrossRef]
- Brody, J.D.; Ai, W.Z.; Czerwinski, D.K.; Torchia, J.A.; Levy, M.; Advani, R.H.; Kim, Y.H.; Hoppe, R.T.; Knox, S.J.; Shin, L.K.; et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: A phase I/II study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4324–4332. [Google Scholar] [CrossRef]
- Abdel-Motal, U.M.; Wigglesworth, K.; Galili, U. Intratumoral injection of alpha-gal glycolipids induces a protective anti-tumor T cell response which overcomes Treg activity. Cancer Immunol. Immunother. 2009, 58, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.S.; Satyanarayanajois, S. Peptides and peptidomimetics as immunomodulators. Immunotherapy 2014, 6, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Camilio, K.A.; Berge, G.; Ravuri, C.S.; Rekdal, O.; Sveinbjørnsson, B. Complete regression and systemic protective immune responses obtained in B16 melanomas after treatment with LTX-315. Cancer Immunol. Immunother. 2014, 63, 601–613. [Google Scholar] [CrossRef]
- Zhang, L.; Tai, Y.T.; Ho, M.Z.G.; Qiu, L.; Anderson, K.C. Interferon-alpha-based immunotherapies in the treatment of B cell-derived hematologic neoplasms in today’s treat-to-target era. Exp. Hematol. Oncol. 2017, 6, 20. [Google Scholar] [CrossRef]
- McDermott, D.F.; Atkins, M.B. Interleukin-2 therapy of metastatic renal cell carcinoma--predictors of response. Semin. Oncol. 2006, 33, 583–587. [Google Scholar] [CrossRef]
- Clark, J.I.; Curti, B.; Davis, E.J.; Kaufman, H.; Amin, A.; Alva, A.; Logan, T.F.; Hauke, R.; Miletello, G.P.; Vaishampayan, U.; et al. Long-term progression-free survival of patients with metastatic melanoma or renal cell carcinoma following high-dose interleukin-2. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2021, 69, 888–892. [Google Scholar] [CrossRef]
- Jackaman, C.; Bundell, C.S.; Kinnear, B.F.; Smith, A.M.; Filion, P.; van Hagen, D.; Robinson, B.W.; Nelson, D.J. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: A novel mechanism for IL-2. J. Immunol. 2003, 171, 5051–5063. [Google Scholar] [CrossRef]
- Yasuda, K.; Nirei, T.; Tsuno, N.H.; Nagawa, H.; Kitayama, J. Intratumoral injection of interleukin-2 augments the local and abscopal effects of radiotherapy in murine rectal cancer. Cancer Sci. 2011, 102, 1257–1263. [Google Scholar] [CrossRef]
- Vaquero, J.; Martínez, R. Intratumoral immunotherapy with interferon-alpha and interleukin-2 in glioblastoma. Neuroreport 1992, 3, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.F.; van der Sluis, T.C.; Ossendorp, F.; Arens, R.; Melief, C.J. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Knorr, D.A.; Dahan, R.; Ravetch, J.V. Toxicity of an Fc-engineered anti-CD40 antibody is abrogated by intratumoral injection and results in durable antitumor immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 11048–11053. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. MCR 2020, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody-drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef]
- Rugo, H.S.; Crossno, C.L.; Gesthalter, Y.B.; Kelley, K.; Moore, H.B.; Rimawi, M.F.; Westbrook, K.E.; Buys, S.S. Real-World Perspectives and Practices for Pneumonitis/Interstitial Lung Disease Associated With Trastuzumab Deruxtecan Use in Human Epidermal Growth Factor Receptor 2-Expressing Metastatic Breast Cancer. JCO Oncol. Pract. 2023, 19, 539–546. [Google Scholar] [CrossRef]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, Ö.; Vormehr, M.; Kranz, L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef]
- Hewitt, S.L.; Bailey, D.; Zielinski, J.; Apte, A.; Musenge, F.; Karp, R.; Burke, S.; Garcon, F.; Mishra, A.; Gurumurthy, S.; et al. Intratumoral IL12 mRNA Therapy Promotes TH1 Transformation of the Tumor Microenvironment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 6284–6298. [Google Scholar] [CrossRef]
- Hewitt, S.L.; Bai, A.; Bailey, D.; Ichikawa, K.; Zielinski, J.; Karp, R.; Apte, A.; Arnold, K.; Zacharek, S.J.; Iliou, M.S.; et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl. Med. 2019, 11, eaat9143. [Google Scholar] [CrossRef]
- Kelly, E.; Russell, S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. J. Am. Soc. Gene Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, X.; Sander, M.; Zhang, H.; Yan, G.; Lin, Y. Oncolytic Viro-Immunotherapy: An Emerging Option in the Treatment of Gliomas. Front. Immunol. 2021, 12, 721830. [Google Scholar] [CrossRef] [PubMed]
- Package Insert—IMLYGIC (Talimogene Laherparepvec). 2015. Available online: https://www.fda.gov/media/94129/download (accessed on 7 November 2023).
- Soliman, H.; Hogue, D.; Han, H.; Mooney, B.; Costa, R.; Lee, M.C.; Niell, B.; Williams, A.; Chau, A.; Falcon, S.; et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: A phase 2 trial. Nat. Med. 2023, 29, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Pless, M.; Cubillo, A.; Calvo, A.; Chon, H.J.; Liu, C.; Snyder, W.; Chan, E.; Chaney, M.F.; Chesney, J.A.; et al. Early safety from a phase I, multicenter, open-label clinical trial of talimogene laherparepvec (T-VEC) injected (inj) into liver tumors in combination with pembrolizumab (pem). J. Clin. Oncol. 2020, 38, 3015. [Google Scholar] [CrossRef]
- Ribas, A.; Chesney, J.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. 1037O MASTERKEY-265: A phase III, randomized, placebo (Pbo)-controlled study of talimogene laherparepvec (T) plus pembrolizumab (P) for unresectable stage IIIB–IVM1c melanoma (MEL). Ann. Oncol. 2021, 32, S868–S869. [Google Scholar] [CrossRef]
- Eissa, I.R.; Naoe, Y.; Bustos-Villalobos, I.; Ichinose, T.; Tanaka, M.; Zhiwen, W.; Mukoyama, N.; Morimoto, T.; Miyajima, N.; Hitoki, H.; et al. Genomic Signature of the Natural Oncolytic Herpes Simplex Virus HF10 and Its Therapeutic Role in Preclinical and Clinical Trials. Front. Oncol. 2017, 7, 149. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Mizuno, T.; Sugiura, S.; Goshima, F.; Kohno, S.; Nakashima, T.; Nishiyama, Y. Intratumoral injection of herpes simplex virus HF10 in recurrent head and neck squamous cell carcinoma. Acta Oto-Laryngol. 2006, 126, 1115–1117. [Google Scholar] [CrossRef]
- Heo, J.; Breitbach, C.J.; Moon, A.; Kim, C.W.; Patt, R.; Kim, M.K.; Lee, Y.K.; Oh, S.Y.; Woo, H.Y.; Parato, K.; et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: Preclinical and clinical demonstration of combination efficacy. Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 1170–1179. [Google Scholar] [CrossRef]
- Heo, J.; Reid, T.; Ruo, L.; Breitbach, C.J.; Rose, S.; Bloomston, M.; Cho, M.; Lim, H.Y.; Chung, H.C.; Kim, C.W.; et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013, 19, 329–336. [Google Scholar] [CrossRef]
- Jiang, H.; Clise-Dwyer, K.; Ruisaard, K.E.; Fan, X.; Tian, W.; Gumin, J.; Lamfers, M.L.; Kleijn, A.; Lang, F.F.; Yung, W.K.; et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE 2014, 9, e97407. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Olszanski, A.J.; Nyakas, M.; Hornyak, T.J.; Wolchok, J.D.; Levitsky, V.; Kuryk, L.; Hansen, T.B.; Jäderberg, M. Pilot Study of ONCOS-102 and Pembrolizumab: Remodeling of the Tumor Microenvironment and Clinical Outcomes in Anti-PD-1-Resistant Advanced Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Rajani, K.; Parrish, C.; Kottke, T.; Thompson, J.; Zaidi, S.; Ilett, L.; Shim, K.G.; Diaz, R.M.; Pandha, H.; Harrington, K.; et al. Combination Therapy With Reovirus and Anti-PD-1 Blockade Controls Tumor Growth Through Innate and Adaptive Immune Responses. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Bekierkunst, A.; Goren, M.B. Immunotherapy of guinea pig line 10 hepatoma with nonliving BCG cells in aqueous medium. Infect. Immun. 1979, 24, 817–820. [Google Scholar] [CrossRef]
- Hong, E.H.; Chang, S.Y.; Lee, B.R.; Pyun, A.R.; Kim, J.W.; Kweon, M.N.; Ko, H.J. Intratumoral injection of attenuated Salmonella vaccine can induce tumor microenvironmental shift from immune suppressive to immunogenic. Vaccine 2013, 31, 1377–1384. [Google Scholar] [CrossRef]
- Ahmed, S.G.; Oliva, G.; Shao, M.; Wang, X.; Mekalanos, J.J.; Brenner, G.J. Intratumoral injection of schwannoma with attenuated Salmonella typhimurium induces antitumor immunity and controls tumor growth. Proc. Natl. Acad. Sci. USA 2022, 119, e2202719119. [Google Scholar] [CrossRef]
- Flickinger, J.C., Jr.; Rodeck, U.; Snook, A.E. Listeria monocytogenes as a Vector for Cancer Immunotherapy: Current Understanding and Progress. Vaccines 2018, 6, 48. [Google Scholar] [CrossRef]
- Vitiello, M.; Evangelista, M.; Di Lascio, N.; Kusmic, C.; Massa, A.; Orso, F.; Sarti, S.; Marranci, A.; Rodzik, K.; Germelli, L.; et al. Antitumoral effects of attenuated Listeria monocytogenes in a genetically engineered mouse model of melanoma. Oncogene 2019, 38, 3756–3762. [Google Scholar] [CrossRef]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111. [Google Scholar] [CrossRef]
- Janku, F.; Fu, S.; Murthy, R.; Karp, D.; Hong, D.; Tsimberidou, A.; Gillison, M.; Adat, A.; Raina, A.; Call, G.; et al. 383 First-in-man clinical trial of intratumoral injection of clostridium Novyi-NT spores in combination with pembrolizumab in patients with treatment-refractory advanced solid tumors. J. ImmunoTherapy Cancer 2020, 8, A233. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Laurell, A.; Lönnemark, M.; Brekkan, E.; Magnusson, A.; Tolf, A.; Wallgren, A.C.; Andersson, B.; Adamson, L.; Kiessling, R.; Karlsson-Parra, A. Intratumorally injected pro-inflammatory allogeneic dendritic cells as immune enhancers: A first-in-human study in unfavourable risk patients with metastatic renal cell carcinoma. J. Immunother. Cancer 2017, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ramakrishnan, R.; Trkulja, M.; Ren, X.; Gabrilovich, D.I. Therapeutic effect of intratumoral administration of DCs with conditional expression of combination of different cytokines. Cancer Immunol. Immunother. CII 2012, 61, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Fröbom, R.; Berglund, E.; Berglund, D.; Nilsson, I.-L.; Åhlén, J.; von Sivers, K.; Linder-Stragliotto, C.; Suenaert, P.; Karlsson-Parra, A.; Bränström, R. Phase I trial evaluating safety and efficacy of intratumorally administered inflammatory allogeneic dendritic cells (ilixadencel) in advanced gastrointestinal stromal tumors. Cancer Immunol. Immunother. 2020, 69, 2393–2401. [Google Scholar] [CrossRef]
- Lindskog, M.; Laurell, A.; Kjellman, A.; Melichar, B.; Rey, P.M.; Zieliński, H.; Villacampa, F.; Bigot, P.; Zoltan, B.; Parikh, O.; et al. Ilixadencel, a Cell-based Immune Primer, plus Sunitinib Versus Sunitinib Alone in Metastatic Renal Cell Carcinoma: A Randomized Phase 2 Study. Eur. Urol. Open Sci. 2022, 40, 38–45. [Google Scholar] [CrossRef]
- Morotti, M.; Albukhari, A.; Alsaadi, A.; Artibani, M.; Brenton, J.D.; Curbishley, S.M.; Dong, T.; Dustin, M.L.; Hu, Z.; McGranahan, N.; et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Br. J. Cancer 2021, 124, 1759–1776. [Google Scholar] [CrossRef]
- Liu, D.L.; Håkansson, C.H.; Seifert, J. Immunotherapy in liver tumors: II. Intratumoral injection with activated tumor-infiltrating lymphocytes, intrasplenic administration of recombinant interleukin-2 and interferon α causes tumor regression and lysis. Cancer Lett. 1994, 85, 39–46. [Google Scholar] [CrossRef]
- Globerson-Levin, A.; Waks, T.; Eshhar, Z. Elimination of progressive mammary cancer by repeated administrations of chimeric antigen receptor-modified T cells. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 1029–1038. [Google Scholar] [CrossRef]
- Huang, C.P.; Yang, C.Y.; Shyr, C.R. Utilizing Xenogeneic Cells As a Therapeutic Agent for Treating Diseases. Cell Transplant. 2021, 30, 9636897211011995. [Google Scholar] [CrossRef]
- Huang, C.P.; Chen, C.C.; Shyr, C.R. Xenogeneic cell therapy provides a novel potential therapeutic option for cancers by restoring tissue function, repairing cancer wound and reviving anti-tumor immune responses. Cancer Cell Int. 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Wolchok, J.D. Tumor antigens for cancer immunotherapy: Therapeutic potential of xenogeneic DNA vaccines. J. Transl. Med. 2004, 2, 12. [Google Scholar] [CrossRef]
- Huang, C.P.; Liu, L.C.; Lu, H.L.; Shyr, C.R. Effects of hepatocyte growth factor on porcine mammary cell growth and senescence. BioMedicine 2023, 13, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.A.; Murthy, R.; Hong, D.S.; Patel, S.; Overman, M.J.; Diab, A.; Hwu, P.; Tam, A. Assessment of Image-Guided Intratumoral Delivery of Immunotherapeutics in Patients With Cancer. JAMA Netw Open 2020, 3, e207911. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.; Nguyen, P.T.; Thompson, J.A.; Kurosaki, T.T.; Casey, L.R.; Leung, E.C.; Granger, G.A. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer 2000, 88, 1325–1335. [Google Scholar] [CrossRef]
- Momin, N.; Palmeri, J.R.; Lutz, E.A.; Jailkhani, N.; Mak, H.; Tabet, A.; Chinn, M.M.; Kang, B.H.; Spanoudaki, V.; Hynes, R.O.; et al. Maximizing response to intratumoral immunotherapy in mice by tuning local retention. Nat. Commun. 2022, 13, 109. [Google Scholar] [CrossRef]
- Gauthier, L.M. Chapter Fifteen—Preclinical and Clinical Toxicity of Immuno-Oncology Therapies and Mitigation Strategies. In Cancer Immunology and Immunotherapy; Amiji, M.M., Milane, L.S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 499–513. [Google Scholar] [CrossRef]
- Goldmacher, G.V.; Khilnani, A.D.; Andtbacka, R.H.I.; Luke, J.J.; Hodi, F.S.; Marabelle, A.; Harrington, K.; Perrone, A.; Tse, A.; Madoff, D.C.; et al. Response Criteria for Intratumoral Immunotherapy in Solid Tumors: itRECIST. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2667–2676. [Google Scholar] [CrossRef]
- Adashek, J.J.; LoRusso, P.M.; Hong, D.S.; Kurzrock, R. Phase I trials as valid therapeutic options for patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 773–778. [Google Scholar] [CrossRef]
| Class | Agent | Target Tumor Type | Development Stage | Main Result | Reference |
|---|---|---|---|---|---|
| Small and macromolecule | Imiquimod | Mesothelioma | Preclinical murine model | 30% Complete resolution | [17] |
| MIW815 (ADU-S100) | Solid tumors or lymphomas | Phase Ib | 10.4% Overall response rate | [18] | |
| G100 | Lymphoma | Phase I/II | 33.3% Overall response rate | [19] | |
| CpG oligodeoxynucleotide | Colon; Breast | Preclinical murine model | Survival benefit | [20] | |
| Vidutolimod | Lung | Phase Ib | 15.4% to 25.0% Response rate | [21] | |
| α-gal glycolipid | Advanced solid tumors | Phase I | No DLT | [22] | |
| Peptides and Proteins | LTX-315 (ruxotemitide) | Advanced solid tumors | Phase I | Immune-mediated anticancer activity | [23] |
| IL-2 | Melanoma | Phase II | 36.7% Overall response rate | [24] | |
| IFNα-2a | Basal cell carcinoma | Clinical study | 55% Complete remission | [25] | |
| IFNα-2b | Basal cell carcinoma | Clinical study | 80% Cured | [26] | |
| IFNγ | Melanoma | Clinical study | Enhance T-cell infiltration and mediated tumor control | [27] | |
| GM-CSF | Melanoma | Phase I | 23% Partial regression | [28] | |
| L19-IL2 and L19-TNF | Melanoma | Phase II | 20 Efficacy-evaluable patients, 32 melanoma lesions complete responses | [29] | |
| Anti-CTLA4 | Glioblastoma | Phase I | 34% Two-year overall survival rate | [30] | |
| Anti-PD-1 | Basal cell carcinoma | Phase I | 45% Tumor reduction ≥25% | [31] | |
| Anti-CD40 | Breast; melanoma; advanced solid tumors | Phase I | Clinical activity observed | [32,33] | |
| Trastuzumab-vc-MMAE | Gastric carcinoma | Preclinical murine model | Increased antitumor activity | [34] | |
| Nucleic acid-based gene products | tavokinogene telseplasmid | Melanoma | Phase II | 35.7–41% Overall response rate | [35,36] |
| MEDI1191 | Advanced solid tumors | Phase I | Preliminary antitumor activity | [37] | |
| mRNA-2752 | Advanced solid tumors | Phase I | 5.8% Overall response rate | [38] | |
| SAR441000 | Advanced solid tumors | Phase I | Generally, well tolerated | [39] | |
| TriMix mRNA | Lymphoma; mastocytoma; lung | Preclinical murine model | Delay the growth of established tumors | [40] | |
| Circular mRNA | Lung; melanoma; colon | Preclinical murine model | Tumor repression | [41] | |
| Viruses | Talimogene laherparepvec | Melanoma (only FDA-approved indication) | Phase III | 31.5% Overall response rate | [42] |
| Talimogene laherparepvec | Breast; liver | Phase II | 31.5–52% Overall response rate | [43,44] | |
| CAVATAK | Melanoma | Phase II | 28.1% Overall response rate | [45] | |
| Hf10 | Breast; melanoma; pancreatic; | Phase I; phase II | 25–41% Overall response rate | [46,47,48] | |
| Pexastimogene devacirepvec | Liver | Phase III | Fail to improve survival | [49,50,51] | |
| Teserpaturev | Glioblastoma | Phase II | 84.2% One-year survival rate | [52] | |
| Tasadenoturev | Glioma | Phase I | 20% Patients survived >3 years | [53,54] | |
| ONCOS-102 | Refractory solid tumors | Phase I | 40% Disease control | [55] | |
| Reolysin | Pancreatic | Phase I | No difference in progression-free survival to chemotherapy | [56] | |
| Cavatak | Melanoma | Phase II | 38.6% Progression-free survival at 6 months | [56] | |
| PVSRIPO | Glioma | Phase 1 | 21% Survival at 24 months | [57] | |
| Bacteria | Bacillus Calmette–Guérin | Non-muscle invasive bladder cancer (only FDA-approved indication) | Phase II | 76% Complete remission | [58] |
| VNP20009 | Advanced or metastatic cancer | Phase I | Report a safe profile | [59]. | |
| C. novyi-NT | Refractory solid tumors | Phase I | 41% Decrease in the size of the injected tumor | [60] | |
| Cells | Autologous dendritic cells | Melanoma; breast | Clinical study | Regression of the injected tumors | [61] |
| Ilixadencel | Gastrointestinal stromal tumor | Phase I | 33% Tumor responses | [62] | |
| Tumor-infiltrating lymphocytes | Melanoma | Clinical study | Tumor regression | [63] | |
| Erbb-targeted CAR-T | Squamous cell carcinoma | Phase I | 60% Stabilization of disease | [64] | |
| C-Met-CAR-T | Breast | Phase 0 | Evoke an inflammatory response within tumors | [65] | |
| Xenogeneic tissue cells | Bladder; breast; pancreatic | Preclinical murine model | Suppress tumor growth | [66,67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyr, C.-R.; Liu, L.-C.; Chien, H.-S.; Huang, C.-P. Immunotherapeutic Agents for Intratumoral Immunotherapy. Vaccines 2023, 11, 1717. https://doi.org/10.3390/vaccines11111717
Shyr C-R, Liu L-C, Chien H-S, Huang C-P. Immunotherapeutic Agents for Intratumoral Immunotherapy. Vaccines. 2023; 11(11):1717. https://doi.org/10.3390/vaccines11111717
Chicago/Turabian StyleShyr, Chih-Rong, Lang-Chi Liu, Hui-Shan Chien, and Chi-Ping Huang. 2023. "Immunotherapeutic Agents for Intratumoral Immunotherapy" Vaccines 11, no. 11: 1717. https://doi.org/10.3390/vaccines11111717
APA StyleShyr, C.-R., Liu, L.-C., Chien, H.-S., & Huang, C.-P. (2023). Immunotherapeutic Agents for Intratumoral Immunotherapy. Vaccines, 11(11), 1717. https://doi.org/10.3390/vaccines11111717





