Characterization of the Antibody and Interferon-Gamma Release Response after a Second COVID-19 Booster Vaccination
Abstract
:1. Introduction
2. Materials and Methods
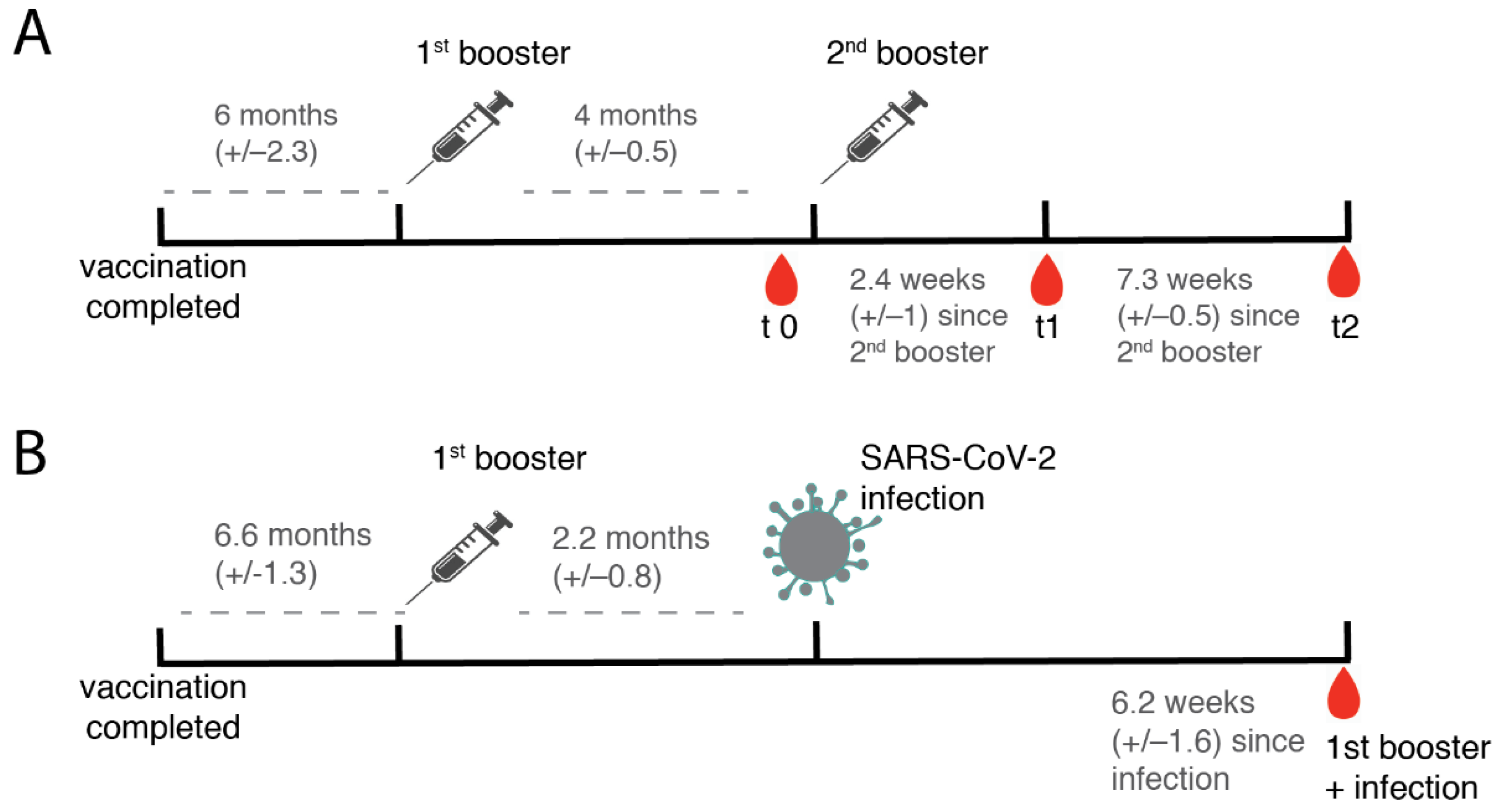
2.1. Collection of Serum Samples
2.2. Cell Culture
2.3. Virus Propagation and SARS-CoV-2 Variants
2.4. Anti-Spike IgG Assay
2.5. Neutralization Assay
2.6. Interferon Gamma Release Assay (IGRA)
2.7. Statistics
3. Results
3.1. Characterization of the Study Cohort after the Second Booster Vaccination
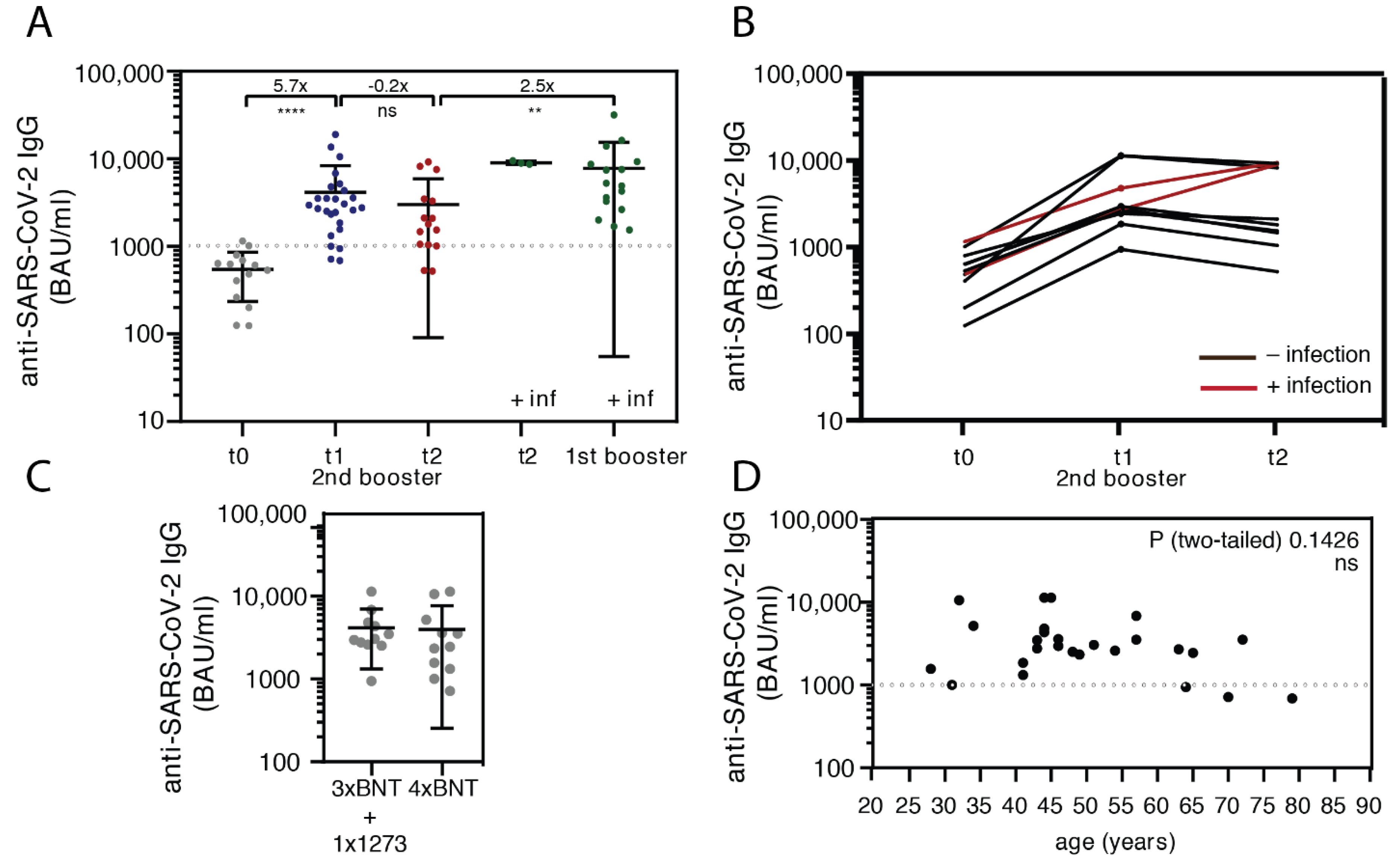
3.2. Anti-Spike IgG Antibody Levels after the Second Booster Vaccination
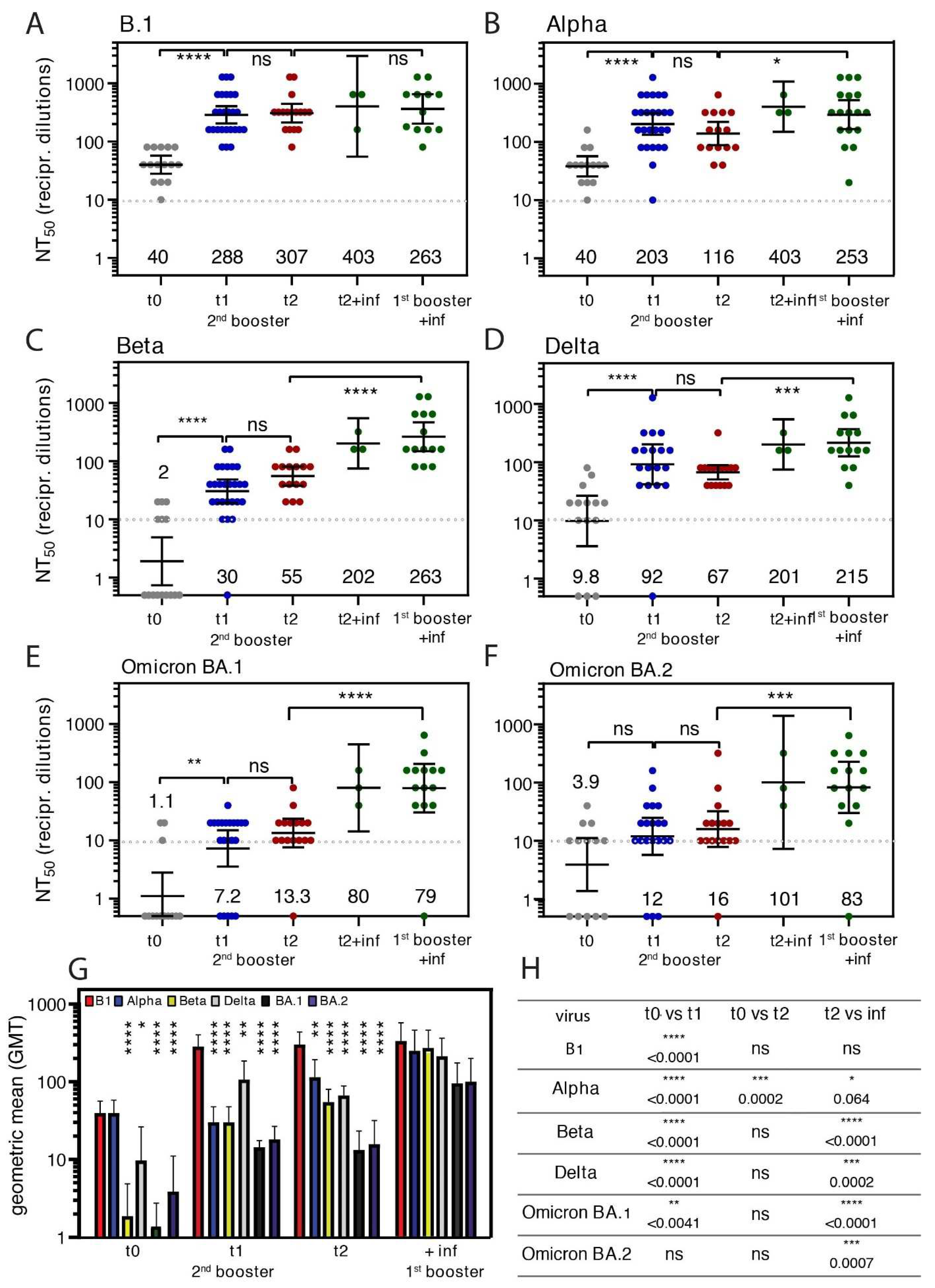
3.3. Booster Vaccination Elicited Neutralizing Antibodies against SARS-CoV-2 Variants
3.4. Neutralizing Antibodies against Diverse Variants of SARS-CoV-2 after Omicron BA.1 Infection
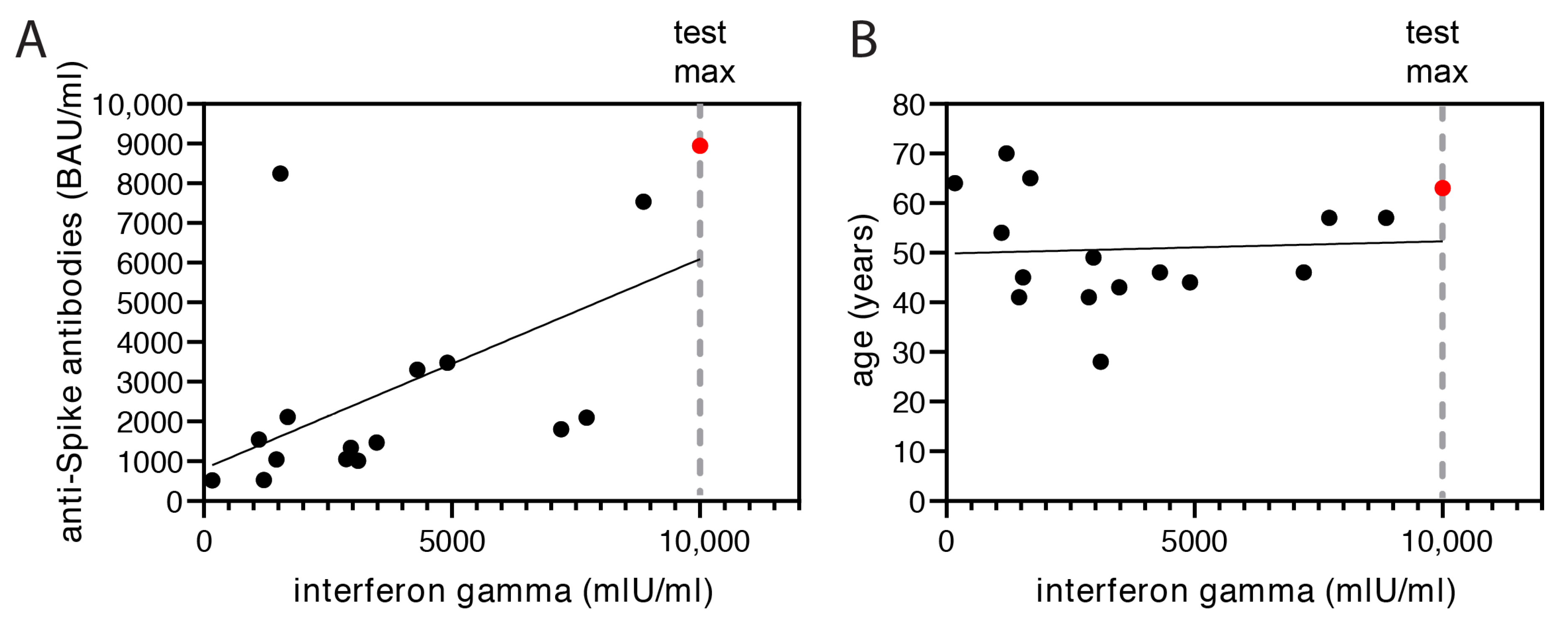
3.5. Evaluation of Interferon-Gamma Release after the Second Booster Vaccination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- German Ministry of Health National COVID19 Vaccination Strategy. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/C/Coronavirus/Impfstoff/National_COVID-19_Vaccination_Strategy_June_2021.pdf (accessed on 30 March 2022).
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological Mechanisms of Vaccine-Induced Protection against COVID-19 in Humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, J.; Qin, X.; Wang, W.; Xu, M.; Wang, L.-F.; Xu, C.; Tang, S.; Liu, P.; Zhang, L.; et al. SARS-CoV-2 Neutralizing Antibody Levels Are Correlated with Severity of COVID-19 Pneumonia. Biomed. Pharmacother. 2020, 130, 110629. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-Neutralizing Antibodies Predict Disease Severity and Survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Faustini, S.; Shields, A.; Banham, G.; Wall, N.; Al-Taei, S.; Tanner, C.; Ahmed, Z.; Efstathiou, E.; Townsend, N.; Goodall, M.; et al. Cross Reactivity of Spike Glycoprotein Induced Antibody against Delta and Omicron Variants before and after Third SARS-CoV-2 Vaccine Dose in Healthy and Immunocompromised Individuals. J. Infect. 2022, 84, 579–613. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Marschalek, R.; et al. Limited Neutralisation of the SARS-CoV-2 Omicron Subvariants BA.1 and BA.2 by Convalescent and Vaccine Serum and Monoclonal Antibodies. eBioMedicine 2022, 82, 104158. [Google Scholar] [CrossRef]
- Tang, J.W.; Tambyah, P.A.; Hui, D.S. Emergence of a New SARS-CoV-2 Variant in the UK. J. Infect. 2021, 82, e27–e28. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 Variant of Concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- Widera, M.; Wilhelm, A.; Hoehl, S.; Pallas, C.; Kohmer, N.; Wolf, T.; Rabenau, H.F.; Corman, V.M.; Drosten, C.; Vehreschild, M.J.G.T.; et al. Limited Neutralization of Authentic Severe Acute Respiratory Syndrome Coronavirus 2 Variants Carrying E484K In Vitro. J. Infect. Dis. 2021, 224, 1109–1114. [Google Scholar] [CrossRef]
- Ferreira, I.A.T.M.; Kemp, S.A.; Datir, R.; Saito, A.; Meng, B.; Rakshit, P.; Takaori-Kondo, A.; Kosugi, Y.; Uriu, K.; Kimura, I.; et al. SARS-CoV-2 B.1.617 Mutations L452R and E484Q Are Not Synergistic for Antibody Evasion. J. Infect. Dis. 2021, 224, 989–994. [Google Scholar] [CrossRef]
- Wilhelm, A.; Toptan, T.; Pallas, C.; Wolf, T.; Goetsch, U.; Gottschalk, R.; Vehreschild, M.J.G.T.; Ciesek, S.; Widera, M. Antibody-Mediated Neutralization of Authentic SARS-CoV-2 B.1.617 Variants Harboring L452R and T478K/E484Q. Viruses 2021, 13, 1693. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.d.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and Epidemiology of the P.1 SARS-CoV-2 Lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid Epidemic Expansion of the SARS-CoV-2 Omicron Variant in Southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Sarkar, R. Mutational and Phylogenetic Analyses of the Two Lineages of the Omicron Variant. J. Med. Virol. 2022, 94, 1777–1779. [Google Scholar] [CrossRef]
- Desingu, P.A.; Nagarajan, K.; Dhama, K. Emergence of Omicron Third Lineage BA.3 and Its Importance. J. Med. Virol. 2022, 94, 1808–1810. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity of, and Effectiveness of MRNA Vaccines against, Covid-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and Immunogenicity of SARS-CoV-2 Variant MRNA Vaccine Boosters in Healthy Adults: An Interim Analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef]
- World Health Organisation (WHO) Interim Statement on Booster Doses for COVID-19 Vaccination. Available online: https://www.who.int/news/item/22-12-2021-interim-statement-on-booster-doses-for-covid-19-vaccination---update-22-december-2021 (accessed on 30 March 2022).
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of Covid-19 MRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Widera, M.; Wilhelm, A.; Toptan, T.; Raffel, J.M.; Kowarz, E.; Roesmann, F.; Grözinger, F.; Siemund, A.L.; Luciano, V.; Külp, M.; et al. Generation of a Sleeping Beauty Transposon-Based Cellular System for Rapid and Sensitive Screening for Compounds and Cellular Factors Limiting SARS-CoV-2 Replication. Front. Microbiol. 2021, 12, 701198. [Google Scholar] [CrossRef]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Münch, C. Proteomics of SARS-CoV-2-Infected Host Cells Reveals Therapy Targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Toptan, T.; Hoehl, S.; Westhaus, S.; Bojkova, D.; Berger, A.; Rotter, B.; Hoffmeier, K.; Cinatl, J.; Ciesek, S.; Widera, M. Optimized QRT-PCR Approach for the Detection of Intra- and Extra-Cellular SARS-CoV-2 RNAs. Int. J. Mol. Sci. 2020, 21, 4396. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Helfritz, F.A.; et al. Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and Monoclonal Antibodies. medRxiv 2021. preprint. [Google Scholar]
- Pinilla, Y.T.; Heinzel, C.; Caminada, L.-F.; Consolaro, D.; Esen, M.; Kremsner, P.G.; Held, J.; Kreidenweiss, A.; Fendel, R. SARS-CoV-2 Antibodies Are Persisting in Saliva for More Than 15 Months After Infection and Become Strongly Boosted After Vaccination. Front. Immunol. 2021, 12, 798859. [Google Scholar] [CrossRef]
- Angyal, A.; Longet, S.; Moore, S.C.; Payne, R.P.; Harding, A.; Tipton, T.; Rongkard, P.; Ali, M.; Hering, L.M.; Meardon, N.; et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously Infected and SARS-CoV-2-Naive UK Health-Care Workers: A Multicentre Prospective Cohort Study. Lancet Microbe 2022, 3, e21–e31. [Google Scholar] [CrossRef]
- Coppeta, L.; Ferrari, C.; Somma, G.; Mazza, A.; D’Ancona, U.; Marcuccilli, F.; Grelli, S.; Aurilio, M.T.; Pietroiusti, A.; Magrini, A.; et al. Reduced Titers of Circulating Anti-SARS-CoV-2 Antibodies and Risk of COVID-19 Infection in Healthcare Workers during the Nine Months after Immunization with the BNT162b2 MRNA Vaccine. Vaccines 2022, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of MRNA-1273 against SARS-CoV-2 Omicron and Delta Variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and Reactogenicity of BNT162b2 Booster in ChAdOx1-S-Primed Participants (CombiVacS): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody Responses and Correlates of Protection in the General Population after Two Doses of the ChAdOx1 or BNT162b2 Vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Hofmann, C.; Ali, A.; Ayoub, P.; Kohn, D.B.; Yang, O.O. Previous Infection Combined with Vaccination Produces Neutralizing Antibodies with Potency against SARS-CoV-2 Variants. mBio 2021, 12, e02656-21. [Google Scholar] [CrossRef]
- Carreño, J.M.; Alshammary, H.; Tcheou, J.; Singh, G.; Raskin, A.J.; Kawabata, H.; Sominsky, L.A.; Clark, J.J.; Adelsberg, D.C.; Bielak, D.A.; et al. Activity of Convalescent and Vaccine Serum against SARS-CoV-2 Omicron. Nature 2022, 602, 682–688. [Google Scholar] [CrossRef]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough Infections with SARS-CoV-2 Omicron despite MRNA Vaccine Booster Dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- Yu, J.; Collier, A.Y.; Rowe, M.; Mardas, F.; Ventura, J.D.; Wan, H.; Miller, J.; Powers, O.; Chung, B.; Siamatu, M.; et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants. N. Engl. J. Med. 2022, 386, 1579–1580. [Google Scholar] [CrossRef] [PubMed]
- Delbrück, M.; Hoehl, S.; Toptan, T.; Schenk, B.; Grikscheit, K.; Metzler, M.; Herrmann, E.; Ciesek, S. Low But Recoverable Markers of Humoral Immune Response to BNT162b2 in Elderly LTCF Residents Five to Seven Months After Two-Dose Vaccination. Front. Aging 2022, 3, 883724. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grikscheit, K.; Rabenau, H.F.; Ghodratian, Z.; Widera, M.; Wilhelm, A.; Toptan Grabmair, T.; Hoehl, S.; Layer, E.; Helfritz, F.; Ciesek, S. Characterization of the Antibody and Interferon-Gamma Release Response after a Second COVID-19 Booster Vaccination. Vaccines 2022, 10, 1163. https://doi.org/10.3390/vaccines10071163
Grikscheit K, Rabenau HF, Ghodratian Z, Widera M, Wilhelm A, Toptan Grabmair T, Hoehl S, Layer E, Helfritz F, Ciesek S. Characterization of the Antibody and Interferon-Gamma Release Response after a Second COVID-19 Booster Vaccination. Vaccines. 2022; 10(7):1163. https://doi.org/10.3390/vaccines10071163
Chicago/Turabian StyleGrikscheit, Katharina, Holger F. Rabenau, Zahra Ghodratian, Marek Widera, Alexander Wilhelm, Tuna Toptan Grabmair, Sebastian Hoehl, Emily Layer, Fabian Helfritz, and Sandra Ciesek. 2022. "Characterization of the Antibody and Interferon-Gamma Release Response after a Second COVID-19 Booster Vaccination" Vaccines 10, no. 7: 1163. https://doi.org/10.3390/vaccines10071163
APA StyleGrikscheit, K., Rabenau, H. F., Ghodratian, Z., Widera, M., Wilhelm, A., Toptan Grabmair, T., Hoehl, S., Layer, E., Helfritz, F., & Ciesek, S. (2022). Characterization of the Antibody and Interferon-Gamma Release Response after a Second COVID-19 Booster Vaccination. Vaccines, 10(7), 1163. https://doi.org/10.3390/vaccines10071163





