Active and Passive Immunization with an Anti-Methamphetamine Vaccine Attenuates the Behavioral and Cardiovascular Effects of Methamphetamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Drug
2.2. Vaccine Preparation and Administration
2.3. Quantification of Anti-Methamphetamine Antibody Levels
2.4. Methamphetamine-Induced Locomotor Activity in Mice
2.5. Passive Immunization
2.6. Intravenous Cannula and Telemetry Probe Implantation
2.7. Tests Following Methamphetamine Administration
2.8. Intravenous Catheter Implantation and Methamphetamine Self-Administration Training
2.9. Reinstatement Tests
2.10. Data Analysis
3. Results
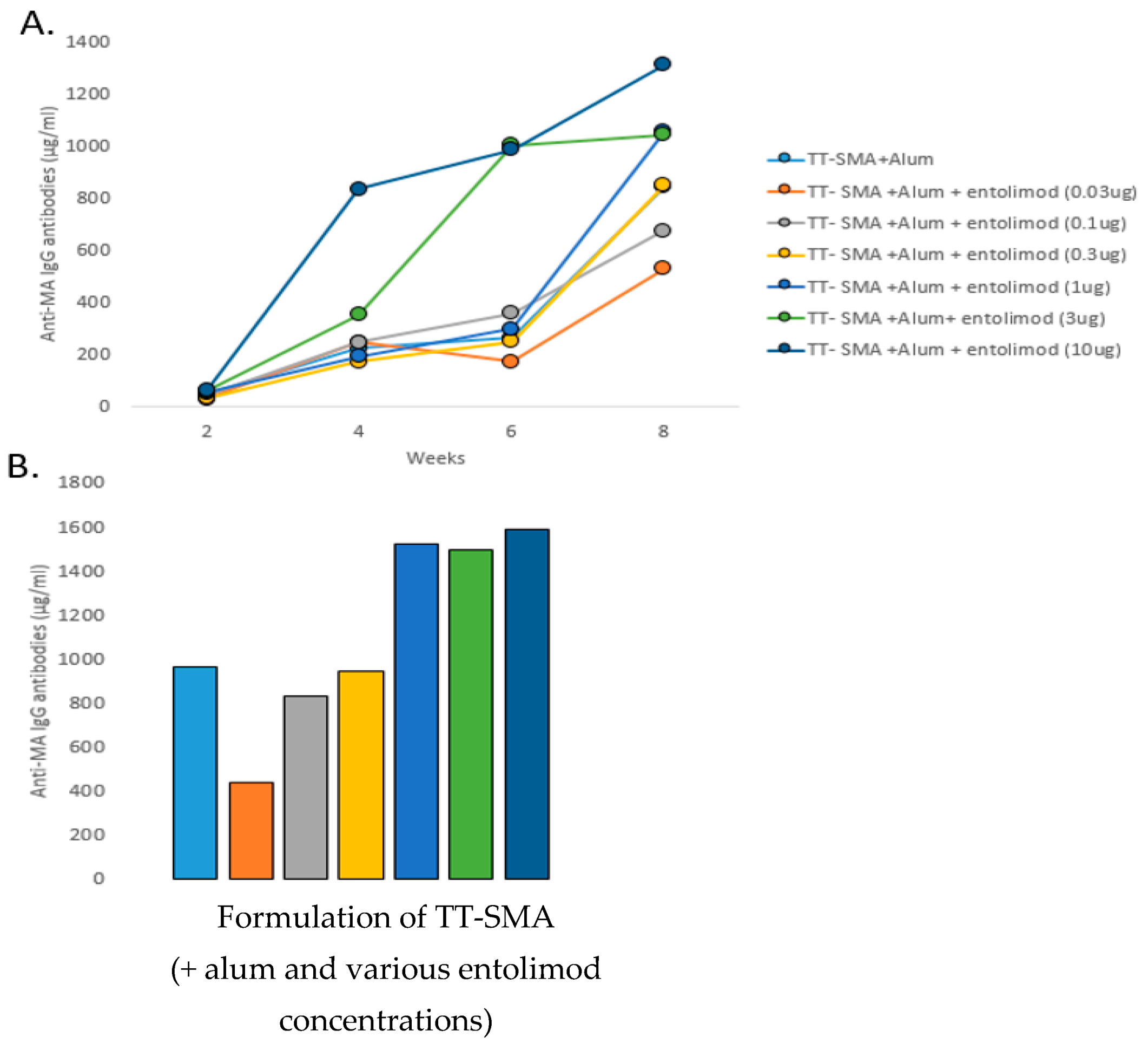
3.1. Anti-MA Response to Different Entolimod Doses
3.2. MA Induced Locomotor Activity
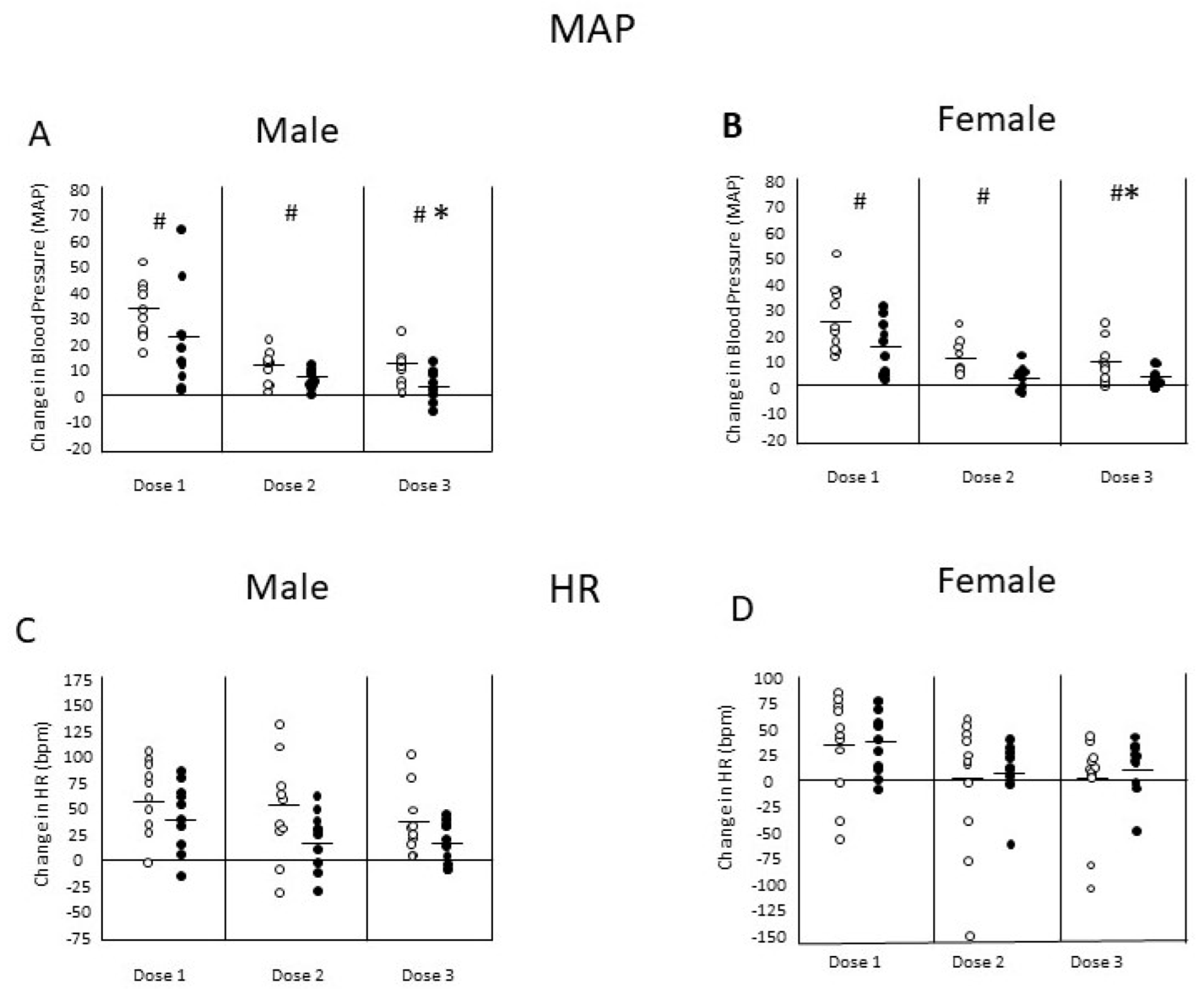
3.3. Passive Immunization and MA Cardiovascular Effects
3.4. Passive Immunization and Drug-Induced Reinstatement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health; (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56); Center for Behavioral Health Statistics and Quality; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2021; Available online: https://www.samhsa.gov/data/ (accessed on 14 August 2022).
- Han, B.; Compton, W.M.; Jones, C.M.; Einstein, E.B.; Volkow, N.D. Methamphetamine Use, Methamphetamine Use Disorder, and Associated Overdose Deaths Among US Adults. JAMA Psychiatry 2021, 78, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.S.; Kasper, Z.A.; Cicero, T.J. Twin epidemics: The surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend. 2018, 193, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Siefried, K.J.; Acheson, L.S.; Lintzeris, N.; Ezard, N. Pharmacological Treatment of Methamphetamine/Amphetamine Dependence: A Systematic Review. CNS Drugs 2020, 34, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Twillman, R.K.; Dawson, E.; LaRue, L.; Guevara, M.G.; Whitley, P.; Huskey, A. Evaluation of Trends of Near-Real-Time Urine Drug Test Results for Methamphetamine, Cocaine, Heroin, and Fentanyl. JAMA Netw. Open 2020, 3, e1918514. [Google Scholar] [CrossRef]
- Kamp, F.; Proebstl, L.; Hager, L.; Schreiber, A.; Riebschlager, M.; Neumann, S.; Straif, M.; Schacht-Jablonowsky, M.; Manz, K.; Soyka, M.; et al. Effectiveness of methamphetamine abuse treatment: Predictors of treatment completion and comparison of two residential treatment programs. Drug Alcohol Depend. 2019, 201, 8–15. [Google Scholar] [CrossRef]
- Soares, E.; Pereira, F.C. Pharmacotherapeutic strategies for methamphetamine use disorder: Mind the subgroups. Expert Opin. Pharmacother. 2019, 20, 2273–2293. [Google Scholar] [CrossRef]
- Byrnes-Blake, K.A.; Carroll, F.I.; Abraham, P.; Owens, S.M. Generation of anti-(+)methamphetamine antibodies is not impeded by (+)methamphetamine administration during active immunization of rats. Int. Immunopharmacol. 2001, 1, 329–338. [Google Scholar] [CrossRef]
- Duryee, M.J.; Bevins, R.A.; Reichel, C.M.; Murray, J.E.; Dong, Y.; Thiele, G.M.; Sanderson, S.D. Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine 2009, 27, 2981–2988. [Google Scholar] [CrossRef]
- Carroll, F.I.; Blough, B.E.; Pidaparthi, R.R.; Abraham, P.; Gong, P.K.; Deng, L.; Huang, X.; Gunnell, M.; Lay, J.O., Jr.; Peterson, E.C.; et al. Synthesis of mercapto-(+)-methamphetamine haptens and their use for obtaining improved epitope density on (+)-methamphetamine conjugate vaccines. J. Med. Chem. 2011, 54, 5221–5228. [Google Scholar] [CrossRef]
- Moreno, A.Y.; Mayorov, A.V.; Janda, K.D. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J. Am. Chem. Soc. 2011, 133, 6587–6595. [Google Scholar] [CrossRef]
- Miller, M.L.; Moreno, A.Y.; Aarde, S.M.; Creehan, K.M.; Vandewater, S.A.; Vaillancourt, B.D.; Wright, M.J.; Janda, K.D.; Taffe, M.A. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol. Psychiatry 2013, 73, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Ruedi-Bettschen, D.; Wood, S.L.; Gunnell, M.G.; West, C.M.; Pidaparthi, R.R.; Carroll, F.I.; Blough, B.E.; Owens, S.M. Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine 2013, 31, 4596–4602. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Y.; Kosten, T.A.; Lopez, A.Y.; Kinsey, B.M.; Kosten, T.R.; Orson, F.M. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013, 129, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.C.; Janda, K.D. Investigating hapten clustering as a strategy to enhance vaccines against drugs of abuse. Bioconjug. Chem. 2014, 25, 593–600. [Google Scholar] [CrossRef]
- Collins, K.C.; Schlosburg, J.E.; Lockner, J.W.; Bremer, P.T.; Ellis, B.A.; Janda, K.D. Lipid tucaresol as an adjuvant for methamphetamine vaccine development. Chem. Commun. 2014, 50, 4079–4081. [Google Scholar] [CrossRef]
- Haile, C.N.; Kosten, T.A.; Shen, X.Y.; O’Malley, P.W.; Winoske, K.J.; Kinsey, B.M.; Wu, Y.; Huang, Z.; Lykissa, E.D.; Naidu, N.; et al. Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. Am. J. Addict. 2015, 24, 748–755. [Google Scholar] [CrossRef]
- Miller, M.L.; Aarde, S.M.; Moreno, A.Y.; Creehan, K.M.; Janda, K.D.; Taffe, M.A. Effects of active anti-methamphetamine vaccination on intravenous self-administration in rats. Drug Alcohol Depend. 2015, 153, 29–36. [Google Scholar] [CrossRef]
- Collins, K.C.; Schlosburg, J.E.; Bremer, P.T.; Janda, K.D. Methamphetamine Vaccines: Improvement through Hapten Design. J. Med. Chem. 2016, 59, 3878–3885. [Google Scholar] [CrossRef]
- Gooyit, M.; Miranda, P.O.; Wenthur, C.J.; Ducime, A.; Janda, K.D. Influencing Antibody-Mediated Attenuation of Methamphetamine CNS Distribution through Vaccine Linker Design. ACS Chem. Neurosci. 2017, 8, 468–472. [Google Scholar] [CrossRef]
- Arora, R.; Haile, C.N.; Kosten, T.A.; Wu, Y.; Ramakrishnan, M.; Hawkins, L.D.; Orson, F.M.; Kosten, T.R. Preclinical efficacy of an anti-methamphetamine vaccine using E6020 adjuvant. Am. J. Addict. 2019, 28, 119–126. [Google Scholar] [CrossRef]
- Olson, M.E.; Sugane, T.; Zhou, B.; Janda, K.D. Consequence of Hapten Stereochemistry: An Efficacious Methamphetamine Vaccine. J. Am. Chem. Soc. 2019, 141, 14089–14092. [Google Scholar] [CrossRef]
- Keller, C.M.; Spence, A.L.; Stevens, M.W.; Owens, S.M.; Guerin, G.F.; Goeders, N.E. Effects of a methamphetamine vaccine, IXT-v100, on methamphetamine-related behaviors. Psychopharmacology 2020, 237, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Gentry, W.B.; Laurenzana, E.M.; Williams, D.K.; West, J.R.; Berg, R.J.; Terlea, T.; Owens, S.M. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int. Immunopharmacol. 2006, 6, 968–977. [Google Scholar] [CrossRef]
- Kosten, T.R.; Domingo, C.B.; Shorter, D.; Orson, F.; Green, C.; Somoza, E.; Sekerka, R.; Levin, F.R.; Mariani, J.J.; Stitzer, M.; et al. Vaccine for cocaine dependence: A randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014, 140, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Martell, B.A.; Orson, F.M.; Poling, J.; Mitchell, E.; Rossen, R.D.; Gardner, T.; Kosten, T.R. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: A randomized, double-blind, placebo-controlled efficacy trial. Arch. Gen. Psychiatry 2009, 66, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Bryant, C.E.; Doyle, S.L. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol. Rev. 2009, 61, 177–197. [Google Scholar] [CrossRef]
- Hennessy, E.J.; Parker, A.E.; O’Neill, L.A. Targeting Toll-like receptors: Emerging therapeutics? Nat. Rev. Drug Discov. 2010, 9, 293–307. [Google Scholar] [CrossRef]
- Duthie, M.S.; Windish, H.P.; Fox, C.B.; Reed, S.G. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2011, 239, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Garaude, J.; Kent, A.; van Rooijen, N.; Blander, J.M. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci. Transl. Med. 2012, 4, 120ra116. [Google Scholar] [CrossRef]
- Bremer, P.T.; Schlosburg, J.E.; Lively, J.M.; Janda, K.D. Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol. Pharm. 2014, 11, 1075–1080. [Google Scholar] [CrossRef]
- Ishizaka, S.T.; Hawkins, L.D. E6020: A synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev. Vaccines 2007, 6, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Morefield, G.L.; Hawkins, L.D.; Ishizaka, S.T.; Kissner, T.L.; Ulrich, R.G. Synthetic Toll-like receptor 4 agonist enhances vaccine efficacy in an experimental model of toxic shock syndrome. Clin. Vaccine Immunol. 2007, 14, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Lockner, J.W.; Ho, S.O.; McCague, K.C.; Chiang, S.M.; Do, T.Q.; Fujii, G.; Janda, K.D. Enhancing nicotine vaccine immunogenicity with liposomes. Bioorg. Med. Chem. Lett. 2013, 23, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.W.; Gunnell, M.G.; Tawney, R.; Owens, S.M. Optimization of a methamphetamine conjugate vaccine for antibody production in mice. Int. Immunopharmacol. 2016, 35, 137–141. [Google Scholar] [CrossRef]
- Yang, F.; Kosten, T.R. Psychopharmacology: Neuroimmune signaling in psychiatric disease-developing vaccines against abused drugs using toll-like receptor agonists. Psychopharmacology 2019, 236, 2899–2907. [Google Scholar] [CrossRef]
- Al Ustwani, O.; Gupta, N.; Bakhribah, H.; Griffiths, E.; Wang, E.; Wetzler, M. Clinical updates in adult acute lymphoblastic leukemia. Crit. Rev. Oncol./Hematol. 2016, 99, 189–199. [Google Scholar] [CrossRef]
- deWit, H.; Stewart, J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 1981, 75, 134–143. [Google Scholar] [CrossRef]
- Shalev, U.; Highfield, D.; Yap, J.; Shaham, Y. Stress and relapse to drug seeking in rats: Studies on the generality of the effect. Psychopharmacology 2000, 150, 337–346. [Google Scholar] [CrossRef]
- Shaham, Y.; Shalev, U.; Lu, L.; DeWit, H. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology 2003, 168, 3–20. [Google Scholar] [CrossRef]
- Haile, C.N.; Kosten, T.A. Differential effects of D1- and D2-like compounds on cocaine self-administration in Lewis and Fischer 344 inbred rats. J. Pharmacol. Exp. Ther. 2001, 299, 509–518. [Google Scholar]
- Zhang, X.Y.; Kosten, T.A. Prazosin, an a-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol. Psychiatry 2005, 57, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Lockner, J.W.; Eubanks, L.M.; Choi, J.L.; Lively, J.M.; Schlosburg, J.E.; Collins, K.C.; Globisch, D.; Rosenfeld-Gunn, R.J.; Wilson, I.A.; Janda, K.D. Flagellin as carrier and adjuvant in cocaine vaccine development. Mol. Pharm. 2015, 12, 653–662. [Google Scholar] [CrossRef]
- Byrnes-Blake, K.A.; Laurenzana, E.M.; Carroll, F.I.; Abraham, P.; Gentry, W.B.; Lanes, R.D.; Owens, S.M. Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur. J. Pharmacol. 2003, 461, 119–128. [Google Scholar] [CrossRef]
- Hambuchen, M.D.; Rüedi-Bettschen, D.; Gunnell, M.G.; Hendrickson, H.; Owens, S.M. Chronic treatment of (+)-methamphetamine-induced locomotor effects in rats using one or a combination of two high affinity anti-methamphetamine monoclonal antibodies. Hum. Vaccines Immunother. 2016, 12, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.D.; Bremer, P.T.; Hwang, C.S.; Vandewater, S.A.; Collins, K.C.; Creehan, K.M.; Janda, K.D.; Taffe, M.A. Effective active vaccination against methamphetamine in female rats. Drug Alcohol Depend. 2017, 175, 179–186. [Google Scholar] [CrossRef]
- Singh, R.A.K.; Kosten, T.A.; Kinsey, B.M.; Shen, X.; Lopez, A.L.; Kosten, T.R.; Orson, F.M. Dose-dependent changes in the locomotor responses to methamphetamine in BALB/c mice: Low doses induce hypolocomotion. Pharmacol. Biochem. Behav. 2012, 103, 230–236. [Google Scholar] [CrossRef]
- Good, R.L.; Radcliffe, R.A. Methamphetamine-induced locomotor changes are dependent on age, dose and genotype. Pharmacol. Biochem. Behav. 2011, 98, 101–111. [Google Scholar] [CrossRef]
- Kosten, T.R.; Newton, T.F.; DeLaGarza, R.; Haile, C.N. Cocaine and Methamphetamine Dependence: Advances in Treatment; American Psychiatric Publishing: Arlington, VA, USA, 2012. [Google Scholar]
- Kantak, K.M.; Collins, S.L.; Lipman, E.G.; Bond, J.; Giovanoni, K.; Fox, B.S. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology 2000, 148, 251–262. [Google Scholar] [CrossRef]
- Carrera, M.R.; Ashley, J.A.; Zhou, B.; Wirsching, P.; Koob, G.F.; Janda, K.D. Cocaine vaccines: Antibody protection against relapse in a rat model. Proc. Natl. Acad. Sci. USA 2000, 97, 6202–6206. [Google Scholar] [CrossRef]
- McMillan, D.E.; Hardwick, W.C.; Li, M.; Gunnell, M.G.; Carroll, F.I.; Abraham, P.; Owens, S.M. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J. Pharmacol. Exp. Ther. 2004, 309, 1248–1255. [Google Scholar] [CrossRef]
- Barrett, A.C.; Miller, J.R.; Dohrmann, J.M.; Caine, S.B. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 2004, 47 (Suppl. 1), 256–273. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, R.; Deroche, V.; Koob, G.F.; Weiss, F. Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology 1996, 126, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Lindenstrøm, T.; Woodworth, J.; Dietrich, J.; Aagaard, C.; Andersen, P.; Agger, E.M. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect. Immun. 2012, 80, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- Køllgaard, T.; Ugurel-Becker, S.; Idorn, M.; Andersen, M.H.; Becker, J.C.; Straten, P.T. Pre-Vaccination Frequencies of Th17 Cells Correlate with Vaccine-Induced T-Cell Responses to Survivin-Derived Peptide Epitopes. PLoS ONE 2015, 10, e0131934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haile, C.N.; Varner, K.J.; Huijing, X.; Arora, R.; Orson, F.M.; Kosten, T.R.; Kosten, T.A. Active and Passive Immunization with an Anti-Methamphetamine Vaccine Attenuates the Behavioral and Cardiovascular Effects of Methamphetamine. Vaccines 2022, 10, 1508. https://doi.org/10.3390/vaccines10091508
Haile CN, Varner KJ, Huijing X, Arora R, Orson FM, Kosten TR, Kosten TA. Active and Passive Immunization with an Anti-Methamphetamine Vaccine Attenuates the Behavioral and Cardiovascular Effects of Methamphetamine. Vaccines. 2022; 10(9):1508. https://doi.org/10.3390/vaccines10091508
Chicago/Turabian StyleHaile, Colin N., Kurt J. Varner, Xia Huijing, Reetakshi Arora, Frank M. Orson, Thomas R. Kosten, and Therese A. Kosten. 2022. "Active and Passive Immunization with an Anti-Methamphetamine Vaccine Attenuates the Behavioral and Cardiovascular Effects of Methamphetamine" Vaccines 10, no. 9: 1508. https://doi.org/10.3390/vaccines10091508
APA StyleHaile, C. N., Varner, K. J., Huijing, X., Arora, R., Orson, F. M., Kosten, T. R., & Kosten, T. A. (2022). Active and Passive Immunization with an Anti-Methamphetamine Vaccine Attenuates the Behavioral and Cardiovascular Effects of Methamphetamine. Vaccines, 10(9), 1508. https://doi.org/10.3390/vaccines10091508



